Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
2015-01-20YudongXuXudongPengLeiWngMinShiYunZhngQunWngSnQiNingDing
Yu-dong Xu,Xu-dong Peng,Lei Wng,Min Shi,Yun Zhng,Qun Wng,Sn Qi, Ning Ding
a.School of Materials Science and Engineering,Hefei University of Technology,Hefei 230009,China
b.Bengbu Yucheng New Materials Science and Technology Ltd.Co.,Bengbu 233000,China
(Dated:Received on April 15,2015;Accepted on July 29,2015)
Effects of Sm Co-doping on Luminescent Properties of Sr4Al14O25:M (M=Mn4+,Cr3+)Phosphors
Yu-dong Xua∗,Xu-dong Penga,Lei Wanga∗,Min Shia,Yuan Zhanga,Quan Wanga,San Qia, Ning Dingb
a.School of Materials Science and Engineering,Hefei University of Technology,Hefei 230009,China
b.Bengbu Yucheng New Materials Science and Technology Ltd.Co.,Bengbu 233000,China
(Dated:Received on April 15,2015;Accepted on July 29,2015)
The Sr4Al14O25:M and doped Sr4Al14O25:M+Sm3+(M=Mn4+,Cr3+)phosphors were synthesized by a solid-state reaction method and their luminescent properties were investigated.The results showed that the co-doping of Sm ions did not change the positions of excitation band and emission band but significantly improved the luminescent properties of Sr4Al14O25:Cr3+phosphors;whereas,the emission intensity of Sr4Al14O25:Mn4+was reduced remarkably when Sm ions were co-doped.In addtion,a radiative-form energy transfer from Sm3+to Cr3+was observed for the first time in the Cr,Sm co-doped Sr4Al14O25phosphors.The results indicated that Sm ions could significantly improve the emission intensity of Sr4Al14O25:Cr3+,making the Sm3+co-doped Sr4Al14O25:Cr3+phosphor a promising candidate for the applications in display and solid state lightening.
Phosphor,Co-doping,Strontium aluminates,Energy transfer
I.INTRODUCTION
In 1996,Nakamura and Fasol in Nichia Company combined a blue LEDs chip with a yellowish phosphor(YAG:Ce)to produce the white light.However, the color rendering index of YAG:Ce phosphor for WLEDs is too low to be used in warm white LEDs because of lacking a red component(color rendering index(CRI≤85))[1].Recently,the rare earth ionsdoped alkali earth aluminates have attracted much attention owing to their high luminescence in blue green to red regions[2,3],which have been widely used in the illumination,medical instruments,displays,etc. Nowadays,the most used activators in the red emitting phosphors are Eu2+and Eu3+,followed by Ce3+and Sm3+etc.Nevertheless,most of these rare earth ions are very expensive and some chlorides,citrates, and oxides of the above rare earth ions are toxic and harmful,which greatly limits their further applications in W-LEDs.In contrast,the transition-metal ions have lower price but higher luminescence property[4].In recent years,transition-metal ions-doped strontium aluminates-based phosphors have been found to exhibit high brightness and already applied in some commercial LEDs[5].A new Sr4Al14O25:Mn4+phosphor has been prepared by high temperature solid state reaction method previously,which showed high brightness and luminescence chromaticity[6].However,it was noticeable that the luminescence intensity of Sr4Al14O25:Mn4+phosphor was relatively lower and the range of absorption band was not very wide in comparison with those commercial phosphors.Consequently, the Sr4Al14O25:Mn4+phosphor has not been widely applied in W-LEDs.
Energy transfer between rare earth ions or rare earth ions and transition-metal ions has also been regarded as an effective method in improving the luminescent properties of aluminate-based phosphors[7–9].Luitel et al.synthesized Sr4Al14O25:Cr3++Eu2++Dy3+phosphor successfully by combustion method and investigated the optical properties and phosphorescence decays of Cr3+-doped Sr4Al14O25[10].Kroon et al. prepared the phosphors of Ce,Tb co-doped LaF3by hydrothermal method and found that the energy transfer occurred through a non-radiative mechanism[11]. Lin et al.investigated the influence of co-doping of different rare earth ions(Dy,Nd,La)on the luminescence of CaAl2O4-based phosphors(CaAl2O4:Eu3+) [12].Subsequently,researchers found that Sm3+ion showed strong emission in variety oflattices in the range from strong yellow to red region[13,14].
