新型芝麻酚并吡喃螺环氧化吲哚拼接衍生物的合成*
2015-02-02郭丰敏俸婷婷刘雄利贵州大学药学院贵州省中药民族药创制工程中心贵州贵阳550025
杨 超,杨 俊,郭丰敏,赵 致,俸婷婷,刘雄利,周 英(贵州大学药学院贵州省中药民族药创制工程中心,贵州贵阳 550025)
新型芝麻酚并吡喃螺环氧化吲哚拼接衍生物的合成*
杨超,杨俊,郭丰敏,赵致,俸婷婷,刘雄利,周英
(贵州大学药学院贵州省中药民族药创制工程中心,贵州贵阳550025)
摘要:以二氯甲烷为溶剂,1,4-二氮杂二环[2.2.2]辛烷为催化剂,取代靛红,芝麻酚和丙二腈经三组分Michael-环化反应合成了16个新型的芝麻酚并吡喃螺环氧化吲哚拼接衍生物——6-胺基-5'-取代基-1'-取代基-2'-氧-7'-取代基螺环[1,3]-二氧亚甲基-[4,5-并]-苯并吡喃-8,3'-吲哚基]-7-腈,产率85%~96%,其结构经1H NMR,13C NMR和HR-ESI-MS表征。讨论了底物上的取代基对反应速度和产率的影响。
关键词:靛红;芝麻酚;丙二腈; Michael-环化反应;氧化吲哚;合成
州省中药现代化科技产业研究开发专项项目{黔科合ZY字[2013]3010} ;贵州民族药物中新颖活性组分的结构测定及NMR
谱仪的应用研发项目(2011YQ12003506) ;贵州省药食同源植物资源研究开发中心项目{[2014(4003)]黔科合字}
通信联系人:周英,教授,博士生导师,E-mail: yingzhou71@ yeah.net
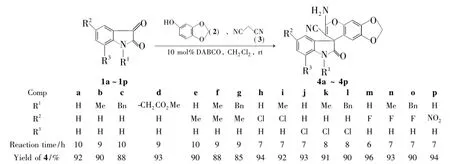
Scheme 1
为了满足活性筛选和新药研究开发的需要,根据药物化学药效团的拼接原理,本文在前期[6-10]工作研究的基础上,以二氯甲烷为溶剂,1,4-二氮杂二环[2.2.2]辛烷(DABCO)为催化剂,取代靛红(1a~1p),2和丙二腈(3)经三组分Michael-环化反应合成了16个新型的芝麻酚并吡喃螺环氧化吲哚拼接衍生物——6-胺基-5'-取代基-1'-取代基-2'-氧-7'-取代基螺环[1,3]-二氧亚甲基-[4,5-并]-苯并吡喃-8,3'-吲哚基]-7-腈(4a~4p,Scheme 1),产率85%~96%,其结构经1H NMR,13C NMR和ESI-MS表征。讨论了底物上的取代基对反应速度和产率的影响。其相关药理活性正在测试中。
1实验部分
1.1仪器与试剂
Bruker-400 MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标) ; MicroTM Q-TOF型质谱仪。
所用试剂均为分析纯;无水溶剂均按标准程序进行脱水处理。
1.2 4a~4p的合成(以4a为例)
在反应试管中依次加入靛红(1a) 147 mg (1.0 mmol),2 152 mg(1.0 mmol),3 132 mg (2.0 mmol),DABCO 11.2 mg (0.1 mmol,10 mmol%)及二氯甲烷10 mL,搅拌下于室温反应至终点(TLC检测)。反应液经硅胶层析柱[洗脱剂: V(乙酸乙酯)∶V(石油醚) = 1∶3]纯化得白色固体6-氨基-2'-螺环{[1,3]-二氧亚甲基[4,5-并]并吡喃-8,3'-吲哚基} -7-腈(4a)。
用类似的方法合成白色或灰色固体4b~4p。
4a:1H NMR δ: 5.99 (s,2H),6.79 (s,1H),6.93(s,1H),6.95(d,J = 7.5 Hz,1H),7.01(s,1H),7.05(d,J = 8.4 Hz,1H),7.19 (m,1H),7.28 (s,1H),10.57 (s,2H) ;13C NMR δ: 53.7,62.5,98.2,102.0,104.5,107.2,110.1,112.3,118.7,122.7,124.8,129.2,134.4,141.9,143.6,147.7,161.3,178.9; HR-ESI-MS m/z: Calcd for C18H11N3O4{[M + Na]+} 356.064 7,found 356.065 9。
评估量表是一种预测、评估、筛选压疮高危人群的工具,根据我院收治患者的实际需求,改良压疮风险评估量表具有实用、直观等特点,且该评估量表内容清晰、操作简单,可降低护理人员的工作强度,从而提高评估正确率[5]。本研究结果显示,对照组患者压疮危险因素评估正确率明显低于观察组,差异有统计学意义(P<0.05),对照组患者的压疮发生率明显高于观察组,差异有统计学意义(P<0.05),证实改良压疮风险评估量表效果显著,有利于压疮规范化管理,达到患者安全管理目标。
