Oxidation of Anatase TiO2(001)(1×4)Surface
2015-01-20KunChenYonglingShiJinZho
Kun-d Chen,Yong-ling Shi,Jin Zho,b,c∗
a.ICQD/Hefei National Laboratory for Physical Sciences at the Microscale,and Key Laboratory of Strongly-Coupled Quantum Matter Physics,Chinese Academy of Sciences,and Department of Physics, University of Science and Technology of China,Hefei 230026,China
b.Synergetic Innovation Center of Quantum Information&Quantum Physics,University of Science and Technology of China,Hefei 230026,China
c.Department of Physics,University of Pittsburgh,Pittsburgh,Pennsylvania 15261,USA
(Dated:Received on May 13,2015;Accepted on May 28,2015)
Oxidation of Anatase TiO2(001)(1×4)Surface
Kuan-da Chena,Yong-liang Shia,Jin Zhaoa,b,c∗
a.ICQD/Hefei National Laboratory for Physical Sciences at the Microscale,and Key Laboratory of Strongly-Coupled Quantum Matter Physics,Chinese Academy of Sciences,and Department of Physics, University of Science and Technology of China,Hefei 230026,China
b.Synergetic Innovation Center of Quantum Information&Quantum Physics,University of Science and Technology of China,Hefei 230026,China
c.Department of Physics,University of Pittsburgh,Pittsburgh,Pennsylvania 15261,USA
(Dated:Received on May 13,2015;Accepted on May 28,2015)
Anatase TiO2(001)surface arouses lots ofresearch interests since it is believed to be the most reactive surface.However,recent STM measurements showed that except the defect sites, anatase TiO2(001)(1×4)reconstructed surface is inert to H2O adsorption.It was indicated that oxidation could be the reason which induces the inert surface reactivity.Therefore,it is strongly motivated to understand the oxidation structures as well as the oxidation process on this surface.In this work,based on first principles calculations,we investigated the oxidized structures and processes of TiO2anatase(001)surface with(1×4)reconstruction. We have discovered two kinds of oxidized structures through the molecular adsorption and dissociated adsorption with different oxidation ratio.To understand the oxidation process, we studied the reaction barrier of oxidation process.We conclude the stability of different oxidized structures with different oxidation ratio by comparing the free energy of the system as a function of oxygen chemical potential.Based on that,a first-principles-based phase diagram of the low-energy oxidized surface structures is provided.The effects of the lattice stress are also studied.Results show that the oxidized structure and oxidation ratio strongly depend on the temperature and pressure.The lattice stress also plays an important role.
Surface phase diagram,Oxidization process,Surface reconstruction
I.INTRODUCTION
TiO2is a semiconductor with many applications in photocatalysis,photoelectrocatalysis,and photovoltaics[1−3].To understand the principle of the photocatalysis,TiO2rutile(110)surface has been intensively studied because of its stability.However,anatase phase of TiO2yields more stable nanoparticles and higher photocatalytic activities[2,4].
A natural anatase crystal typically exhibits majority (101)surface and minority(001)surfaces.The minority (001)surface has been suggested to be the most reactive surface by first principles calculations[5−7].This surface is known to exhibit(1×4)surface reconstruction because of the existing surface stress[8].Selloni et al.presented a reconstructed structure model labeled as“ad-molecule”(ADM)model[8].Based on ADM model,H2O molecules dissociated directly on the reconstruction ridge,suggesting a high catalytic reactivity[9].
Motivated by this exciting hypothesis,lots of efforts have been devoted to the synthesis of(001)-rich anatase nanocrystals[10−13].However,the validity of these theoretical predictions is still controversial.Actually, they are even in contrast to some recent experimental observations.For example,a comparison of the activity of epitaxial anatase(001)and rutile(110)surfaces revealed nearly equal photochemical rate constants[14], and a clean anatase(001)surface exhibited a lower reactivity than the anatase(101)surface in photocatalytic reactions[15,16].Among these investigations,recent atomic-resolved STM measurements on anatase(001) (1×4)reconstructed surface grown on SrTiO3substrate gave direct evidence that this surface is inert to H2O adsorption[17].