It is known that the emission range of Sm3+is overlapped with the excitation range of Cr3+,which possibly results in the occurrence of energy transfer between Sm3+ions and Cr3+ions.Consequently,it is believed that co-doping of Cr3+and Sm3+will further enhance the photoluminescence properties of strontium aluminate-based phosphors.However,up to now,the effects of co-doping of Sm3+on the photoluminescenceof Sr4Al14O25:M(M=Mn4+,Cr3+)phosphors have not been reported.In this work,four kinds of Sr4Al14O25-based phosphors(Sr4Al14O25:Mn4+, Sr4Al14O25:Mn4++Sm3+,Sr4Al14O25:Cr3+and Sr4Al14O25:Cr3++Sm3+)were prepared by solid state reaction method at high temperature and the influences of co-doping of Sm3+on the photoluminescence properties of Sr4Al14O25:M(M=Mn4+,Cr3+)were studied by means of XRD,SEM(scanning electron microscopy)and photoluminescence excitation spectra and emission spectra.Furthermore,a feasible energy transfer mechanism from Sm3+to Cr3+was put forward.
II.EXPERIMENTS
In this work,samples were synthesized by high temperature solid state reaction.The starting materials include MnCO3(99%),Al2O3(99.99%),SrCO3(99%), Sm2O3(99.5%),Cr2O3(99.5%)and H3BO3(99%,as a flux).The molar ratio of SrCO3,Al2O3and H3BO3is chosen as 4:7:0.2.The mixing was performed thoroughly with the help of ethanol in agate mortar for 1 h and then preheated at 900◦C in air for 2 h,and finally sintered in a tubular furnace at 1350◦C for 5 h in an oxidizing atmosphere(O2flowing gas).
The crystal phases of phosphors were identified with an X-ray diffractometer(D/MAX2500V).SEM characterization was carried out using a JSM-6490LV SEM instrument.The excitation spectra and emission spectra of the phosphors were measured with a HITACHI F-4500 fluorescence photometer equipped with a 150 W Xe lamp as the excitation source.Allthe measurements were performed at ambient temperature.
III.RESULTS AND DISCUSSION
A.XRD characterization of the prepared phosphors
XRD patterns of the un-doped,0.1%Mn4+doped, 0.1%Cr3+doped,0.1%Mn4++1.0%Sm3+co-doped and 0.1%Cr3++1.0%Sm3+co-doped Sr4Al14O25phosphors synthesized by conventionalsolid state reaction method at 1350◦C were presented in Fig.1,respectively.It was shown that single phase of Sr4Al14O25(JCPDS card No.52-1876)was obtained and no second phase was detected,implying that the host structure was not significantly changed by the doping of Mn4+ions,Cr3+and Sm3+ions.Sr4Al14O25was reported to crystallize in an orthorhombic crystal structure with Pmma space group[15]in which octahedral sites(AlO6)and tetrahedral sites(AlO4)are embodied,as shown in Fig.2. According to the differences of ionic radii,it can be deduced that Mn4+and Cr3+ions have a pronounced tendency to occupy the Al3+positions in AlO6octahedra,and Sm3+ions tend to occupy the Sr2+sites in the Sr4Al14O25(see Fig.2),even though it will cause a little charge imbalance effect.