4b:1H NMR δ: 3.19 (s,3H),5.97 (s,2H),6.77(s,1H),6.93(s,1H),6.95(d,J = 7.4 Hz,1H),7.10(d,J = 8.5 Hz,1H),7.22 (s,1H),7.38 (s,1H),10.58 (br s,2H) ;13C NMR δ: 26.5,53.5,61.3,98.1,102.0,104.5,107.2,109.2,112.1,118.5,123.3,124.5,129.3,133.7,141.9,143.6,147.8,161.4,177.8; HR-ESI-MS m/z: Calcd for C19H13N3O4{[M +Na]+}370.080 4,found 370.080 0。
4c:1H NMR δ: 4.86 (s,2H),5.03 (s,1H),5.98 (s,2H),6.80 (s,1H),6.98 (s,1H),7.00(d,J =7.7 Hz,2H),7.06(d,J =8.2 Hz,2H),7.08(s,1H),7.14(s,1H),7.20(s,1H),7.27(s,1H),7.29(br s,2H) ;13C NMR δ: 43.2,53.4,98.3,102.1,104.5,107.2,109.7,110.6,112.1,118.7,123.5,124.7,127.2,129.2,132.5,133.7,136.1,138.4,142.3,143.6,144.5,147.9,157.2,161.5,177.4; HR-ESI-MS m/z: Calcd for C25H17N3O4{[M + Na]+} 446.111 7,found 446.112 0。
4d:H NMR δ: 2.51 (s,3H),3.85 (s,2H),5.93 (s,2H),6.72 (s,1H),6.90 (s,1H),7.15(d,J =7.5 Hz,1H),7.31(d,J =7.7 Hz,2H),7.45 (s,1H),9.48 (br s,2H) ;13C NMR δ: 44.8,50.2,53.3,98.2,102.1,104.9,109.3,111.1,114.6,118.7,122.7,125.9,128.5,134.4,136.8,137.6,138.4,144.7,148.0,161.4,178.1; HR-ESI-MS m/z: Calcd for C21H15N3O6Na{[M + Na]+} 428.085 9,found 428.086 4。
4e:1H NMR δ: 3.18 (s,3H),6.07 (s,2H),6.78(s,1H),6.91(s,1H),7.07(d,J = 7.5 Hz,1H),7.09(d,J = 7.4 Hz,1H),7.17 (m,1H),7.23 (s,1H),10.38 (br s,2H) ;13C NMR δ: 28.5,53.6,61.8,98.2,102.0,104.7,109.2,110.4,112.2,118.6,122.9,124.4,129.3,133.9,141.9,143.4,147.8,161.4,177.9; HR-ESI-MS m/z: Calcd for C19H13N3O4{[M +Na]+}370.080 4,found 370.082 7。
4f:1H NMR δ: 2.26(s,3H),3.16(s,3H),5.97(s,2H),6.79(s,1H),6.92(s,1H),7.03 (d,J =7.5 Hz,1H),7.09(d,J =7.9 Hz,1H),7.13(m,1H),7.21(br s,2H) ;13C NMR δ: 20.6,26.5,53.6,98.1,102.0,104.9,108.9,110.2,112.1,118.6,124.5,129.5,132.4,133.9,140.9,143.5,144.6,147.7,161.3,177.0; HR-ESI-MS m/z: Calcd for C20H15N3O4{[M + Na]+} 384.096 0,found 384.094 9。