In order to understand the experimental results,oxidation of the anatase(001)(1×4)reconstructed surface has been considered.The simplest oxidation structure model in which an additional O atom is added to each bridging O on the ridge can qualitatively explain the experimental results[17].However,the oxidized structures with different oxidation ratio as well as the oxidation process have not been systematically studied. In this work,using first principles calculations,we investigated the oxidized structures with different oxidation ratio of TiO2anatase(001)surface with(1×4)reconstruction.To understand the oxidation process,westudied the reaction barrier of oxidation process.To compare the stability of different oxidized structures with different oxidation ratio,we calculated the free energy of the system as a function of oxygen chemical potential.Based on that,a first-principles-based phase diagram of the low-energy oxidized surface structures was provided.Since the anatase(001)(1×4)reconstructed surface was grown on SrTiO3substrate in the STMmeasurements,there existed lattice mismatch[11, 12].Therefore,we also investigated the effects oflattice stress.
II.METHOD
First principles calculations were performed using Vienna ab initio simulation package within the generalized gradient approximation[18−20].The exchangecorrelation functional of Perdew-Burke-Ernzerhof[21] and the projector-augmented wave methods[22]were used.The plane-wave basis set cutoff energy is 400.0 eV.The criteria of convergence for electronic structure and geometrical optimization are set to be 10−5and 10−3eV,respectively,and the relaxation will stop if forces on all the atoms are smaller than 0.05 eV/˚A.For a(4×4)or larger area of the anatase surface,reciprocal space sampling was restricted to the Gamma point.Climbing image nudged elastic band method[23]was used for transition state location and barrier height determination.Finite difference method was used to calculate vibrationalfrequencies for the calculation of free energy,during which only those atoms on ridge is allowed to relax.The Gibbs free energy was calculated using the same method presented in Ref.[24]. The oxidation formation energy is calculated as

where n is the number of extra O2in one surface unit cell of the oxidized surface.Finite differences were used to calculate vibrational frequencies for the free energy calculation,during which only those atoms on ridge are allowed to relax.In this study,we use two different lattice constants.The first is the lattice constant a=0.378 nm which is obtained from anatase bulk structure optimized by PBE functional.The other is lattice constant of a=0.393 nm i.e.the lattice of SrTiO3[11, 12].It was investigated by considering the lattice mismatch between the anatase(001)and SrTiO3.
III.RESULTS AND DISCUSSION
A.Oxidized structure

FIG.1(a)Optimized M and D-type oxidation structures with different oxidation ratio(a=0.378 nm).(b)Formation energy for M and D type oxidized structures with different oxidation ratio(a=0.378 nm and a=0.393 nm).O:red ball, Ti:silver ball.
A(4×4)anatase surface cell was used to study the oxidized structure.We generate two kinds of oxidized structures by inserting O2molecules(labeled as M)or dissociated O atom(labeled as D)into the(1×4)ridge. The ratio of oxidation is defined as the proportion of extra O2number relative to the Tiridgeatom(as shown in Fig.1(a))number in one unit cell.Therefore,using a(4×4)unit cell,we studied the oxidation ratio from 1/8 ML to 1/2 ML for D type of oxidation.For M type of oxidation,(4×4)unit cell is used to calculate the 1/4 and 1/2 ML.And a(8×4)unit cell is used to calculate the 1/8 ML M type oxidation as needed.The optimized structures with a=0.378 nm is shown in Fig.1(a). The formation energy corresponding to oxidation ratio is presented in Fig.1(b).One can see that for both M and D oxidation structures,oxidation with an extensive lattice(a=0.393 nm)has lower formation energy.This is because that the Ti−O bond is also lengthened when the lattice is extended.Therefore it will be easier to insert O2or O atom into the ridge.The formation energy decreases with the increase of the oxidation ratio, and becomes positive when the oxidation ratio is larger than 1/4 ML.For 1/8 ML,the formation energy is as large as 1.2−2.1 eV,indicating the low ratio oxidation is easy.The M oxidation structure has higher formation energy compared with D oxidation structures for 1/4 and 1/2 ML because of extra Ti−O and O−O bond formation.

