Patterns of cancer recurrence in localized resected hepatocellular carcinoma
2015-02-06
Cleveland, USA
Patterns of cancer recurrence in localized resected hepatocellular carcinoma
Aryavarta MS Kumar, Elisha T Fredman, Christopher Coppa, Galal El-Gazzaz, Federico N Aucejo and May Abdel-Wahab
Cleveland, USA
BACKGROUND: Tumor resection in non-metastatic hepatocellular carcinoma (HCC) patients with adequate liver reserve offers a potential cure, but has a high 5-year recurrence rate. We analyzed the patterns of cancer relapse after partial hepatectomy to guide post-operative management.
METHODS: A total of 144 HCC patients (1996-2011) after partial hepatectomy were reviewed. Statistical correlations were determined using univariate and partition analyses.
RESULTS: A median follow-up of 20 months showed recurrence in 71 (49%) patients, and the median time to recurrence was 11.9 months. Vascular invasion (P<0.01) and number of lesions (P<0.01) predicted for recurrence. Histologic grade was not correlated with recurrence. Twenty-two (31%) patients developed both surgical margin (SM) and concurrent intrahepatic recurrences, and 28 (40%) had non-SM intrahepatic recurrences with no other signs of recurrence. On partition analysis, the risk of marginal recurrence in patients with SM <1 mm and SM ≥1 mm was 35% and 13.5% respectively. Approximately 57% of patients with intrahepatic recurrence had recurrence ≤2.5 cm from SM.
CONCLUSIONS: Intrahepatic recurrence after partial hepatectomy is common and is signifcantly associated with vascular invasion and tumor stage. About 57% of patients with intrahepatic relapse had a recurrence close (≤2.5 cm) to the SM. Additionally, patients with SM <1 mm have a higher recurrence rate and may beneft from adjuvant local therapy.
(Hepatobiliary Pancreat Dis Int 2015;14:269-275)
hepatocellular carcinoma; patterns of failure; partial hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is responsible for over 12 000 deaths annually in the USA and is currently one of the leading causes of cancer death worldwide.[1]The majority of HCC occurs in developing countries where hepatitis B (HBV) infection is the main etiology.[2]In developed countries, the rising incidence of HCC is attributed to hepatitis C (HCV) and the increase of non-alcoholic steatohepatitis (NASH).[3]Regardless of etiology, global mortality rates from HCC have steadily increased.
Progress in early diagnosis of HCC has given more patients the chance for cure despite challenges in access to healthcare worldwide. For surgical candidates, liver transplantation and partial hepatectomy are curative options, each presenting challenges.[4-6]While transplantation offers excellent cure rates especially in cirrhotic patients, donor organ shortage limits wide adoption. On the other hand, in patients with good liver reserve, partial hepatectomy avoids long-term immunosuppression from transplantation but has higher rates of recurrence; this could represent micrometastatic disease in the unresected liver or a new primary HCC in a cirrhotic liver. Identifcation of risk factors that predispose to recurrence, in both cirrhotic[7]and non-cirrhotic livers,[7-9]would help guide future treatment decisions. For example, retrospective studies have correlated liver reserve (Child-Pugh class), vascular invasion, size and the number of lesions with recurrent disease. Margin status and serum liver function tests remain controversial.[7,8,10-12]However, there have been limited reports evaluating the patterns of cancer recurrence. Understanding these patterns may guide decisions for local, regional, and systemic treat-ment after a partial hepatectomy.
This study analyzed patterns of cancer recurrence and examined outcomes from a prospective database of newly diagnosed non-metastatic HCC patients after partial hepatectomy. Patient, tumor, and treatment factors that correlate with recurrence were evaluated.
Methods
HCC patients who were surgical candidates were enrolled in a Institutional Review Board-approved prospective registry at our institution. Patients were diagnosed according to the American Association for the Study of Liver Diseases (AASLD) guidelines which include biopsy, radiographic changes, and/or pre-operative serum alphafetoprotein (AFP).[13]After imaging, dedicated hepatobiliary surgeons planned the operation and determined the necessary extent of resection. Cirrhosis was determined by radiographic evidence or clinical documentation before surgery. Patients with primary resection between January 1996 and January 2011 were analyzed.
Primary liver resection
Each patient had a pre-operative medical history and physical examination, an HBV and HCV screening, and other studies including complete blood count, metabolic panel, hepatic panel, and serum AFP. None of the patients received neoadjuvant treatment for HCC. Followup included routine post-operative visit(s) and multiphase contrast-enhanced cross-sectional liver imaging (CT or MRI) at each follow-up.
Defnition of recurrence
Tumor recurrence was defned as one of the following: 1) biopsy proven recurrence, 2) new lesion on follow-up imaging with characteristic features of HCC including early arterial contrast enhancement with contrast washout on CT or hypointensity with arterial phase contrast on MRI, or 3) indeterminate lesion with measurable growth on serial scans. The date of recurrence was either the date of biopsy proven HCC, the date of imaging diagnosing HCC, or for indeterminate lesions when a recommendation for treatment was made after serial scans.