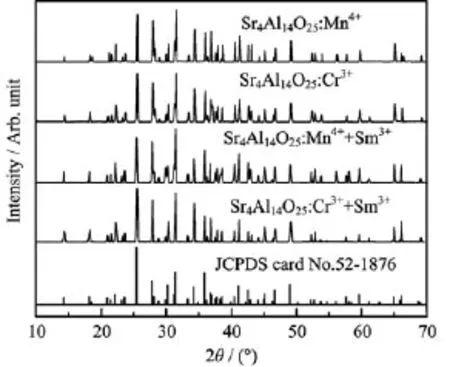
FIG.1 XRD patterns of Sr4Al14O25:0.1%Mn4+, Sr4Al14O25:0.1%Cr3+,Sr4Al14O25:0.1%Mn4++1.0%Sm3+, and Sr4Al14O25:0.1%Cr3++1.0%Sm3+compared with the standard Sr4Al14O25(JCPDS card No.52-1876).
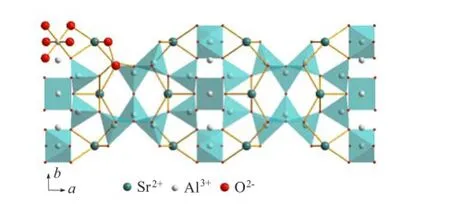
FIG.2 Projection of the structure of Sr4Al14O25on the (001)plane.
B.SEM characterization of the prepared phosphors
SEM images of the Sr4Al14O25:M+Sm3+(M=Mn4+, Cr3+)samples are shown in Fig.3.It was shown that the phosphor sample prepared with 0.1%Mn4+consisted of irregular shape particles with an average size of 2.5µm (Fig.3(a)).By contrast,the particles in the sample prepared with 0.1%Cr3+were somewhat homogeneous in grain size(Fig.3(b)).However,it should be noted that there are little or indistinct differences in grain sizes and morphologies when Sm3+ions or Cr3+ions were co-doped into the above samples.
C.Photoluminescence properties of Sr4Al14O25:Cr3++ Sm3+phosphor

FIG.3 SEM images of Sr4Al14O25-based phosphors with (a)0.1%Mn4+,(b)0.1%Cr3+,(c)0.1%Mn4++1.0%Sm3+, (d)0.1%Cr3++1.0%Sm3+.
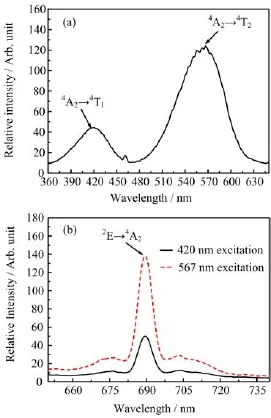
FIG.4(a)Excitation spectrum at 689 nm and(b)emission spectra at 420 and 567 nm of 0.1%Cr3+-doped Sr4Al14O25.
Figure 4 shows the excitation spectra and emission spectra of Sr4Al14O25:Cr3+phosphors.For the excitation band,420 and 567 nm could be ascribed to the transition of4A2→4T1and4A2→4T2,respectively. For its emission band,the main peak was located at 689 nm which was in the deep red region and could be ascribed to a typical emission of Cr3+[16].However, its emission intensity under the excitation band from 360 nm to 460 nm was relatively weak,implying that Sr4Al14O25:Cr3+phosphor could not be effectively excited by an UV-light and blue light.Therefore,the ions which would efficiently transfer its energy to Cr3+were very important in improving the application feasibility of Sr4Al14O25:Cr3+phosphor.
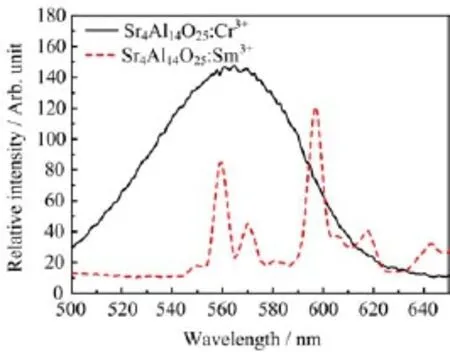
FIG.5 Spectra overlap of single-doped Sr4Al14O25with Cr3+or Sm3+.