FIG.2 The reaction path and corresponding structures 1−9 for the oxidation process from 0 ML to 1/2 ML.
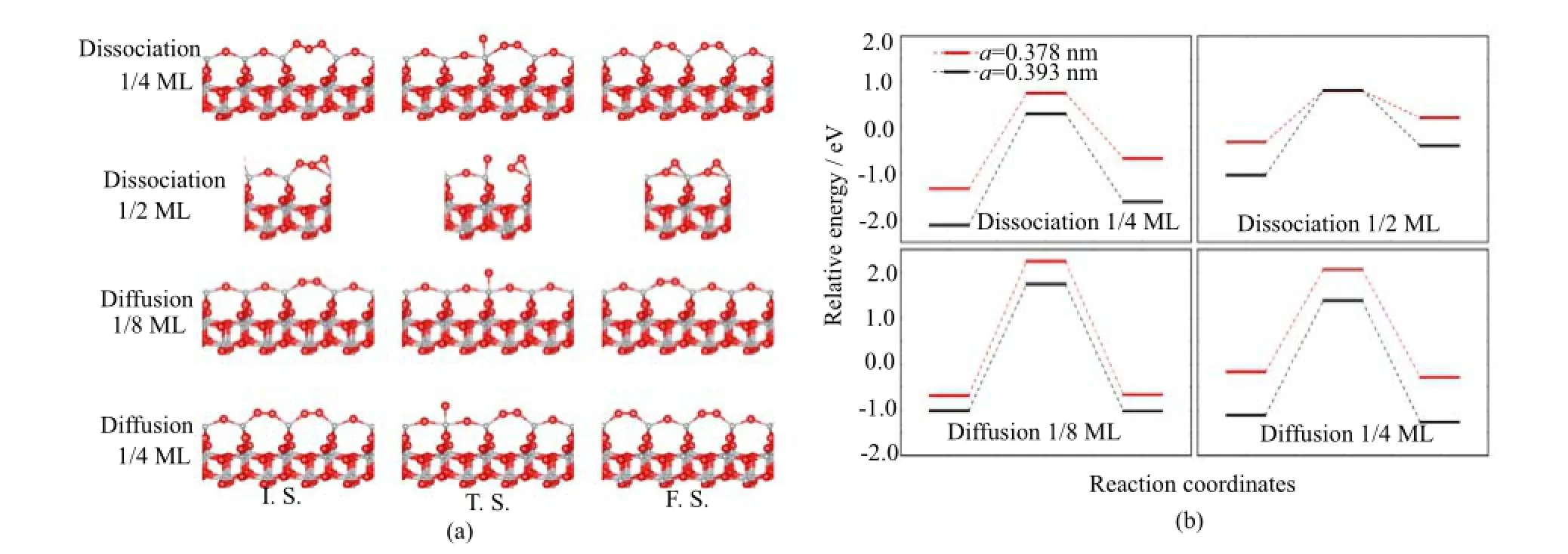
FIG.3 The reaction path and corresponding structures for the dissociation process and diffusion process.
B.Reaction barrier for oxidation
To understand the oxidation process,we investigated the reaction barrier of oxidation using a(4×4)unit cell. Figure 2 shows the oxidation process of0−1/4−1/2 ML for M type oxidation structure with two different lattice constant.We put two O2molecules physically adsorbed on the surface as the initial structure(structure 1 in Fig.2(a)).For both lattice parameters we investigated, the oxidation process from 0 ML to 1/4 ML is very easy.The first O2molecule needs to overcome a very small energy barrier(less than 0.1 eV for both lattice constants)to form a meta-stable oxidized structure(structure 3 in Fig.2(a)).Then,another minor energy barrier(less than 0.05 eV)needs to be overcome to reach the stable 1/4 ML oxidized structure (structure 5 in Fig.2(a)).Compared to that,the oxidation from 1/4 ML to 1/2 ML is much more difficult.The second O2need to overcome a large energy barrier(1.7 eV when a=0.378 nm and 1.0 eV when a=0.393 nm)to reach a meta-stable 1/2 ML oxidized structure(structure 7 in Fig.2(a)).After that, a small energy barrier(0.2 eV when a=0.378 nm and 0.1 eV when a=0.393 nm)needs to be overcome to reach the stable 1/2 ML oxidized structure(structure 9 in Fig.2(a)).