Radiographic evaluation of recurrence
Post-operative contrast-enhanced MRI or CT scans in patients with known disease recurrence were displayed on an AGFA Impax picture archiving and communication system (PACS) and analyzed in a non-blinded fashion by a board-certifed radiologist specialized in abdominal imaging. After review of operative reports, the presence of surgical clips and altered hepatic contour were used to defne the margins of the surgical bed on the post-operative scans. The PACS measurement tool was used to determine the shortest distance (cm) from the closest margin of the surgical bed to the center of the recurrent lesion. If the surgical margin and recurrence were not on the same axial image, the shortest distance was determined after creating a multiplanar reformatted image with the PACS 3-D reconstruction tool. If there was more than one intrahepatic recurrence, the distance from the surgical bed to the farthest recurrence was measured as a proxy for the range of recurrent intrahepatic disease.
Statistical analysis
All statistical analyses were done with JMP Pro v10.0 (SAS, Cary, NC, USA). Demographics, clinical features, and tumor characteristics were analyzed using Fisher's exact test for categorical variables and Wilcoxon's ranksum test for continuous variables. Descriptive statistics were reported as median (range) or mean±standard deviations. Using these data sets, partition analyses were used to locate data trends and to obtain important threshold values. The Kaplan-Meier method and log-rank test were used to analyze survival. Patients were censored based on their last oncologic or surgical follow-up date.
Two different local recurrences were analyzed. A surgical margin (SM) recurrence was defned by any sized lesion at the SM. A local recurrence was defned as a recurrence within 2.5 cm from the SM to the center of the lesion. This may include some small and intermediate sized (<5 cm) SM recurrences. Local recurrences are defned this way to address the effective range of intraoperative radiation.Pvalues <0.05 were considered statistically signifcant.