For the previously reported Sr4Al14O25:Sm3+[17],it was shown that the Sm3+could be excited by 365 and 420 nm.Thus,Cr3+with red emission could be incorporated in the same host crystal.Therefore,it is reasonable to investigate the effect of co-doping of Sm3+ions and Cr3+ions on the photoluminescence of Sr4Al14O25phosphors.It can be found from Fig.5 and Fig.6 that both Cr3+-doped and Sm3+-doped Sr4Al14O25phosphors could be excited by 415 nm light.Moreover,the co-doping of Sm3+ions and Cr3+ions did not change the positions and shapes of luminescence band of phosphors.Noticeably,the emission intensity of Cr3+ions was greatly enhanced,whereas the emission intensity of Sm3+ions was greatly reduced.That meant there was an energy transfer process from Sm3+ions to Cr3+ions,which resulted in the enhancement effect in the co-doped phosphors.
As far as the so-called energy transfer processes in the co-doped phosphors are concerned,resonance transfer and radiative transition have been regarded as two primary mechanisms in this system.Through the energy levels of Sm3+and Cr3+we can deduce that the resonance transfer is impossible between Cr3+and Sm3+owing to the level mismatch.In fact,the enhancement effect is mostly likely related to the radiative transition process in the Sr4Al14O25:Cr3++Sm3+system.It is apparent that spectral overlap can be readily found between the emission band of Sm3+ions and the excitation spectrum of Cr3+ions(see Fig.5),implying that a radiative energy transfer possibly occurs from the Sm3+ions to the doped Cr3+ions.In other words, it can be reasonably deduced that Sm3+ions can absorb the energy and emit lights at wavelengths of 560, 570,and 597 nm,and then the emitted lights are reabsorbed by Cr3+ions and thus a red light at 689 nm is emitted.Nowadays,radiative transition is to some ex-tent believed to be an efficient method to enhance the photoluminescence property of Sr4Al14O25:Cr3++Sm3+system though there is some energy loss,which has been revealed by some literatures,such as the radiative transition from Mn2+to Cr3+in the MgAl2O4system[18] and the inner energy transfer from the host to Eu3+ions in CaGa2O4:Eu3+[19].

FIG.6 The emission spectra atλex=415 nm of single doped Cr3+,Sm3+ions and co-doping in Sr4Al14O25.
In addition,the influence of the concentration of Sm3+ions on the emission intensity of Sr4Al14O25:0.1%Cr3++x%Sm3+phosphor is shown in Fig.7.It is shown that the luminescence intensity was the greatest when the concentration of the doped Sm3+ions is 1.0%.With any further increment of Sm3+concentration,the emission intensity decreased owing to the concentration quenching effect. Therefore,the optimum concentration of Sm ions in Sr4Al14O25:0.1%Cr3++x%Sm3+system was 1.0%.
D.Photoluminescence properties of co-doping Cr3+and Sm3+in Sr4AL14O25
Since the co-doping of Sm3+could effectively improve the emission intensity of Sr4Al14O25:Cr3+,it is worth knowing whether Sm3+has the similar effects on the luminescent properties of Mn4+doped Sr4Al14O25phosphor or not.In order to make it clear,we tentatively prepared Sm3+and Mn4+co-doped phosphor.The excitation spectrum and emission spectrum of 0.1%Mndoped Sr4Al14O25are shown in Fig.8.It could be seen that the excitation spectrum of Sr4Al14O25:Mn4+was attributed to the transitions of4A2→4T1(300 nm to 400 nm)and4A2→4T2(400 nm to 450 nm)of Mn4+ions,and emission peak which was located at 651 nm were ascribed to the transition of2E→4A2of Mn4+ions.Obviously,the results were highly close to the previous investigations[6].

FIG.7(a)The emission spectra atλex=415 nm of Sr4Al14O25:0.1%Cr3++x%Sm3+and(b)the variation of relative intensity of Sr4Al14O25:0.1%Cr3++x%Sm3+with different concentration of Sm3+.
The photoluminescence properties of Sr4Al14O25: Mn4++Sm3+are shown in Fig.9.What was unexpected was that the co-doping of Sm3+ions decreased the emission intensity of Sr4Al14O25:Mn4+dramatically,while the photoluminescence band position of the phosphor was not altered.In fact,it was noticeable that there was no overlapping between the luminescence spectra of Sr4Al14O25:Mn4+and those of Sr4Al14O25:Sm3+,which was apparently different from Cr3+-doped and Sm3+-doped Sr4Al14O25phosphors. Therefore,it could also be deduced that there was little energy transfer process from Sm3+ions to Mn4+ions in the Sr4Al14O25:Mn4++Sm3+phosphor.