4g:1H NMR δ: 3.39 (s,3H),4.86 (s,2H),5.03 (s,1H),5.98 (s,2H),6.80 (s,1H),6.98(m,1H),7.00(d,J =7.6 Hz,2H),7.06(d,J = 8.0 Hz,2H),7.14(s,1H),7.20 (s,1H),7.27 (s,1H),7.29 (br s,2H) ;13C NMR δ: 43.2,59.8,98.3,102.1,104.6,107.5,109.2,110.8,112.5,118.1,123.4,124.6,127.3,129.2,132.5,133.7,136.0,138.1,142.3,143.6,144.6,147.5,157.8,159.4,161.3,178.9; HR-ESIMS m/z: Calcd for C26H19N3O4{[M + Na]+} 460.127 3,found 460.127 3。
4h:1H NMR δ: 4.02 (s,1H),4.89 (s,1H),5.98(s,2H),6.78(s,1H),7.00(d,J = 7.5 Hz,1H),7.15(d,J = 7.4 Hz,1H),7.27 (s,1H),10.73(br s,2H) ;13C NMR δ: 53.2, 65.1,98.3,102.1,104.6,109.2,111.6,118.6,124.9,126.6,129.2,136.4,140.8,143.6,144.6,147.9,161.3,178.6; HR-ESI-MS m/z: Calcd for C18H10N3O4Cl{[M + Na]+} 390.025 8,found 390.026 8。
4i:1H NMR δ: 2.51(s,3H),4.77(s,1H),5.98(s,2H),6.78(s,1H),7.02(d,J = 7.4 Hz,1H),7.17(d,J = 8.5 Hz,1H),7.36(s,1H),11.03(br s,2H) ;13C NMR δ: 41.5,53.5,98.2,102.1,104.7,107.5,112.1,114.3,118.5,123.6,124.0,129.2,136.1,139.7,141.9,143.5,147.9,161.3,178.8; HR-ESI-MS m/z: Calcd for C19H12N3O4Cl{[M + Na]+} 404.041 4,found 404.042 1。
4j:1H NMR δ: 4.89(s,1H),5.98(s,2H),6.81(s,1H),6.98(s,1H),7.00(d,J = 7.5 Hz,1H),7.06(d,J = 7.9 Hz,1H),7.28(s,1H),9.39(br s,2H) ;13C NMR δ: 53.3,98.3,102.2,105.0,107.5,111.5,114.7,118.7,123.6,126.0,128.5,136.8,138.5,143.6,144.9,148.1,161.4,178.2; HR-ESI-MS m/z: Calcd for C18H10N3O4Cl{[M + Na]+} 390.025 8,found 390.026 1。
4k:1H NMR δ: 3.37 (s,3H),4.89 (s,1H),5.98(s,2H),6.80(s,1H),7.17(d,J = 7.4 Hz,1H),7.20(d,J = 7.8 Hz,1H),7.43 (s,1H),9.30(br s,2H) ;13C NMR δ: 26.7,52.9,98.2,102.1,104.9,109.2,111.2,118.5,123.3,124.5,127.3,129.2,135.7,142.3,143.6,144.6,147.9,161.3,176.8; HR-ESI-MS m/z: Calcd for C19H12N3O4Cl{[M + Na]+} 404.041 4,found 404.041 0。
4l:1H NMR δ: 4.01(s,2H),6.00(s,2H),6.78(s,1H),6.94(s,1H),7.01(d,J = 7.4 Hz,2H),7.10(d,J = 8.4 Hz,2H),7.11(s,1H),7.15(m,1H),7.20(s,1H),7.26(s,1H),10.61(br s,2H) ;13C NMR δ: 51.2,53.3,59.8,98.3,102.1,104.6,107.4,109.2,111.6,112.6,115.8,118.6,123.3,124.5,129.4,132.6,136.0,138.1,143.6,144.6,147.9,157.8,159.4,161.3,178.9; HR-ESI-MS m/z: Calcd for C25H16N3O4Cl{[M +Na]+}480.072 7,found 480.071 7。