From here we can see that the oxidation from 0 ML to 1/4 ML(structure 1 to 5 in Fig.2(a))is an exothermic reaction.The energy barrier is less than 0.1 eV. This indicates it is very easy to form a low ratio oxidized structure.However,the oxidation from 1/4 ML to 1/2 ML(structure 5 to 9 in Fig.2(a))is an endothermic reaction.The highest energy barrier that needs to be overcome is above 1 eV.Considering the inverse process,only 0.4 eV energy barrier needs to be overcome to release a O2from 1/2 ML oxidized structure.Therefore,the high ratio oxidation is not easy to be achieved.
From our study of reaction barrier of oxidation,we found that it is difficult to decompose a O2molecule and form a D type oxidized structure directly.However,D type oxidized structure can be obtained by the dissociation of M type oxidized structure.Figure 3 shows the dissociation barrier of M type oxidized structure into D type oxidized structure for 1/4 and 1/2 ML(the energy of un-oxidized structure is set to zero energy).All the dissociation reactions are endothermic.For 1/4 ML, the dissociation energy barrier is as high as 2.1 eV for a=0.378 nm and 2.5 eV for a=0.393 nm.For 1/2 ML, the dissociation energy barrier is 1.1 eV for a=0.378 nm and 1.8 eV for a=0.393 nm.The reverse process,i.e. the re-combination process from D type to M type is easier.For 1/4 ML,it is 1.4 eV for a=0.378 nm and1.9 eV for a=0.393 nm.For 1/2 ML,it is as small as 0.6 eV for a=0.378 nm and 1.2 eV for a=0.393 nm.We also studied the diffusion energy barrier of the extra O in D type oxidized structure with 1/8 ML and 1/4 ML. For 1/8 ML,the diffusion energy barrier is as large as 2.9 and 2.8 eV for a=0.378 and a=0.393 nm.For 1/4 ML, it is 2.2 and 2.5 eV for a=0.378 and a=0.393 nm.From these calculations one can see that the dissociation of M type to D type oxidized structure is not easy.The diffusion of extra O atom in D type structure is even more difficult.Therefore,if only considering the ground state energy and reaction barrier at zero temperature,Mtype oxidized structure is more favorable than D type.
C.Surface phase diagram
To understand the stability of different oxidized structure in a real environment,including the effects of temperature and pressure,we calculated the Gibbs free energy of different oxidized structure as:

here Etotalis the total energy of the system,which can be obtained by DFT calculations.The configurational free energy Fconf,is almost always negligible when temperature is sufficiently low(T<1000 K)[25].pV in Eq.(2)could be elided even in very high temperature and pressure.Fvibis the free energy contributed by the vibration,which can be handled with harmonic approximation:

whereωican be calculated using the finite differences method.Including the chemical potential of O2(µO), the difference of the Gibbs free energy before and after surface oxidation can be expressed as:

whereΔEtotal=−θEformation,θis the oxidation ratio. And in equilibrium with O2gas,µOis expressed as in Ref.[26]:
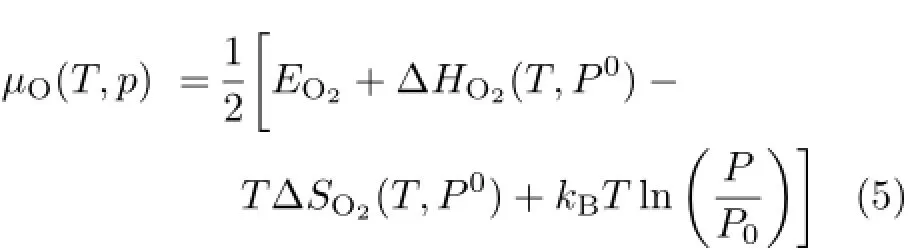
where kB,PO,PO,and P are the Boltzmann constant, standard atmospheric pressure,atmospheric pressure, and oxygen partial pressure,respectively.EO2was obtained from a spin-polarized DFT calculation,considering that the ground state of O2is spin triplet. ΔHO2(T,PO)and TΔSO2(T,PO)were taken from a thermodynamic database[27].The fourth term is the contribution coming from the partial pressure of oxygen.