Results
One hundred and forty-seven patients newly diagnosed with HCC had upfront partial hepatectomy. Three patients (2%) were excluded from further analysis due to regional nodal or distant metastatic disease. Patient and tumor characteristics along with surgery details and pathology results are summarized in Table 1. The median follow-up was 20 months and recurrence was seen in 71 patients (49%) with a median time to recurrence of 11.9 months (range 1.6-69.9).
With regard to diagnosis, 50 (35%) patients presented with an incidental fnding; some of these patients had previously documented cirrhosis. Forty-six (32%) patients did not have a biopsy and were diagnosed by se-rum AFP and/or radiographic methods.
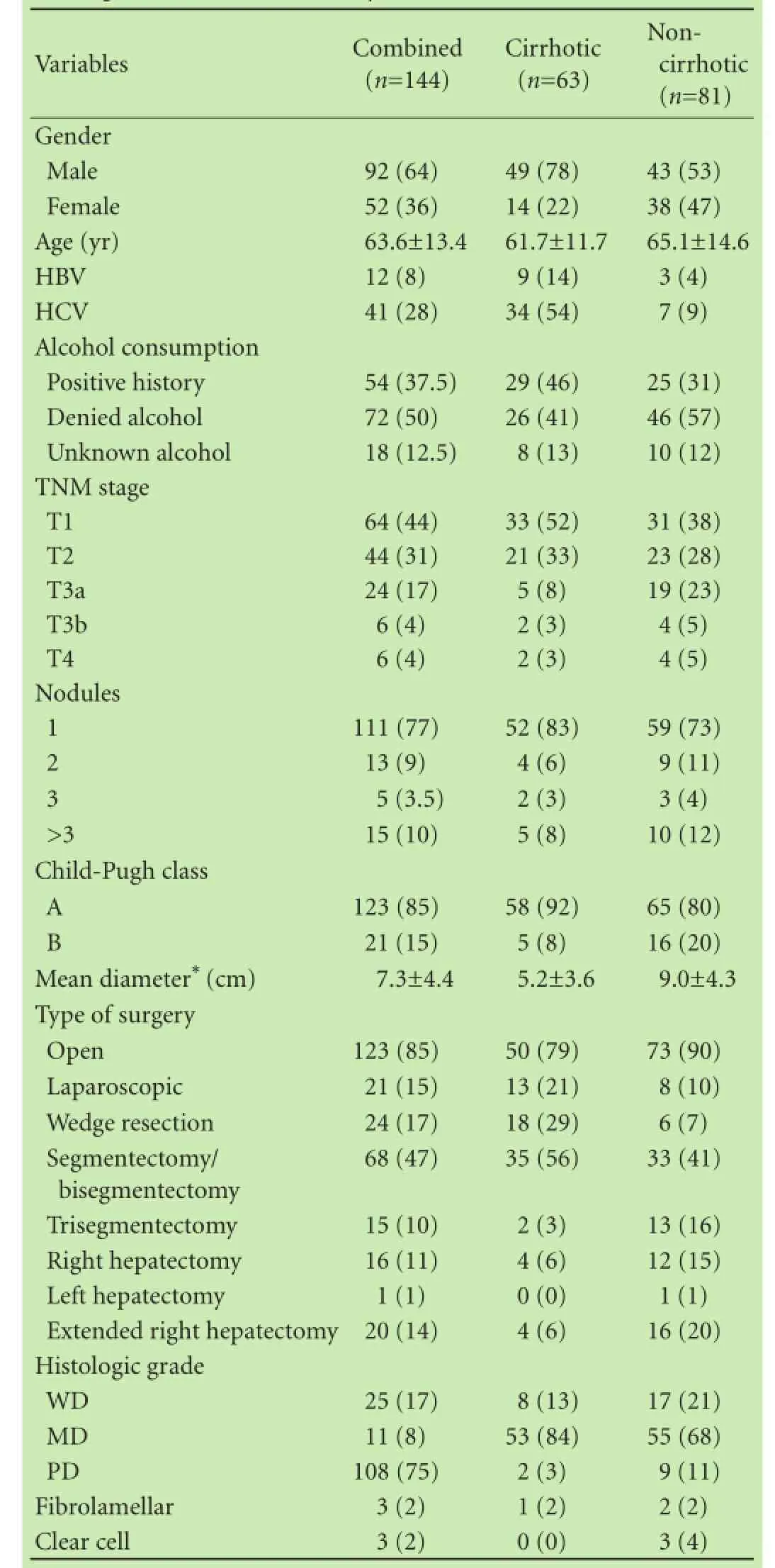
Table 1.Patient, tumor, surgery, and pathology characteristics for all patients and stratifed by cirrhosis (n, %)
Median time from initial HCC diagnosis to partial hepatectomy was 4.3 months. Sixty (42%) patients had vascular invasion (major vascular structures), 8 (6%) had microportal (microvascular) invasion on fnal pathology, and 2 (1%) had tumor rupture.
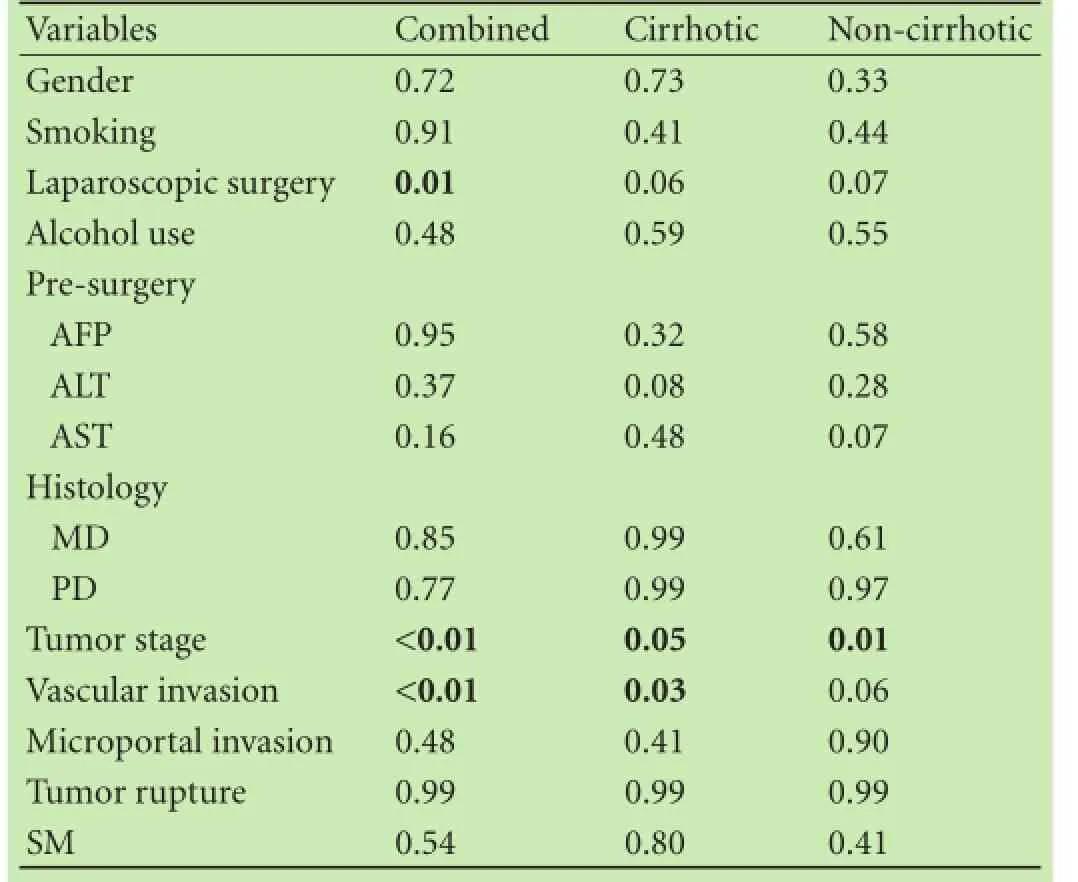
Table 2.Univariate analysis of patient and tumor factors correlated with recurrence
Correlation with recurrence
Univariate analysis showed that many patient and tumor factors were not signifcantly associated with tumor recurrence; however, vascular invasion (P<0.01), tumor stage (P<0.01), and laparoscopic surgery (P=0.01) were signifcantly correlated to recurrence (Table 2). Open surgery was correlated with a higher recurrence compared to laparoscopic surgery (P<0.01); mean diameter of largest tumor was 3.6 cm in the laparoscopic group and 8.0 cm in the open surgery group. Recurrence in the open and laparoscopic surgery groups were 67 (54%) and 5 (24%), respectively. Non-anatomic resection was not signifcantly correlated to recurrence. Microportal invasion, histologic grade/differentiation, degree of SM involvement, and tumor capsular rupture were not signifcantly associated with recurrence (P>0.05).
