Adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma: a systematic review and a meta-analysis
2015-02-06
Nanchang, China
Adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma: a systematic review and a meta-analysis
Hua-Shan Lin, Ren-Hua Wan, Liang-Hui Gao, Jian-Feng Li, Ren-Feng Shan and Jun Shi
Nanchang, China
BACKGROUND: Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide and liver transplantation (LT) is considered as the best therapeutic option for patients with HCC combined with cirrhosis. However, tumor recurrence after LT for HCC remains the major obstacle for long-term survival. The present study was to evaluate the effcacy and necessity of adjuvant chemotherapy in patients with HCC who had undergone LT.
DATA SOURCES: Several databases were searched to identify comparative studies fulflling the predefned selection criteria before October 2014. Suitable studies were chosen and data extracted for meta-analysis. Three authors independently evaluated the bias of each study according to the Cochrane Handbook for Systematic Review of Intervention. Stata 12 was used for statistical analysis. Hazard ratio (HR) was considered as a summary statistic for overall survival, disease-free survival and recurrence rate.
RESULTS: Three prospective studies and 5 retrospective studies including 360 patients (166 in the adjuvant chemotherapy group, and 194 in the control group) were included. Compared with the control group, post-LT adjuvant chemotherapy conferred signifcant beneft for overall survival (HR: 0.34; 95% CI: 0.22-0.52;P=0.000). Meanwhile, the results showed an improvement for disease-free survival on favoring adjuvant chemotherapy (HR: 0.87; 95% CI: 0.78-0.95;P=0.004). However, no signifcant difference in HCC recurrence rate was observed between the two groups (HR: 1.26; 95% CI: 0.40-4.00;P=0.696). Descriptions of adverse events were of anecdotal nature and did not allow meta-analytic calculations.
CONCLUSIONS: Adjuvant chemotherapy after LT for HCC can signifcantly prolong patient's survival and delay the recurrence of HCC. For advanced HCC with poor differentiation, patients may perhaps beneft from the early implantation of adjuvant chemotherapy after LT.
(Hepatobiliary Pancreat Dis Int 2015;14:236-245)
adjuvant chemotherapy; liver transplantation; hepatocellular carcinoma; recurrence; meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide and a major cause of morbidity and mortality.[1]Because of a high propensity of vascular invasion and distant metastasis, conventional therapies including resection, radiofrequency ablation, and chemoembolization for liver malignancies have not achieved satisfactory results.[2-4]In HCC patients combined with cirrhosis, liver transplantation (LT) is considered as the best therapeutic option, since it treats HCC as well as the underlying liver disease. HCC patients within the Milan criteria can achieve excellent overall survival (OS) and recurrence-free survival rates. However, the results of transplantation alone for advanced HCC are disappointing, with a 1-year recurrence rate of 30%-60% and a 5-year survival rate of less than 30%.[5,6]Because of the high recurrence rate of advanced HCC and the scarcity of liver donor, many Transplant Centers have adopted strict selection criteria for LT in patients with HCC.
In an attempt to reduce HCC recurrence, many studies have investigated the effcacy of adjuvant chemother-apy for patients who have undergone LT. A diverse group of agents have been evaluated in the post-LT adjuvant chemotherapy, for instance, doxorubicin, cisplatin, gemcitabine, licartin and sorafenib. However, the clinical use of these agents is controversial or requires further evaluation.[7,8]Several studies[9-16]have suggested a modest beneft from post-LT adjuvant chemotherapy in contrast to other trials.[17-20]However, the side effect of adjuvant chemotherapy should be carefully evaluated because of the necessity of immunosuppression after LT. To date, several systemic reviews[8,21-24]have evaluated the role of adjuvant chemotherapy after LT. Nevertheless, none of them quantitatively measured the effects of adjuvant chemotherapy on survival or recurrence using time-to-event outcomes. Furthermore, emerging evidence from clinical controlled trials is currently available for accessing the role of adjuvant chemotherapy in post-LT patients.
In this study, a systematic review and a comprehensive meta-analysis were performed according to the Cochrane Handbook.[25]The extracted data were analyzed using hazard ratios (HRs). This systematic review aimed to evaluate the effcacy of adjuvant chemotherapy in terms of the disease-free survival (DFS), OS and recurrence rate of HCC patients after LT.
Methods
Study selection
We conducted a systematic search using electronic databases (MEDLINE, Embase, Cochrane Library Search, Ovid and Google Scholar) for all published studies without language restriction (the last published work search date: April 10, 2014). The keywords used were as follows:“liver transplantation“, “chemotherapy“, “hepatocellular carcinoma“, and “recurrence“. The “related articles“ function was used to widen the search, and reference lists of included trials were manually searched. In addition, published reviews and meta-analysis of relevance were scrutinized for potential studies. The detailed strategies are given in Fig. 1.
Inclusion and exclusion criteria
Inclusion criteria were as follows: 1) comparative studies with available full text evaluating adjuvant chemotherapy in patients with HCC who had undergone LT were considered, no matter whether preoperative adjuvant therapies were given or not; 2) patients in adjuvant chemotherapy group received one or more adjuvant agents by intravenous injection or oral administration, while the control group did not accept any modality of adjuvant chemotherapy after LT; and 3) follow-up data were available on DFS , OS or recurrence rate.
Exclusion criteria were as follows: 1) studies including cholangiocellular carcinoma participants; 2) studies that the control group received any modality of adjuvant chemotherapy after LT; 3) studies that adjuvant chemotherapy performed only before LT; 4) studies that patients received adjuvant chemotherapy because of recurrent HCC after LT; 5) mTOR inhibitor could function not only as immunosuppressor but also probably as chemotherapeutic drug, so studies that mTOR inhibitor was employed for immunosuppressive protocol were excluded; and 6) study overlapped with previous reportfrom same institution, and only the most informative and/or recent article was chosen for meta-analysis.