FIG.4 Oxygen pressure-temperature phase diagram of anatase(001)(1×4)reconstructed surface.(a)a=0.368 nm and(b)a=0.393 nm.

TABLE IΔFvib(in eV)of M-type oxidized and D-type oxidized structures at two temperatures(300 K as room temperature and 800 K as annealing temperature).
First,we consider the contribution of the vibrations ΔFvib.In Table I we giveΔFvibfor M-type and D-type oxidized structure with different oxidation ratio at 300 K(room temperature)and 800 K(annealing temperature in the experiments[17]).It can be seen that compared with M-type oxidized structure,the vibration can lower the Gibbs energy of D-type oxidized structure more distinctly at 800 K.This indicates that D-type oxidized structure can be more stable at high temperature. This trend is more distinct for low coverage(1/4 ML) and larger lattice constant(a=0.393 nm).
Including the chemical potential of O2molecule, we plot out a phase diagram in the range of the temperature T=100−1300 K,and oxygen pressure P=10−2O−0PO(Fig.4).One can see that within this re-gion,the un-oxidized structure(ADM model),1/8 ML D-type oxidized structure and 1/4 ML M-type oxidized structure are the three most stable structures at different temperature and oxygen pressure.Comparing the two different lattice constants,one can see that the oxidation is easier to happen with larger lattice constant.
From the phase diagram,one can see that most stable structure depends on the temperature and pressure a lot.We noticed that for this surface,the experimental results from different group can be very different. For example,Liu et al.and Zhang et al.found that this surface is reactive for azo dye molecules[28]and 4-chlorophenol[29].However,there are other results showing this surface being inert[16,17,30−32].In addition,both Wang et al.and Zhang et al.studied this surface using atomic resolved STM[17,29].However, their STM measurements show completely different images.It is not difficult to be understood in a uniform picture based on our calculations.It is possible to obtain different surface structures at different temperature and oxygen pressure.Some of the structures are reactive(ADM model)and some others are inert(oxidized structure).
IV.CONCLUSION
In summary,we systematically studied the oxidized structure and oxidization process of TiO2anatase(001) surface with 1×4 reconstruction.By considering different oxidation ratio with different lattice constant,two kinds of oxidized structures(M-type and D-type)by inserting O2or O into the chain have been studied. The formation energy,oxidation reaction barrier and the free energy profile have been studied to understand the oxidation possibility.A first-principles based phase diagram of the low-energy oxidation surface structures is provided.All these studies show that the oxidation with low ratio is easy to happen.The extensive stress of the surface also helps the oxidation.From the phase diagram one can see that the most stable structure relies on the temperature and pressure strongly.Our results may explain the different experimental results from different groups.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.11322434).Calculations were performed at the Shanghai Supercomputer Center and Environmental Molecular Sciences Laboratory at the PNNL,a user facility sponsored by the DOE Office of Biological and Environmental Research.
[1]A.Fujishima and K.Honda,Nature 238,37(1972).
[2]U.Diebold,Surf.Sci.Rep.48,53(2003).
[3]A.Fujishima,X.Zhang,and D.Tryk,Surf.Sci.Rep. 63,515(2008).
[4]M.A.Henderson,Surf.Sci.Rep.66,185(2011).
[5]A.Vittadini,A.Selloni,F.P.Rotzinger,and M. Gratzel,Phys.Rev.Lett.81,2954(1998).
[6]G.S.Herman,Z.Dohnalek,N.Ruzycki,and U. Diebold,J.Phys.Chem.B 107,2788(2003).
[7]X.Q.Gong and A.Selloni,J.Phys.Chem.B 109, 19560(2005).
[8]M.Lazzeri and A.Selloni,Phys.Rev.Lett.87,266105 (2001).
[9]X.Q.Gong,A.Selloni,and A.Vittadini,J.Phys. Chem.B 110,2804(2006).