When stratifying patients by cirrhosis, there was no statistically signifcant correlation with recurrence. Postoperative serum AFP was not correlated with recurrence. In the 12 HBV positive patients, 7 recurred and 5 did not.
In the group of cirrhotic patients, the presence of vascular invasion was signifcantly correlated with recurrence (P=0.03). As expected, univariate analysis showed vascular invasion and recurrence correlated with survival (Fig. 1,P=0.03 on log-rank test). Cirrhotic patients tended to have a shorter median survival than non-cirrhotic patients (Fig. 2;P=0.09). The size of tumor in noncirrhotic patients was signifcantly correlated with recurrence (P<0.01). There was no recurrence or survival dif-ference between anatomic and non-anatomic resections between cirrhotic and non-cirrhotic patients.
Patterns of recurrence
The 71 documented recurrences were recorded as 1) SM relapses, 2) non-SM intrahepatic recurrences, and/or 3) distant recurrences (Fig. 3). Twenty-two patients (31%) had SM recurrence, 63 (89%) had an intrahepatic recurrence, and 29 (41%) had distant recurrence. After review of the location of the surgical bed, two of the metastases appeared to result from SM recurrences close to the capsule leading to peritoneal metastases. Of the 63 intrahepatic recurrences, 49 (78%) had suffcient abdominal imaging in the medical record to conduct a more detailed patterns of relapse analysis. The mean closest recurrence was 3.6±4.3 cm and the mean farthest recurrence was 11.7±3.5 cm. The distance of closest recurrences was not correlated with vascular invasion or cirrhosis (Figs. 4 and 5). No association was seen between distance of recurrence from the surgical bed and presence or absence of cirrhosis.
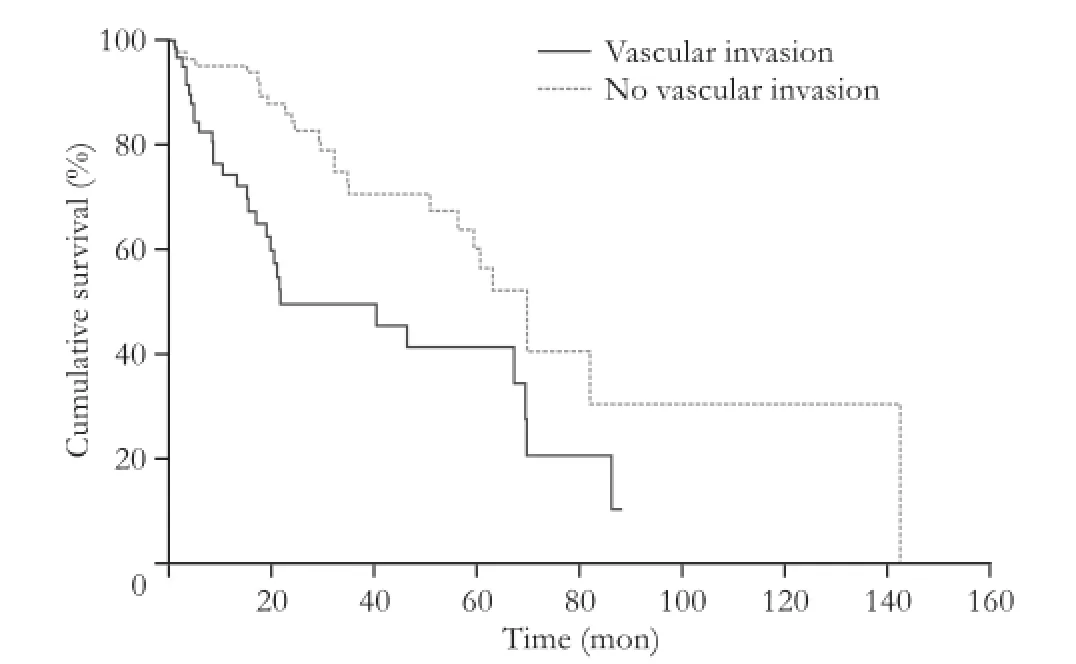
Fig. 1.Kaplan-Meier survival curve stratifed by vascular invasion. The two curves are significantly different on the log-rank test (P=0.03).