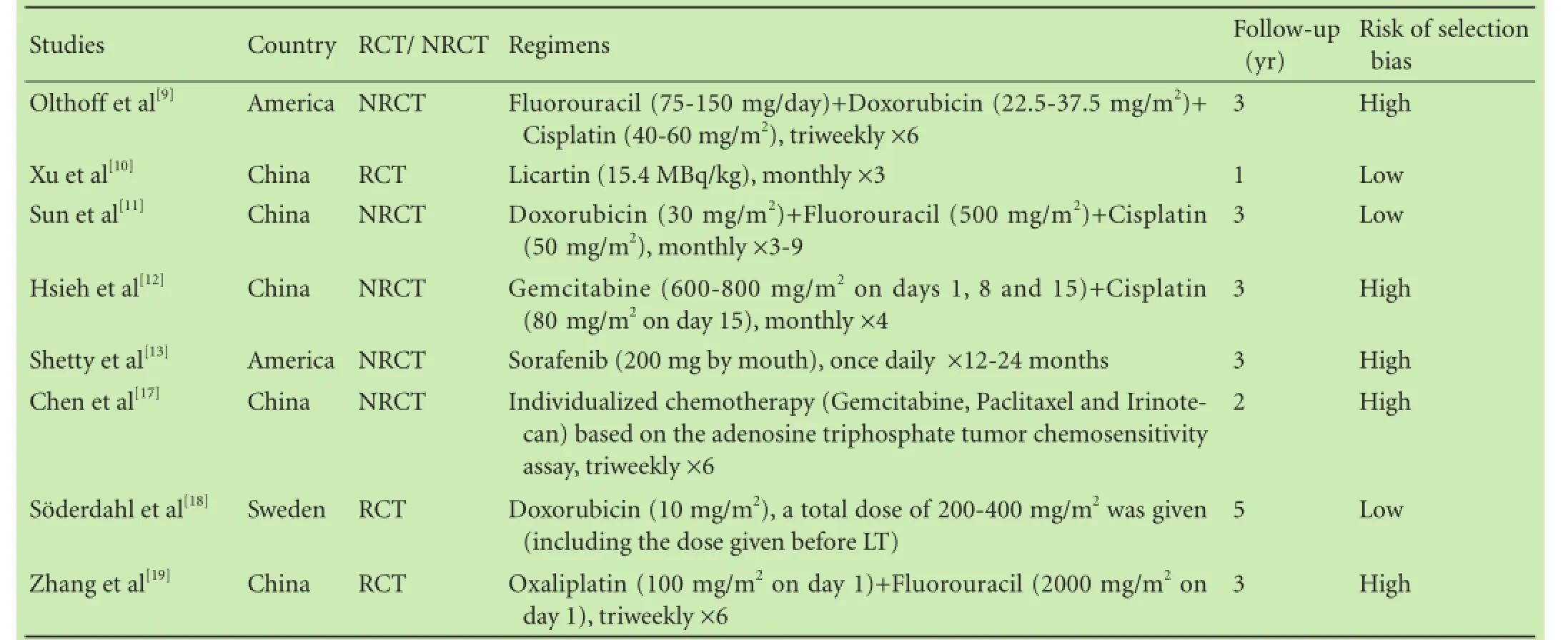
Table 1.Characteristics of the included studies
Data extraction
Three authors (LHS, WRH and SJ) selected the studies according to the criteria described above and extracted data independently using a predesigned data extraction form. Details of adjuvant chemotherapy protocols, tumor histological factors (size, number, vascular invasion), alpha fetoprotein (AFP) level, underlying liver disease (HBV, cirrhosis) and staging of HCC were frst evaluated. Furthermore, the number of patients developing events (death or recurrence) was recorded during the follow-up time, and the survival and recurrence rates at different time points (one, two, three, fve years) in each group were reported. If essential data were missing in a published trial, we tried to conduct the original author for more details.
Assessment of bias
Three authors (LHS, WRH and SJ) evaluated the quality of each included trial independently according to the process described in the Cochrane Handbook for Systematic Review of Intervention.[26]The following items were used for bias assessment: generation of allocation sequence, allocation concealment, completeness of outcomes selective reporting, and other bias. Adequate sequence generation and allocation concealment reduces the risk of selection bias. Therefore, trials with adequate randomization procedure (i.e., sequence generation plus allocation concealment) were classifed as trials with a low risk of selection bias.[26]However, unclear or inadequate randomization is associated with a risk of biased overestimation of treatment effects.[26,27]Accordingly, trials with uncertainty or lack of information were classifed as trials with a high risk of bias.
Statistical analysis
HR was considered as a summary statistic for OS, DFS and recurrence rate. The HR and 95% confdence interval (CI) were directly extracted from the study or estimated indirectly using the reported number of events and the correspondingPvalue for the log-rank statistics, or by reading survival curves, as described by Parmar et al.[27]A pooled HR represented the overall risk of an event on adjuvant chemotherapy over control. Interstudy heterogeneity was assessed using theI2.[28,29]When heterogeneity was not statistically signifcant, a fxedeffects model was adopted. If heterogeneity was signifcant (I2>50% andP<0.05), a random-effects model was adopted instead after exploring the causes of the heterogeneity. Statistical analyses were performed with Stata 12.
Results
Description of studies
A total of 360 relevant articles were identifed in a combined search of MEDLINE, Embase, Cochrane Library Search, Ovid and Google Scholar, and by a manual approach through previous reviews and of reference lists of the identifed articles. Totally 328 articles were excluded through scanning the title/abstract. Full-length articles could not be retrieved for 9 of the 32 accepted articles for various reasons. Of the 23 full text articles retrieved, 15 articles were excluded and reasons were as follows: 9 because of no controlled trials, 4 because of no available essential data (no more information could be obtained for the author),[14-16,20]1 because of the control group receiving routinely epirubicin after LT,[30]and 1 because of mTOR inhibitors were employed for immunosuppressive protocol.[31]Eventually, 8 articles with a total of 360 subjects were included in the meta-analysis, including 3 randomized controlled trials (RCTs) and 5 non-randomized controlled trials (NRCTs). The detailed process of study selection showed in Fig. 1.