4m:1H NMR δ: 4.21 (s,1H),6.00 (s,2H),6.80(s,1H),7.04(s,1H),7.27(d,J = 7.0 Hz,1H),7.30(d,J = 8.4 Hz,1H),7.35 (s,1H),11.02(br s,2H) ;13C NMR δ: 53.5,98.3,102.1,104.7,107.2,111.6,115.7,118.5,124.5,127.3,129.3,133.7,138.5,141.9,143.6,147.8,161.4,177.8; HR-ESI-MS m/z: Calcd for C18H10N3O4F{[M + Na]+} 374.055 3,found 374.055 4。
4n:1H NMR δ: 3.39 (s,3H),4.89 (s,1H),5.95 (s,2H),6.90 (s,1H),7.01 (s,1H),7.13(d,J =7.6 Hz,1H),7.15(d,J =8.0 Hz,1H),7.36(br s,2H) ;13C NMR δ: 39.5,53.0,98.3,102.2,104.5,107.5,111.6,115.8,118.6,124.5,127.2,128.7,135.9,138.5,143.6,144.6,148.0,161.5,177.4; HR-ESI-MS m/z: Calcd for C19H12N3O4F{[M + Na]+} 388.071 0,found 388.071 9。
4o:1H NMR δ: 3.85 (s,2H),6.00 (s,1H),6.15(s,2H),6.81(s,1H),7.16(d,J = 7.4 Hz,2H),7.26(s,1H),7.37(d,J = 7.5 Hz,2H),7.93(m,1H),8.26(s,1H),9.05(s,1H),11.32(br s,2H) ;13C NMR δ: 49.8,52.7,59.8,98.3,102.2,104.8,107.4,110.6,114.6,115.5,118.4,120.4,124.5,126.5,129.4,135.3,136.0,138.1,143.4,144.7,148.3,157.8,159.4,161.4,179.3; HR-ESI-MS m/z: Calcd for C25H16N3O4F{[M +Na]+}464.102 3,found 464.104 0。
4p:1H NMR δ: 4.45 (s,1H),5.98 (s,2H),6.02(s,1H),6.78(s,1H),7.17(d,J = 7.4 Hz,1H),7.30(d,J =7.6 Hz,1H),7.35(s,1H),9.84(br s,2H) ;13C NMR δ: 53.3,98.3,102.2,105.0,107.2,111.5,114.7,118.7,126.0,128.5,129.3,136.8,137.5,138.5,143.6,148.1,161.4,178.2; HR-ESI-MS m/z: Calcd for C18H10N4O6{[M +Na]+}401.049 8,found 401.049 1。
2 结果与讨论
2.1构效关系
1苯环上的取代基对反应速度和产率都有一定的影响(Scheme 1)。当苯环上为吸电子取代基时,其反应速率和产率较为供电子取代基时高。如以5-氯靛红(1h)做底物时,产物4h的产率(94%)比4a(92%)和以5-甲基-靛红(1e)的产物4e(90%)高,反应时间较短。这可能是因为靛红5-位的氯原子使苯环电子云密度降低,增强其亲电性,从而提高了反应速度和产率; 5-位的甲基使靛红苯环电子云密度升高,减弱其亲电性,从而降低了反应速度和产率。
参考文献
[1]Horton D A,Bourne G T,Smythe M L.The combinatorial synthesis of bicyclic privileged structures or privileged substructures[J].Chem Rev,2003,103:893-930.