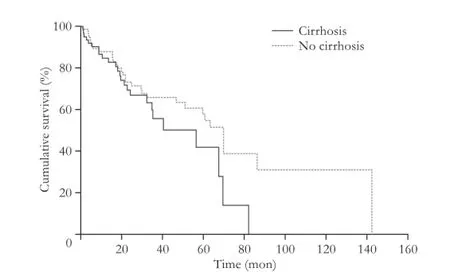
Fig. 2.Kaplan-Meier survival curve stratifed by cirrhosis. Curves are not signifcantly different on the log-rank test (P=0.09).
There were 22 patients who had an SM recurrence. SM involvement was not a signifcant predictor for recurrence as a continuous variable, but we found, out of a total of 22 SM recurrences, that 7 (35%) patients with <1 mm margin had SM recurrences; this compares to 15 (13.5%) patients with ≥1 mm margins that had SM re-currences. This difference was not statistically signifcant likely due to our small sample size. Out of the non-SM intrahepatic failures, 10 (14%) patients had only a single site of recurrence, whereas 30 (42%) had over fve recurrent lesions.
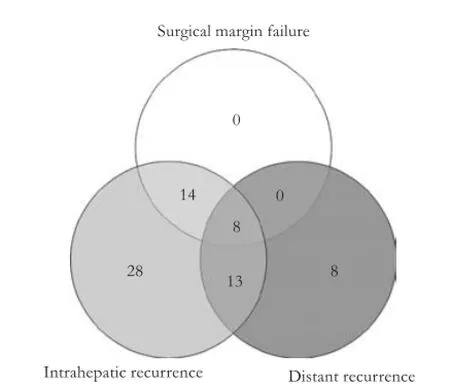
Fig. 3.Patterns of recurrence grouped by surgical margin, intrahepatic, and distant recurrence.
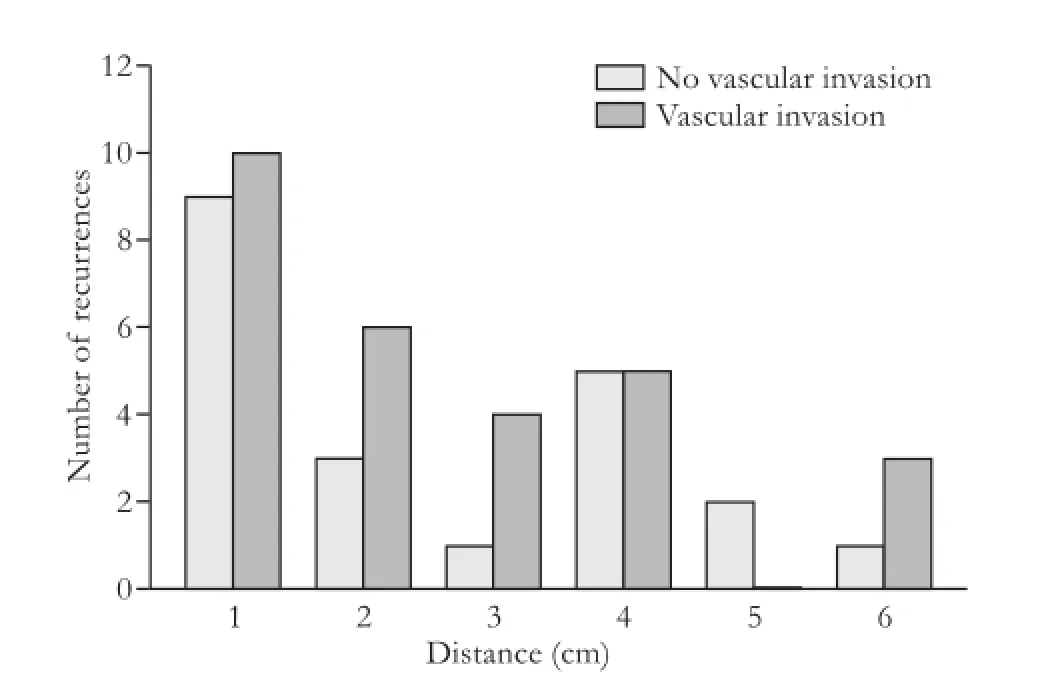
Fig. 4.Closest recurrence measured from surgical bed (cm) stratifed by vascular invasion.