All of the patients included in the 8 studies were diagnosed with HCC, and underwent LT. After the operation, 166 patients were treated with adjuvant chemotherapy, whereas 194 did not receive any modality of chemotherapy. A diverse group of agents were included in these trials: conventional adjuvant chemotherapy for HCC was based on doxorubicin and cisplatin[15]and was used in 3 studies;[9,11,18]novel agents including gemcitabine, oxaliplatin, licartin and sorafenib[8,15]were used in 5 studies.[10,12,13,17,19]It was explicitly stated in 3 studies[11,18,19]that part of the patients received doxorubicinor transarterial chemoembolization (TACE) before LT. The shortest follow-up time was 12 months and the longest follow-up time was 60 months. The smallest sample size was 19 patients and the largest size was 73. The data in terms of OS, DFS and recurrence rate were collected and at least one essential data regarding of HR should be available. The applications of adjuvant chemotherapy are listed in Table 1, and clinical backgrounds of the 8 studies included in the meta-analysis are listed in Table 2. Table 3 detailed the results regarding survival and recurrence.

Fig. 1.Flow chart of selection of studies for inclusion in metaanalysis.
Overall survival
In terms of OS, the heterogeneity between all seventrials was not statistically signifcant (χ2=9.34, degrees of freedom [df]=6;P=0.155;I2=35.8%). Pooling of HRs of the seven trials showed a signifcant difference favoring adjuvant chemotherapy on OS (HR: 0.34; 95% CI:0.22-0.52;P=0.000) (Fig. 2A). This result was confrmed by the following subgroup analysis. Pooling of HRs from four NRCTs showed a signifcant difference favoring adjuvant chemotherapy on OS (HR: 0.32; 95% CI:0.19-0.53;P=0.004). Similarly, three RCTs also showed a signifcant effect (HR: 0.38; 95% CI: 0.19-0.77;P=0.007) (Fig. 2B). Pooling of HRs from three trials with conventional agents showed a signifcant