[2]Zheng S,Chan C,Furuuchi T,et al.Stereospecific formal total synthesis of ecteinascidin 743[J].Angew Chem Int Ed,2006,45: 1754-1759.
[3]Chen J,Chen X,Willot M,et al.Asymmetric total syntheses of ecteinascidin 597 and ecteinascidin 583 [J].Angew Chem Int Ed,2006,45: 8028-8032.
[4]Paolis M D,Chiaroni A,Zhu J.Synthetic studies on ecteinascidin 743: Rapid access to the fully functionalized tetrahydroisoquinoline with a bridged 10-membered sulfur containing macrocycle[J].Chem Commun,2003: 2896-2897.
[5]Hitotsuyanagi Y,Ichihara Y,Takeya K,et al.Synthesis of 4-oxa-2-azapodophyllotoxin,a novel analog of the antitumor lignan podophyllotoxin[J].Tetrahedron Lett,1994,35: 9401-9402.
[6]Liu X L,Yuan W C.A simple and eco-friendly method for the aminomethylation of 3-substituted oxindoles via three-component Mannich reaction in aqueous media[J].Tetrahedron Letters,2011,52: 903-906.
[7]Liu X L,Liao Y H,Wu Z J,et al.Organocatalytic enantioselective hydroxymethylation of oxindoles with paraformaldehyde as C1unit[J].J Org Chem,2010,75: 4872-4875.
[8]Liu X L,Wu Z J,Du X L,et al.Amino-indanol-catalyzed asymmetric Michael additions of oxindoles to protected 2-amino-1-nitroethenes for the synthesis of 3,3'-disubstituted oxindoles bearing a,β-diamino functionality[J].J Org Chem,2011,76: 4008-4017.
[9]Liu X L,Han W Y,Zhang X M,et al.Highly efficient and stereocontrolled construction of 3,3'-pyrrolidonyl spirooxindoles via organocatalytic domino Michael/cyclization reaction[J].Org Lett,2013,12:1246-1249.
[10]朱铭,杨超,余章彪,等.芝麻酚与3-羟基氧化吲哚拼接衍生物的合成及其抗肿瘤活性[J].合成化学,2014,22(4) : 444-447.
·快递论文·
Synthesis of Novel Spiro-Fused Pyranyl Benzo[d][1,3]dioxol Oxindoles
YANG Chao,YANG Jun,GUO Feng-min,ZHAO Zhi,FENG Ting-ting,LIU Xiong-li,ZHOU Ying
(Guizhou Engineering Center for Innovative Traditional Chinese Medicine and Ethnic Medicine,College of Pharmacy,Guizhou University,Guiyang 550025,China)
Abstract:Sixteen novel spiro-fused pyranyl benzo[d][1,3]dioxol oxindole derivatives,6-amino-1'-substituting group-2'-oxospiro{[1,3]dioxolo[4,5-g]chromene-8,3'-indoline} -7-carbonitrile,were synthesized in yield of 85%~96% by tandem Michael-cyclization reaction of sesamol,malononitrile and substituted isatins,using methylene chloride as the solvent,1,4-diazabicyclo[2.2.2]octane as the catalyst,at room temperature.The structures were characterized by1H NMR,13C NMR and HRESI-MS.Relationship of reaction speed,yields and substituent groups in reactions were investigated.
Keywords:Isatin; sesamol; malononitrile; Michael-cyclization reaction; oxindole; synthesis
作者简介:杨超(1988-),男,汉族,安徽亳州人,硕士研究生,主要从事天然活性物质的全合成及结构修饰研究。E-mail: 1024121527@ qq.com
基金项目:国家自然科学基金青年基金资助项目(21302024) ;教育部“新世纪人才支持计划”项目[教技函(2011) 95号];贵
*收稿日期:2014-09-23;
修订日期:2015-05-04
中图分类号:O626; O621.3
文献标识码:A
DOI:10.15952/j.cnki.cjsc.1005-1511.2015.07.0599