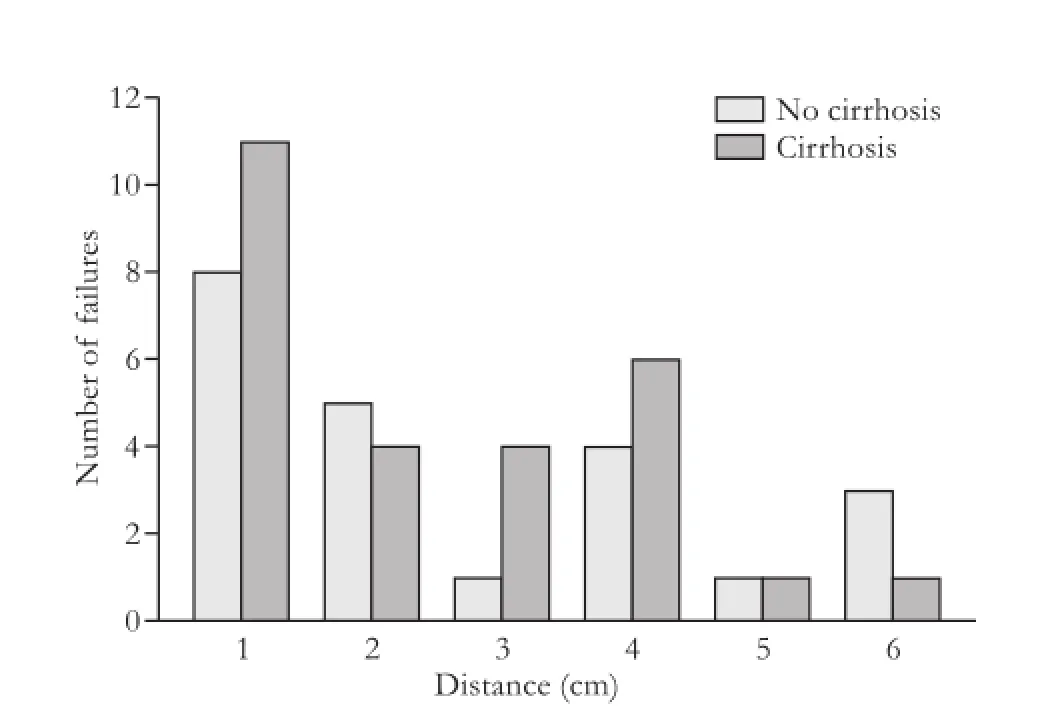
Fig. 5.Closest recurrence measured from surgical bed (cm) stratifed by cirrhosis.
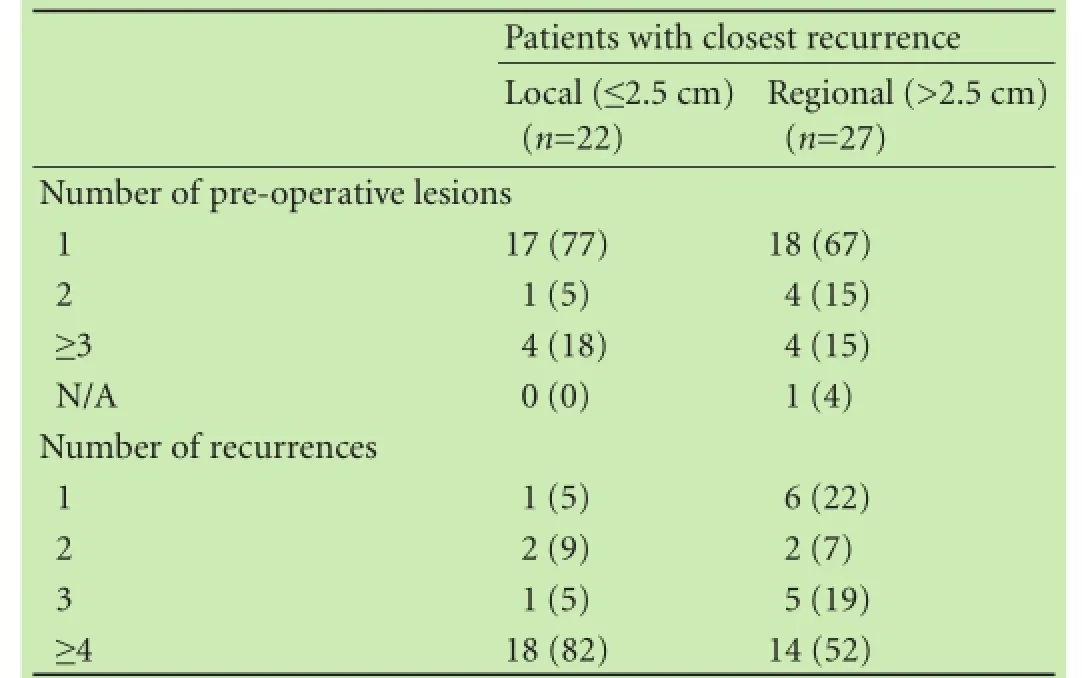
Table 3.Distance of closest intrahepatic relapse grouped by local or regional recurrence (n, %)
Results of both SM and non-SM recurrences occurring within a 2.5 cm radius of the surgical bed are stratifed by the number of lesions on pre-operative scan and by the number of lesions found on recurrence (Table 3). From these data, 45% of patients who recurred have a closest intrahepatic recurrence within 2.5 cm of the surgical bed with no signifcant trend based on the number of pre-operative lesions or the number of recurrences.