difference favoring adjuvant chemotherapy on OS (HR: 0.17; 95% CI:0.08-0.39;P=0.000), and the result was the same in four trials with novel agents (HR: 0.43; 95% CI: 0.26-0.70;P=0.001) (Fig. 2C). When the seven studies divided into a single agent group and a multiple agents group, two trials with single agent (HR: 0.34; 95% CI: 0.21-0.54;P=0.029) and fve trials with multiple agents (HR: 0.35; 95% CI: 0.13-0.90;P=0.000) showed a signifcant difference favoring adjuvant chemotherapy on OS (Fig. 2D).
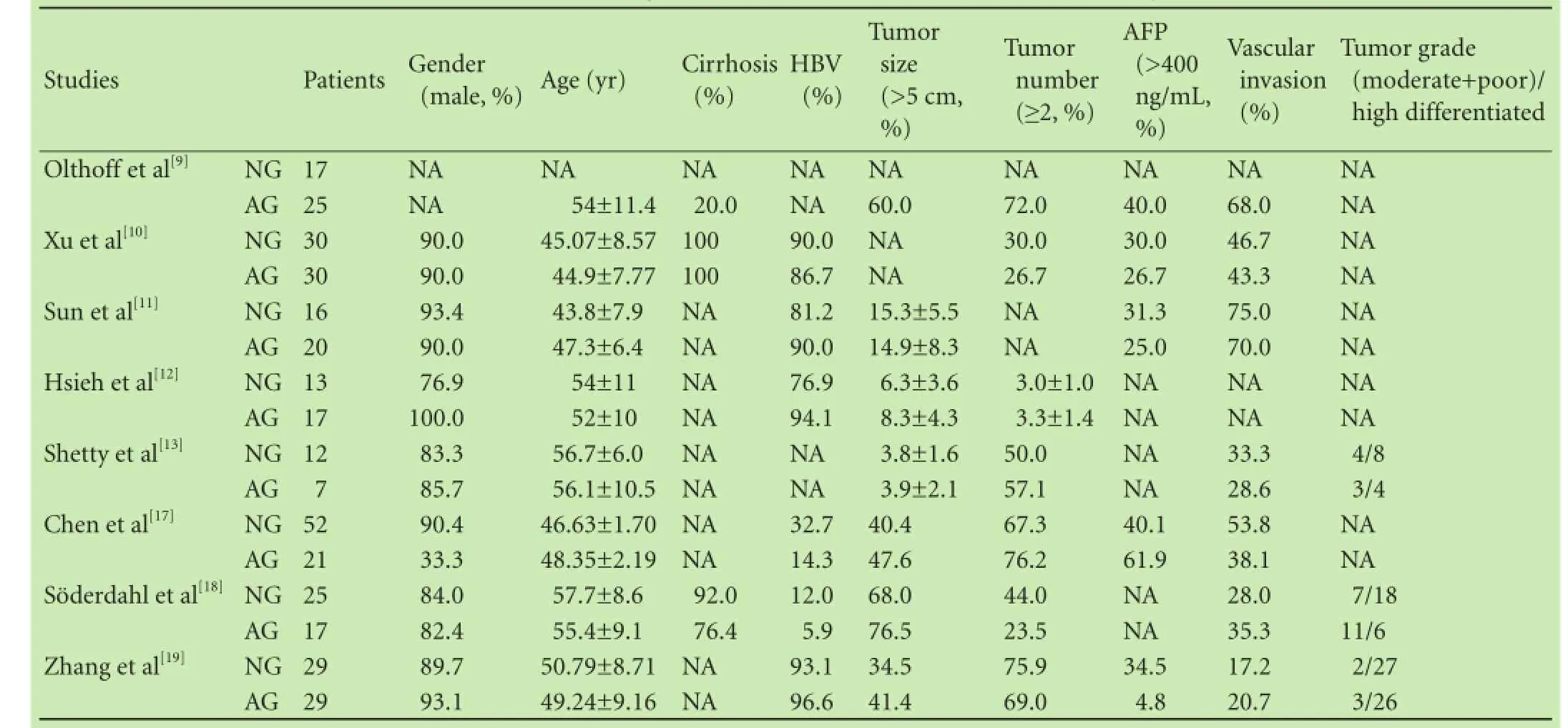
Table 2.Clinical backgrounds of studies included in the meta-analysis
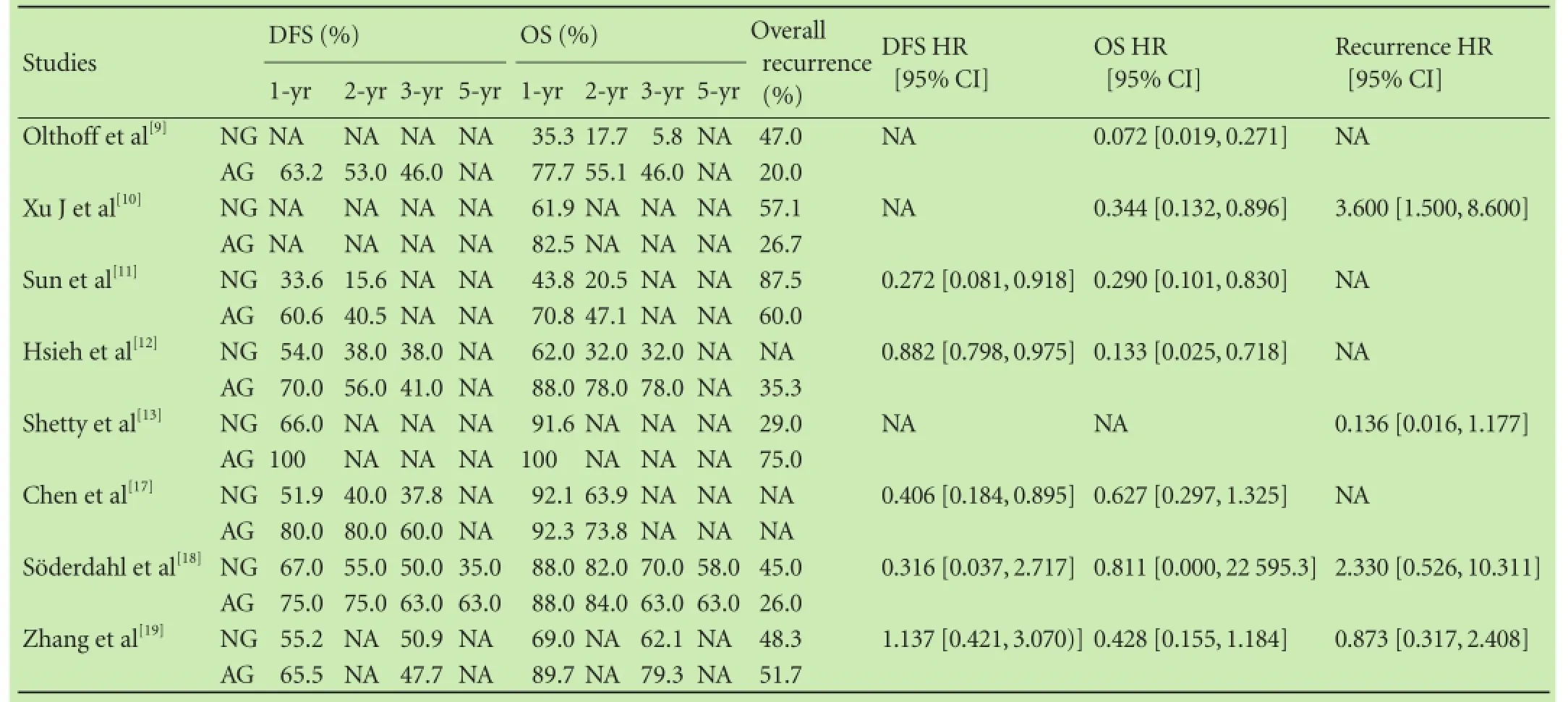
Table 3.Summary of study results regarding survival and recurrence
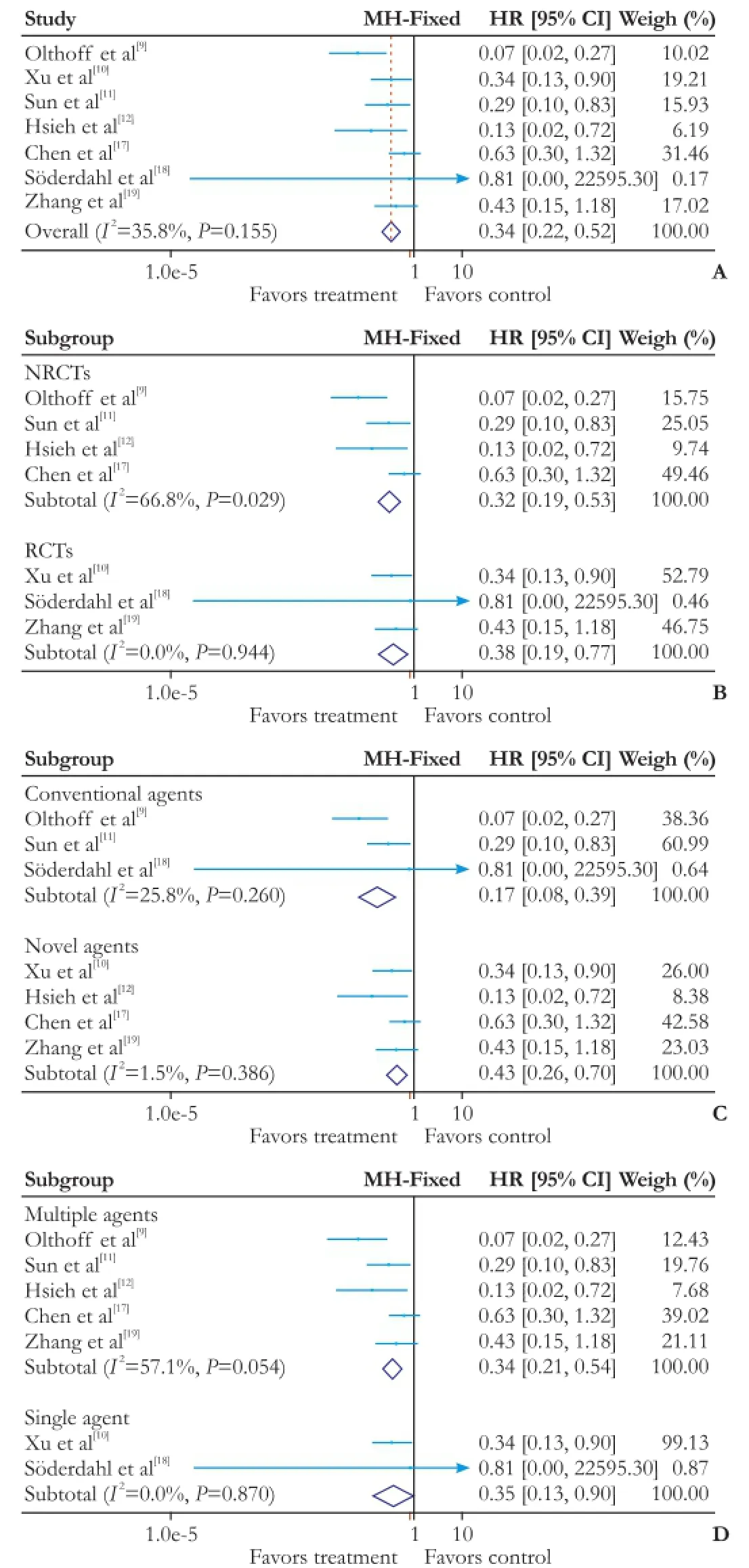
Fig. 2.Forest plot of the meta-analysis on the OS.A: the overall analysis of OS;B: subgroup: RCTs or NRCTs;C: subgroup: conventional agents or novel agents;D: subgroup: multiple agents or single agent. OS: overall survival; CI: confdence interval; RCT: randomized controlled trial; NRCT: non-randomized controlled trial.
Disease-free survival
In terms of DFS, the heterogeneity between all fve trials was moderately elevated but not statistically signifcant (χ2=8.27, degrees of freedom [df]=4;P=0.082;I2=51.6%). Pooling of HRs of the fve trials showed a signifcant difference favoring adjuvant chemotherapy on DFS (HR: 0.87; 95% CI: 0.78-0.95;P=0.004) (Fig. 3A). In subgroup analysis, pooling of HRs from three NRCTs showed a signifcant difference favoring adjuvant chemotherapy on DFS (HR: 0.86; 95% CI: 0.78-0.95;P=0.004). In contrast, the two RCTs showed no signifcant effect (HR:0.91; 95% CI: 0.37-2.24;P=0.834) (Fig. 3B). Pooling of HRs from three trials with novel agents showed a signifcant difference favoring adjuvant chemotherapy on DFS (HR: 0.87; 95% CI: 0.78-0.96;P=0.007) (Fig. 3C), and similar result was found in two trials with conventional agents (HR: 0.28; 95% CI: 