In addition,it can be deduced from the radii of Mn4+ions that Mn4+ions had tendencies to occupy the sites of Al3+ions in Al−O octahedra(AlO6)in Sr4Al14O25and thus led to the charge imbalance(i.e.,an extra positive charge was produced).Simultaneously,a Sr2+vacancy(two negative charges)was generated correspondingly to keep the overall charge balance of crystal.It is worth noticing that a Sr2+vacancy can be generated more easily in comparison with the Al3+vacancy in Sr4Al14O25because the bond energy of Sr−O is lower than that of Al−O bond.By contrast,Sm3+ions tended to occupy the positions of Sr2+ions and thus extra Sr2+vacancies(two negative charges)wereinduced to keep the charge balance when Sm3+ions were co-doped into Sr4Al14O25:Mn4+phosphors,which deteriorated the charge imbalance in Sr4Al14O25and thus had a remarkably negative effect on the luminescent properties of phosphor.

FIG.8(a)Excitation spectrum monitored at 651 nm,and (b)emission spectra excited by 325 nm light of 0.1%Mndoped Sr4Al14O25.
E.Luminescence chromaticity
To evaluate the chromaticity properties of Sr4Al14O25:Cr3++Sm3+,and Sr4Al14O25:Mn4++Sm3+we calculated chromaticity coordinates and presented CIE chromaticity diagram,which are shown in Fig.10.It was found that the chromaticity coordinate of Sr4Al14O25:Cr3++Sm3+was close to that of Sr4Al14O25:Cr3+in deep red region.In contrast,the chromaticity coordinate of Sr4Al14O25:Mn4++Sm3+was in the lower saturated red region compared with that of Sr4Al14O25:Mn4+.In addition,it could be seen that the chromaticity(x,y)of the prepared red phosphor Sr4Al14O25:Cr3++Sm3+was 0.728956 and 0.271038,which was close to that of commercial 3.5MgO·0.5MgF2·GeO2:Mn4+phosphor.Apparently, the prepared Sr4Al14O25:Cr3++Sm3+phosphor could enlarge the deep red region and improve the rendering index.Consequently,it could be concluded that Sr4Al14O25:Cr3++Sm3+is suitable to be used as a high efficient red phosphor in W-LEDs.
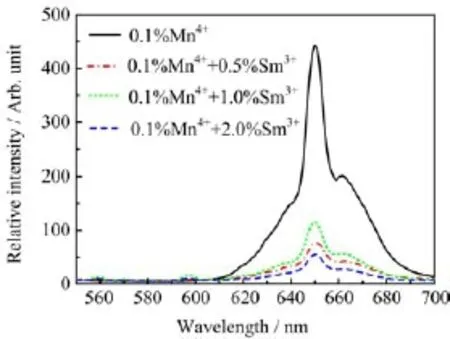
FIG.9 Dependence of emission spectra of Sr4Al14O25: Mn4++Sm3+phosphor on the concentrations of Sm3+ions excited by a 325 nm light.

FIG.10 CIE chromaticity diagram of different phosphors.
IV.CONCLUSION
The Sr4Al14O25:M and Sr4Al14O25:M+Sm3+(M=Mn4+,Cr3+)phosphors were synthesized by solid-state reaction method at 1350◦C for 5 h in the air.It was found that the excitation and emission band positions were not altered and the emission intensity was significantly enhanced when Sm3+ions were co-doped in Sr4Al14O25:Cr3+phosphors,which was mainly ascribed to the radiation and reabsorption process from Sm3+ions to Cr3+ions.In contrast,the emission intensity of Sr4Al14O25:Mn4+was reduced remarkably when Sm3+ions were co-doped.Based on the chromaticity coordinates and CIE chromaticity diagram of the prepared phosphors,we believed that the Sm3+-doped Sr4Al14O25:Cr3+phosphors showed promising applications in displays and solid state lightening.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51302059)and the Natural Science Foundation of Anhui Province (No.1208085QE99).