Discussion
Recurrence after partial hepatectomy for localized HCC occurs frequently and management remains controversial. Risk factors and patterns of HCC recurrence need to be analyzed to decide a strategy for local and regional/ systemic therapy. To our knowledge, this study was the frst to analyze the patterns of recurrence in HCC patients after partial hepatectomy.
Our analysis suggests that patients with SM <1 mm have an increased risk of SM recurrence and would likely beneft from adjuvant local therapy such as stereotactic body radiotherapy or intra-operative radiotherapy. This is especially true of tumors that are close to the liver capsule with inadequate SM that could lead to peritoneal metastases. Our analysis of local recurrences (within 2.5 cm of surgical bed) was focused on identifying appropriate patients for intra-operative radiotherapy. Additional studies are underway to determine whether adjuvant radiotherapy to the surgical bed reduces the risk of SM recurrence.
This study confrmed that vascular invasion and T-stage were correlated with recurrence. We also found that there was a trend towards increasing size correlating with recurrence likely due to the increased likelihood of vascular invasion with increased tumor size. Type of surgery (open or laparoscopic) was signifcantly correlated with recurrence and likely has selection bias as patients with smaller liver tumors were better candidates for laparoscopic surgery. Further subgroup analysis did not show statistical signifcance likely due to the small number of patients.
Chronic active hepatitis and cirrhosis are signifcant risk factors for new HCC lesions through multicentric carcinogenesis.[14]Indeed, reports have shown multicentric disease to have poor prognosis.[15,16]If viral hepatitis is the causative agent, then adjuvant antiviral treatment is recommended. Mechanistically, higher degrees of infammation enhance cellular proliferation and accelerate new foci of HCC. In the non-cirrhotic patients, factors such as tumor size, status of the resection margin, intrahepatic metastases, and portal vein invasion are independent predictors for survival.[16,17]While these risk factors are correlated in cirrhotic and non-cirrhotic subgroups. In our series, there was no overall signifcant correlation between recurrence and cirrhosis.
Patterns of HCC recurrence
While vascular invasion was correlated with the risk of recurrence, as expected, it was not correlated with the location of intrahepatic recurrence. We expected that in patients without vascular invasion, the risk of SM and local recurrence was higher than that of regional intrahepatic recurrence. However, patients with SM recurrence had other intrahepatic recurrences making it diffcult to assess which recurrence occurred frst. Many of the patients in this study were followed up for more than 12 months, and it is possible that those with multiple recurrences may have recurred at a single site between follow-up scans. AASLD guidelines specify 6-12 month follow-up intervals based on tumor doubling times; thus a 6-month follow-up would be recommended to better characterize patients who may have had an SM recurrence frst.
Overall trends indicated that larger SMs produce better control rates with the caveat that more normal liver tissue needs to be resected. A randomized trial suggested that a 2-cm SM could better control the recurrence compared to a 1-cm SM. However, the recurrence rates on the trial were higher than expected thus leading some to question the surgical technique.[18]This hasprompted many to reconsider exploring smaller margins to minimize the volume of resected normal liver, but this lower SM range remains controversial. Some reports[19,20]showed that an SM <5 mm has not negatively affected recurrence and survival; it may, however, increase the risk of surgical margin recurrences. In the present study, univariate analysis showed no correlation between SM as a continuous variable and SM recurrence (P>0.05). Partition analysis showed a higher rate of SM recurrence for SM <1 mm. These fndings suggest that SM ≥1 mm could reduce the risk of recurrence, but additional validation is needed because of the small sample size. For scenarios in which further resection is needed to obtain adequate margins, this presents an opportunity for further local treatments. If surgery is not feasible, radiotherapy may be an option as it has been used in other sites to decrease the risk of local recurrence with a positive or close surgical margin. Adjuvant radiotherapy is used in several other anatomic sites to reduce the risk of recurrence in a close or positive surgical margin in head and neck, thoracic, and pelvic malignancies. Similarly, as radiotherapy techniques have improved signifcantly to reduce toxicity by providing conformal radiation planning, this may provide a beneft for HCC patients.[21]A prospective randomized trial demonstrated that adjuvant radiotherapy after partial hepatectomy with <1 cm SM improves recurrence-free survival in patients with HCC less than 5 cm,[22]but additional study is needed to evaluate this further.
Of the patients with intrahepatic recurrences, 28 (57%) have an intrahepatic recurrence within 2.5 cm (Fig. 3). These recurrences are measured by distance from the surgical bed may be located in an adjacent liver segment. This distinction separates recurrences at the SM from those within 2.5 cm of the surgical bed. We noted that both single and multiple lesions on pre-operative imaging can lead to recurrences within 2.5 cm. Our data indicate that there were no pre-operative variables correlated with the risk of recurrence within 2.5 cm.
The location of a recurrence can infuence the choice of salvage treatment. After intrahepatic-only recurrence, transplantation is a reasonable option.[23]However, in patients with localized disease who may not have the option of transplantation, additional resection[24]and local treatments such as radiofrequency ablation and radiotherapy options such as intra-operative radiotherapy, external beam radiation therapy, and stereotactic body radiotherapy are under investigation;[22,25]these modalities may ultimately be combined with regional/systemic therapy.