0.10-0.81;P=0.019). When trials were stratifed according to single agent or multiple agents, pooling of HRs from four multiple agents showed a signifcant difference favoring adjuvant chemotherapy on DFS (HR: 0.87; 95% CI: 0.78-0.96;P=0.004); whereas the one trial with single agent showed no signifcant effect (HR: 0.32; 95% CI: 0.04-2.72;P=0.294) (Fig. 3D).
Recurrence
In terms of recurrence rate, as statistical heterogeneity (χ2=9.92, degrees of freedom [df]=3;P=0.019;I2=69.8%) was found, the random-effects model was applied to the data from four studies. Pooling of HRs of the four trials showed no signifcant difference in recurrence rate (HR: 1.26; 95% CI: 0.40-4.00;P=0.696) (Fig. 4A). Subgroup analysis was performed for further investigation. The heterogeneity was much diminished when tri-als were stratifed according to RCTs (χ2=4.34, degrees of freedom [df]=2;P=0.114;I2=54.0%) or NRCTs (χ2=0, degrees of freedom [df]=0;P=1;I2=0%), and there was no signifcant difference between the two subgroups (RCTs: HR 1.95, 95% CI: 0.77-4.95,P=0.158; NRCTs: HR 0.14, 95% CI: 0.02-1.18,P=0.070) (Fig. 4B). Pooling of HRs from three novel agents showed no signifcant difference in recurrence rate (HR 1.57; 95% CI: 0.83-2.95;P=0.165), and similarly no remarkable distinction was found in one trial with conventional agents (HR: 2.33; 95% CI: 0.53-10.31;P=0.256) (Fig. 4C). Pooling of HRs from three trials with single agent (HR: 1.35; 95% CI:0.26-6.86;P=0.720) or one trial with multiple agents (HR:0.87; 95% CI: 0.32-2.41;P=0.793) showed no difference in recurrence rate (Fig. 4D).
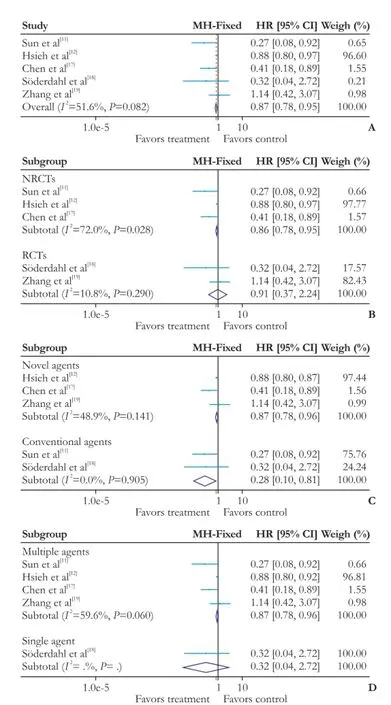
Fig. 3.Forest plot of the meta-analysis on the DFS.A: the overall analysis of DFS;B: subgroup: RCTs or NRCTs;C: subgroup: conventional agents or novel agents;D: subgroup: multiple agents or single agent. DFS: disease-free survival; CI: confidence interval; RCT: randomized controlled trial; NRCT: non-randomized controlled trial.