[1]S.Nakamura,T.Mukai,and M.Senoh,Appl.Phys. Lett.64,1687(1994).
[2]W.Chung,H.J.Yu,and S.H.Park,J.Cryst.Growth 326,73(2011).
[3]H.Ryu and K.S.Bartwal,J.Alloys Compd.480,966 (2009).
[4]Y.Karabulut,A.Canimoglu,Z.Kotan,O.Akyuz,and E.Ekdal,J.Alloys Compd.583,91(2014).
[5]M.Y.Peng,X.W.Yin,P.A.Tanner,C.Q.Liang,P. F.Li,Q.Y.Zhang,and J.R.Qiu,J.Am.Ceram.Soc. 96,2870(2013).
[6]Y.D.Xu,D.Wang,L.Wang,N.Ding,M.Shi,J.G. Zhong,and S.Qi,J.Alloys Compd.550,226(2013).
[7]C.J.Zha,P.Osvath,A.Launikonis,and A.D.Scully, J.Alloys Compd.603,136(2014).
[8]J.Q.Liu,X.J Wang,T.T.Xuan,H.L.Li,and Z.Sun, J.Alloys Compd.593,(2014).
[9]V.Singh,G.Sivaramaiah,J.L.Rao,and S.H.Kim, Appl.Magn.Reson.45,37(2014).
[10]H.N.Luitel,T.Watari,T.Torikai,and M.Yada,J. Opt.Mater.31,1200(2009).
[11]R.E.Kroon,H.C.Swart,O.M.Ntwaeaborwa,and H. A.A.Seed Ahmed,J.Phys.:Condens.Matter 439,83 (2014).
[12]Y.H.Lin,Z.L.Tang,Z.T.Zhang,and C.W.Nan,J. Eur.Ceram.Soc.23,175(2003).
[13]K.P.Wang,J.X.Zhang,J.Y.Wang,W.T.Yu,H.J. Zhang,Z.P.Wang,and Z.S.Shao,Mater.Res.Bull. 41,1695(2006).
[14]H.Lin,X.Y.Wang,L.Lin,D.L.Yang,T.K.Xu,J. Y.Yu,and E.Y.B.Pun,J.Lumin.116,139(2006).
[15]B.Smets,J.Rutten,G.Hokes,and J.Verlijsdonk,J. Electrochem.Soc.136,2119(1989).
[16]R.X.Zhong,J.H.Zhang,X.Zhang,S.Z.Lu,and X. J.Wang,Appl.Phys.Lett.88,201916(2006).
[17]H.N.Luitel,T.Watari,R.Chand,T.Torikai,and M. Yada,Opt.Mater.34,1375(2012).
[18]Q.L.Sai,C.T.Xia,H.Rao,X.D.Xu,G.P.Zhou, and P.Xu,J.Lumin.131,2359(2011).
[19]D.H.Ye,Z.F.Hu,W.Zhang,Y.P.Cui,L.Luo,and Y.H.Wang,Opt.Mater.36,1879(2014).
∗Authors to whom correspondence should be addressed.E-mail:drxuyudong@126.com,leiw03@126.com,Tel./FAX:+86-551-62901362
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Visualization of Melting of Antiferromagnetic Insulator Phase in Phase-Separated Manganite Film using Magnetic Force Microscopy
- Oxidation of Anatase TiO2(001)(1×4)Surface
- One-Dimensional Scanning of Electronic Wavefunction in Carbon Nanotubes by Molecular Encapsulation
- Chemical Empiricism 2.0 at Age of Big Data:Large-scale Prediction of Reaction Pathways Based on Bond Dissociation Energies
- First-Principles Study of La Doping Effects on the Electronic Structures and Photocatalytic Properties of Anatase TiO2
- Reconstruction of Smoke Plume Concentration Peaks Based on Modified MAX-DOAS Tomography