In conclusion, partial hepatectomy of non-metastatic HCC offers a curative modality for well selected patients. When these patients recur, intrahepatic recurrences are more common than surgical margin relapse or distant metastases. Vascular invasion is correlated with any intrahepatic recurrence on univariate analysis; however, close SM <1 mm have a higher risk of surgical margin recurrence. Forty-fve percent of patients with an intrahepatic relapse had a recurrence within 2.5 cm of the surgical margin. Although additional studies will be helpful to clearly identify the subgroup of patients that develops surgical bed marginal recurrence, the data suggested a role for additional local therapy to reduce the risk of a local recurrence.
Contributors:KAMS and AWM proposed the study and wrote the frst draft. KAMS, FET, CC and AWM analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. KAMS is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-2576.
3 Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer:worldwide incidence and trends. Gastroenterology 2004;127:S5-S16.
4 Adam R, Bhangui P, Vibert E, Azoulay D, Pelletier G, Duclos-Vallée JC, et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: does size defne the best oncological strategy? Ann Surg 2012;256:883-891.
5 Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg 2003;238:315-323.
6 Morris-Stiff G, Gomez D, de Liguori Carino N, Prasad KR. Surgical management of hepatocellular carcinoma: is the jury still out? Surg Oncol 2009;18:298-321.
7 Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis:univariate and multivariate analysis. J Surg Oncol 2002;81:195-202.
8 Nara S, Shimada K, Sakamoto Y, Esaki M, Kishi Y, Kosuge T, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery 2012;151:526-536.
9 Ardiles V, Sánchez Clariá R, Mazza OM, Ciardullo MA, Pekolj J, De Santibañes E. Prognostic factors after resection of hepatocellular carcinoma in the non-cirrhotic liver: presentation of 51 cases. Cir Esp 2010;87:148-154.
10 Lee KT, Wang SN, Su RW, Chen HY, Shi HY, Ker CG, et al. Is wider surgical margin justifed for better clinical outcomes in patients with resectable hepatocellular carcinoma? J Formos Med Assoc 2012;111:160-170.
11 Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656-665.
12 Matsui Y, Terakawa N, Satoi S, Kaibori M, Kitade H, Takai S, et al. Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg 2007;142:596-603.
13 Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-1022.
14 Kobayashi T, Ishiyama K, Ohdan H. Prevention of recurrence after curative treatment for hepatocellular carcinoma. Surg Today 2013;43:1347-1354.
15 Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, et al. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg 2013;17:702-711.
16 Wörns MA, Bosslet T, Victor A, Koch S, Hoppe-Lotichius M, Heise M, et al. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol 2012;47:718-728.
17 Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg 2001;88:515-522.
18 Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43.
19 Jeng KS, Jeng WJ, Sheen IS, Lin CC, Lin CK. Is less than 5 mm as the narrowest surgical margin width in central resections of hepatocellular carcinoma justifed? Am J Surg 2013;206:64-71. 20 Tang YH, Wen TF, Chen X. Resection margin in hepatectomy for hepatocellular carcinoma: a systematic review. Hepatogastroenterology 2012;59:1393-1397.
21 Jacob R, Turley F, Redden DT, Saddekni S, Aal AK, Keene K, et al. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥3 cm. HPB (Oxford) 2015;17:140-149.
22 Yu W, Wang W, Rong W, Wang L, Xu Q, Wu F, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg 2014;218:381-392.
23 Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl 2013;19:411-419.
24 Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, et al. Is hepatic resection for recurrent or persistent hepatocellular carcinoma justifed? Hepatogastroenterology 2012;59:2255-2259.
25 Chen K, Xia Y, Wang H, Xiao F, Xiang G, Shen F. Adjuvant iodine-125 brachytherapy for hepatocellular carcinoma after complete hepatectomy: a randomized controlled trial. PLoS One 2013;8:e57397.
Received September 29, 2014
Accepted after revision April 2, 2015
AuthorAffliations:Department of Radiation Oncology, 9500 Euclid Ave, T28, Cleveland Clinic (Kumar AMS, Fredman ET and Abdel-Wahab M); Department of Radiology, 9500 Euclid Ave, Hb6, Cleveland Clinic (Coppa C); and Department of Hepato-pancreato-biliary & Transplant Surgery, 9500 Euclid Ave, A100 (El-Gazzaz G and Aucejo FN), Cleveland OH 44195, USA
Aryavarta MS Kumar, MD, PhD, Radiation Oncology, 6525 Powers Blvd, Parma OH 44129, USA (Tel: 440-743-4749; Fax: 440-743-4716; Email: aryavarta.kumar@gmail.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60382-4
Published online May 21, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer
- Histological examination of frozen sections for patients with acute cholecystitis during cholecystectomy
- Omental faps reduces complications after pancreaticoduodenectomy
- Endoscopic ultrasound-guided fne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic effcacy of cell-block immunocytochemistry
- Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines
- miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