Adverse events evaluation
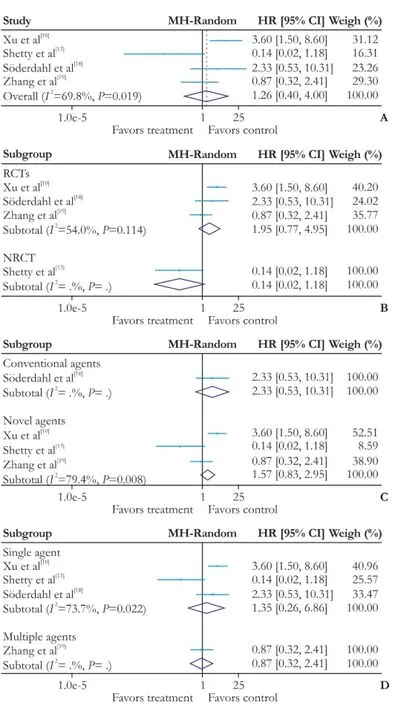
Fig. 4.Forest plot of the meta-analysis on the recurrence rate.A: the overall analysis of recurrence rate;B: subgroup: RCTs or NRCTs;C: subgroup: conventional agents or novel agents;D:subgroup: multiple agents or single agent. CI: confdence interval; RCT: randomized controlled trial; NRCT: non-randomized controlled trial.
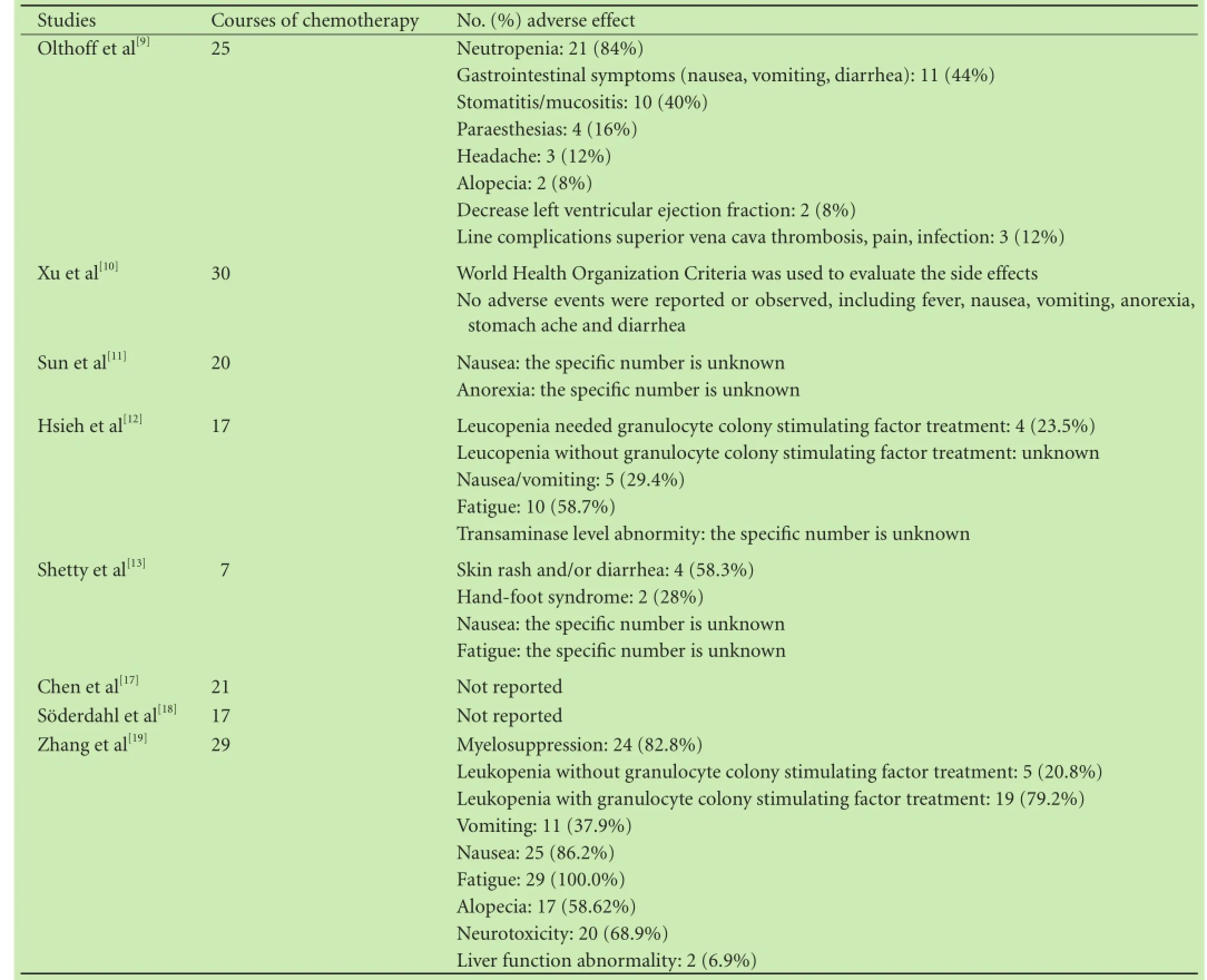
Table 4.Adverse effects of adjuvant chemotherapy
Six trials reported evaluation of adverse events, whereas the other two did not. However, only one study[12]evaluated adverse events by quantitative methods according to World Health Organization Criteria. Therefore, quantitative analysis was considered nearly impossible. Descriptions of adverse events were of anecdotal nature and did not allow meta-analytic calculations. Neutropenia, gastrointestinal symptoms and aminotransferase level abnormity were frequently reported among participants undergoing post-LT chemotherapy. Additional, several albeit rare but severe adverse events were reported, including myelosuppression, neurotoxicity and infection. The detailed descriptions are given in Table 4.