[10]H.G.Yang,C.H.Sun,S.Z.Qiao,J.Zou,G.Liu,S.C. Smith,H.M.Cheng,and G.Q.Lu,Nature 453,638 (2008).
[11]Sugiharto,S.Yamamoto,T.Sumita,and A.Miyashita, J.Phys.Condens.Matter 13,2875(2001).
[12]S.A.Chambers,C.M.Wang,S.Thevuthasan,T. Droubay,D.E.McCready,A.S.Lea,V.Shutthanandan,and C.F.Windisch,Thin Solid Films 418,197 (2002).
[13]M.D.McDaniel,A.Posadas,T.Wang,A.A.Demkov, and J.G.Ekerdt,Thin Solid Films 520,6525(2012).
[14]T.Ohsawa,I.V.Lyubinetsky,M.A.Henderson,and S.A.Chambers,J.Phys.Chem.C 112,20050(2008).
[15]J.Pan,G.Liu,G.Q.M.Lu,and H.M.Cheng,Angew. Chem.Int.Ed.50,2133(2011).
[16]T.Tachikawa,S.Yamashita,and T.Majima,J.Am. Chem.Soc.133,7197(2011).
[17]Y.Wang,H.J.Sun,S.J.Tan,H.Feng,Z.W.Cheng, J.Zhao,A.D.Zhao,B.Wang,Y.Luo,J.L.Yang,and J.G.Hou,Nat.Commun.4,2214(2013).
[18]G.Kresse and J.Hafner,Phys.Rev.B 48,13115(1993).
[19]G.Kresse and J.Hafner,Phys.Rev.B 47,558(1993).
[20]G.Kresse and J.Hafner,Phys.Rev.B 49,14251(1994).
[21]J.P.Perdew,K.Burke,and M.Ernzerhof,Phys.Rev. Lett.77,3865(1996).
[22]G.Kresse and D.Joubert,Phys.Rev.B 59,1758 (1999).
[23]G.Henkelman,B.P.Uberuaga,and H.J´onsson,J. Chem.Phys.113,9901(2000).
[24]J.Rogal and K.Reuter,Ab initio Atomistic Thermodynamics for Surfaces:A Primer,Report No.RTO-ENAVT-142,(2006).
[25]K.Reuter and M.Scheffler,Phys.Rev.B 68,045407 (2003).
[26]J.M.Sanchez,F.Ducastelle,and D.Gratias,Physica A 128,334(1984).
[27]A.Zunger,in Statics and Dynamics of Alloy Phase Transformations,Berlin:Springer,361(1994).
[28]S.Liu,J.Yu,and M.Jaroniec,J.Am.Chem.Soc.132, 11914(2010).
[29]D.Zhang,G.Li,X.Yang,and J.C.Yu,Chem.Commun.4381(2009).
[30]T.Ohsawa,I.V.Lyubinetsky,M.A.Henderson,and S.A.Chambers,J.Phys.Chem.C 112,20050(2008).
[31]J.Pan,G.Liu,G.M.Lu,and H.M.Cheng,Angew. Chem.Int.Ed.50,2133(2011).
[32]Z.Zheng,B.Huang,J.Lu,X.Qin,X.Zhang,and Y. Dai,Chemistry 17,15032(2011).
∗Author to whom correspondence should be addressed.E-mail: zhaojin@ustc.edu.cn
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Visualization of Melting of Antiferromagnetic Insulator Phase in Phase-Separated Manganite Film using Magnetic Force Microscopy
- One-Dimensional Scanning of Electronic Wavefunction in Carbon Nanotubes by Molecular Encapsulation
- Chemical Empiricism 2.0 at Age of Big Data:Large-scale Prediction of Reaction Pathways Based on Bond Dissociation Energies
- First-Principles Study of La Doping Effects on the Electronic Structures and Photocatalytic Properties of Anatase TiO2
- Reconstruction of Smoke Plume Concentration Peaks Based on Modified MAX-DOAS Tomography
- Electronic Structure and Circular Dichroism of Natural Alboatisins Isolated from Aerial Parts of Isodon Albopilosus:DFT and TDDFT Study