Discussion
Tumor recurrence after LT for HCC remains the major obstacle for long-term survival of patients. This metaanalysis aims to evaluate the effcacy of adjuvant chemotherapy for HCC after LT. Although HCC generally responds poorly to chemotherapy, demonstrating extensive multidrug resistance,[32,33]chemotherapeutic effectiveness, in theory, can be increased if the tumor burden is removed and only microscopic deposits remain.[15]
This article systematically reviewed the published comparable studies of adjuvant chemotherapy for HCC patients after LT and sought to evaluate the benefcial and harmful effects using meta-analysis and a descriptive approach. Our results suggest that OS was much better when adjuvant chemotherapy had been given to post-LT HCC patients. Meanwhile, compared with the non-adjuvant chemotherapy group, the present analysis indicates that adjuvant chemotherapy offered potential benefts for DFS. However, we should notice that although therewas no obvious heterogeneity in terms of DFS (χ2=8.27, df=4;P=0.082;I2=51.6%), theI2was up to 51.6%. The signifcant difference for DFS between the adjuvant chemotherapy and control groups should be taken with a grain of salt. Furthermore, there was no evidence demonstrating that whether using adjuvant chemotherapy or not can affect the tumor recurrence rate. In addition, quantitative analysis of side effect failed because the criteria regarding adverse effect evaluation varied substantially among trials. Only description approach of side events was allowed.
Though the main conclusions are consistent with prior systematic reviews,[8,21-24]our systematic review and comprehensive meta-analysis pose more advantages than previous reviews. Firstly, compared with previous descriptive approach, this is the frst time to evaluate the effect of adjuvant chemotherapy on survival of post-LT HCC patients by quantitative methods. Secondly, we used the HR as the point estimate for all-cause mortality in our meta-analysis. The HR is the most appropriate summary statistic for survival data, since it takes into account both the participant censoring the whole followup period.[27,34]Thirdly, the last but not the least, our data implies that adjuvant chemotherapy signifcantly delayed tumor recurrence but did not completely prevent it. It is generally known that among all the factors affecting HCC survival, tumor biology and grade are predominant for HCC recurrence. This study was hardly to evaluate the association between tumor grade and HCC recurrence because of the incomplete data. However, this did not prevent us from putting forward two scientifc proposals. For early stage and well-differentiated HCC, the routine use of chemotherapy is not recommended. Whereas for advanced HCC with poor differentiation, patients may beneft from the early implantation of adjuvant chemotherapy after LT.
HCC metastatic recurrence is a multistage process involving the acquisition of aggressive phenotypes, reduction of intercellular adhesion in the primary tumor, degradation of the extracellular matrix, and proliferation and angiogenesis of metastasis.[35]It is generally believed that the high risk factor for HCC metastasis and recurrence is the escaped tumor cells in the circulation.[36]Chemo-drugs mainly affect well-differentiated HCC cells without completely killing poorly-differentiated ones.[37]Meanwhile, a signifcant problem with LT is that any residual tumor growth rate is markedly increased under immunosuppressive therapy.[38]Taken together, these factors could largely account for why post-LT adjuvant chemotherapy could markedly delay HCC recurrence but could not help patients escape from it.
As opposed to a chemotherapeutic, mTOR inhibitor is primarily an immunosuppressant drug, so mTOR inhibitor based immunosuppression protocols were excluded in this study. The drug is however thought to be an important future component in immunosuppressive and chemotherapeutics in LT patients with HCC. Two recent meta-analyses[39,40]showed that compared with calcineurin inhibitor (CNI), sirolimus was associated with a lower incidence of HCC recurrence after LT. A more recent meta-analysis[41]favored the use of mTOR inhibitor instead of CNI to control HCC recurrence after LT. However, some experts[42]alarmed that the association of mTOR inhibitor and sorafenib could lead to severe bleeding, and the anti-cancer attitude of mTOR inhibitor was still controversial. Comparative studies with longer follow-up are needed to further explore the role of mTOR inhibitor in HCC recurrence.
Though the tolerance of post-LT chemotherapy was acceptable in most trials, more attention should be paid to the adverse effects of chemotherapy. It was reported that conventional chemotherapy based on doxorubicin resulted in severe complications such as septicemia and cardiotoxicity,[43]whereas the toxicity of novel agents was less severe. Moreover, the use of immunosuppressive drugs after LT was associated with side effects. Post-LT immunosuppression and systemic chemotherapy could lead to gastrointestinal syndromes, severe liver dysfunction and especially bone marrow suppression with increased risk of infection. In our study, the heterogeneity of regimens and the varied criteria made quantitative analysis of adverse effects diffcult. Future trials should pay more attention to the adverse effects of post-LT chemotherapy by using World Health Organization Criteria.[10]
It should be noted that this study has several limitations. First, the small sample sizes, the regimens and the courses of chemotherapy, the baseline characteristic of HCC patients consisted of the main heterogeneity in our study. Future RCTs with larger sample size, longer follow-up are needed to confrm the effects of adjuvant chemotherapy on the prognosis of post-LT HCC patients. Second, most studies did not present HR and associated 95% CIs for OS, DFS or recurrence rate directly. Bias may have been produced when calculating the HR with the use of indirect methods. Third, due to the relatively small number of included trials in the subgroup analysis, there is no effective way to analyze publication bias. The risk of publication bias is unclear.
In conclusion, the systematic review and metaanalysis suggested that adjuvant chemotherapy after LT for HCC can signifcantly prolong patient's survival and delay the recurrence of HCC. For advanced HCC with poor differentiation, patients may perhaps beneft from the early application of adjuvant chemotherapy after LT. However, given that existing studies are heterogeneousand of low quality, larger and well designed RCTs are needed to detect realistically achievable treatment effects.
Contributors:SJ proposed the study. LHS and WRH collected and analyzed the data. GLH, LJF and SRF wrote the draft manuscript. All authors reviewed and contributed to the fnal manuscript. SJ is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Asham EH, Boktour M, Ghobrial RM. Liver transplantation for hepatocellular carcinoma: past, present, and future. Clin Transpl 2012:173-183.
2 Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24.
3 Coelho GR, Vasconcelos KF, Vasconcelos JB, Barros MA, Costa PE, Borges GC, et al. Orthotopic liver transplantation for hepatocellular carcinoma: one center's experience in the Northeast of Brazil. Transplant Proc 2009;41:1740-1742.
4 Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer 2012;1:144-158.
5 Fernández JA, Robles R, Marin C, Sánchez-Bueno F, Ramirez P, Pons JA, et al. Can we expand the indications for liver transplantation among hepatocellular carcinoma patients with increased tumor size? Transplant Proc 2003;35:1818-1820.
6 Chu F, Morris DL. Single centre experience of liver resection for hepatocellular carcinoma in patients outside transplant criteria. Eur J Surg Oncol 2006;32:568-572.
7 Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 2010;28:3994-4005.
8 Duvoux C, Kiuchi T, Pestalozzi B, Busuttil R, Miksad R. What is the role of adjuvant therapy after liver transplantation for hepatocellular carcinoma? Liver Transpl 2011;17:S147-158.
9 Olthoff KM, Rosove MH, Shackleton CR, Imagawa DK, Farmer DG, Northcross P, et al. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg 1995;221:734-743.
10 Xu J, Shen ZY, Chen XG, Zhang Q, Bian HJ, Zhu P, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007;45:269-276.
11 Sun J, Hou BH, Jian ZX, Ou YL, Ou JR. Value of perioperative adjuvant therapy in liver transplantation for advanced hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:471-473.
12 Hsieh CB, Chou SJ, Shih ML, Chu HC, Chu CH, Yu JC, et al. Preliminary experience with gemcitabine and cisplatin adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma. Eur J Surg Oncol 2008;34:906-910.
13 Shetty K, Dash C, Laurin J. Use of adjuvant sorafenib in liver transplant recipients with high-risk hepatocellular carcinoma. J Transplant 2014;2014:913634.
14 Saab S, McTigue M, Finn RS, Busuttil RW. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and effcacy. Exp Clin Transplant 2010;8:307-313.
15 Wu J, Sun H, Han Z, Peng Z. A single center experience: posttransplantation adjuvant chemotherapy impacts the prognosis of hepatocellular carcinoma patients. Chin Med J (Engl) 2014;127:430-434.
16 Wang Z, Fan J, Zhou J, Wu ZQ, Qiu SJ, Huang XW, et al. Preventive chemotherapy for hepatocellular carcinoma exceeding Milan criteria after liver transplantation [abstract in English]. Chin J Dig Surg 2008;7:268-270.
17 Chen GH, Lu MQ, Cai CJ, Yang Y, He XS, Zhu XF. Clinical study of adjuvant individualized chemotherapy for hepatocellular carcinoma after liver transplantation. Zhonghua Wai Ke Za Zhi 2004;42:1040-1043.
18 Söderdahl G, Bäckman L, Isoniemi H, Cahlin C, Höckerstedt K, Broomé U, et al. A prospective, randomized, multi-centre trial of systemic adjuvant chemotherapy versus no additional treatment in liver transplantation for hepatocellular carcinoma. Transpl Int 2006;19:288-294.
19 Zhang Q, Chen H, Li Q, Zang Y, Chen X, Zou W, et al. Combination adjuvant chemotherapy with oxaliplatin, 5-fuorouracil and leucovorin after liver transplantation for hepatocellular carcinoma: a preliminary open-label study. Invest New Drugs 2011;29:1360-1369.
20 Pokorny H, Gnant M, Rasoul-Rockenschaub S, Gollackner B, Steiner B, Steger G, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005;5:788-794.
21 Cherqui D. Role of adjuvant treatment in liver transplantation for advanced hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 1998;5:35-40.
22 Schlitt HJ, Schnitzbauer AA. Hepatocellular carcinoma: agents and concepts for preventing recurrence after curative treatment. Liver Transpl 2011;17:S10-12.
23 Li SF, Hawxby AM, Kanagala R, Wright H, Sebastian A. Liver transplantation for hepatocellular carcinoma: indications, bridge therapy and adjuvant therapy. J Okla State Med Assoc 2012;105:12-16.
24 Fujiki M, Aucejo F, Kim R. Adjuvant treatment of hepatocellular carcinoma after orthotopic liver transplantation: do we really need this? Clin Transplant 2013;27:169-177.
25 Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-989.
26 Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes:meta-epidemiological study. BMJ 2008;336:601-605.
27 Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-2834.
28 Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.
29 Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol 2013;27:351-363.
30 Li N, Zhou J, Weng D, Zhang C, Li L, Wang B, et al. Adjuvantadenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2007;13:5847-5854.
31 Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 2008;14:633-638.
32 Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol 2004;5:409-418.
33 Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-1917.
34 Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
35 Yu L, Dai Z, Wang Z, Fan J, Zhou J. Prognostic indicators for tumor recurrence after liver transplantation in hepatocellular carcinoma and related molecular targeted therapy. Oncology 2011;81:116-122.
36 Kar S, Carr BI. Detection of liver cells in peripheral blood of patients with advanced-stage hepatocellular carcinoma. Hepatology 1995;21:403-407.
37 Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27:1578-1583.
38 Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer 1991;68:2095-2100.
39 Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37:411-419.
40 Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:62-69.
41 Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int 2014;27:1039-1049.
42 Mancuso A, Perricone G. Time to resize the role of everolimus as treatment of hepatocellular carcinoma recurrence after liver transplant. Transpl Int 2015;28:502.
43 Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 1988;62:479-483.
Received August 20, 2014
Accepted after revision February 24, 2015
AuthorAffliations:Department of Hepatobiliary and Pancreatic Surgery, The First Affliated Hospital, College of Medicine, Nanchang University, Nanchang 330000, China (Lin HS, Wan RH, Gao LH, Li JF, Shan RF and Shi J)
Jun Shi, MD, The First Affliated Hospital, College of Medicine, Nanchang University, 17# Yongwai Road, Nanchang 330000, China (Tel/Fax: +86-791-88695528; Email: sj88692702@ sina.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60373-3
Published online May 21, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer
- Histological examination of frozen sections for patients with acute cholecystitis during cholecystectomy
- Omental faps reduces complications after pancreaticoduodenectomy
- Endoscopic ultrasound-guided fne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic effcacy of cell-block immunocytochemistry
- Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines
- miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
