c-Jun N-terminal kinase 3 expression in the retina of ocular hypertension mice: a possible target to reduce ganglion cell apoptosis
2015-01-18YueHeJieChenShuguangZhangYuanshengYuanYanLiHongbinLvJinhuaGan
Yue He, Jie Chen, Shu-guang Zhang, Yuan-sheng Yuan, Yan Li, Hong-bin Lv Jin-hua Gan
1 Department of Ophthalmology, the Affliated Hospital of Luzhou Medical College, Luzhou, Sichuan Province, China
2 Department of Rheumatology and Immunology, the Affliated Hospital of Luzhou Medical College, Luzhou, Sichuan Province, China
3 Department of Ophthalmology, the Second People’s Hospital of Zhengzhou City, Zhengzhou, Henan Province, China
4 Department of Ophthalmology, the First Affliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China
c-Jun N-terminal kinase 3 expression in the retina of ocular hypertension mice: a possible target to reduce ganglion cell apoptosis
Yue He1,*,#, Jie Chen2,#, Shu-guang Zhang3, Yuan-sheng Yuan4, Yan Li4, Hong-bin Lv1, Jin-hua Gan1
1 Department of Ophthalmology, the Affliated Hospital of Luzhou Medical College, Luzhou, Sichuan Province, China
2 Department of Rheumatology and Immunology, the Affliated Hospital of Luzhou Medical College, Luzhou, Sichuan Province, China
3 Department of Ophthalmology, the Second People’s Hospital of Zhengzhou City, Zhengzhou, Henan Province, China
4 Department of Ophthalmology, the First Affliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China
Glaucoma, a type of optic neuropathy, is characterized by the loss of retinal ganglion cells. It remains controversial whether c-Jun N-terminal kinase (JNK) participates in the apoptosis of retinal ganglion cells in glaucoma. This study sought to explore a possible mechanism of action of JNK signaling pathway in glaucoma-induced retinal optic nerve damage. We established a mouse model of chronic ocular hypertension by reducing the aqueous humor followed by photocoagulation using the laser ignition method. Results showed signifcant pathological changes in the ocular tissues after the injury. Apoptosis of retinal ganglion cells increased with increased intraocular pressure, as did JNK3 mRNA expression in the retina. These data indicated that the increased expression of JNK3 mRNA was strongly associated with the increase in intraocular pressure in the retina, and correlated positively with the apoptosis of retinal ganglion cells.
nerve regeneration; ocular hypertension; JNK3; retinal ganglion cell; glaucoma; laser photocoagulation; intraocular pressure; neural regeneration
Funding:This work was supported by grants from the Youth Foundation of Luzhou Medical College of Luzhou City of Sichuan Province of China, No. [2010]108; the Talent Fund of the Affiliated Hospital of Luzhou Medical College of Luzhou City of Sichuan Province of China, No. [2011]43.
He Y, Chen J, Zhang SG, Yuan YS, Li Y, Lv HB, Gan JH (2015) c-Jun N-terminal kinase 3 expression in the retina of ocular hypertension mice∶ a possible target to reduce ganglion cell apoptosis. Neural Regen Res 10(3)∶432-437.
Introduction
Glaucoma is a type of optic neuropathy and a major cause of blindness in the world. It is characterized by the vertical expansion of the cupping of the optic nerve head and the loss of retinal ganglion cells (Hatt et al., 2006). Over the years, there have been two accepted theories addressing the pathogenesis of glaucoma: the mechanical theory (Flammer et al., 1992; Halpern and Grosskreutz, 2002) and the hemorheological theory (Chung et al., 1999; Emre et al., 2004; Grieshaber and Flammer, 2005). The current deeper understanding of glaucoma pathogenesis suggests that the traditional mechanical pressure and vascular theories are insufficient to explain the pathogenesis of glaucomatous optic nerve damage. Other pathological mechanisms are likely to lead to the injuries to retinal ganglion cells and the optic nerve.
c-Jun N-terminal kinase (JNK) is in a class of serine/ threonine kinases. The JNK signaling pathway plays an important role in cellular stress response, and can be activated by a variety of extracellular stress signals. It is also known as stress-activated protein kinase (SAPK) (Johnson and Nakamura, 2007). JNK is associated with the pathogenesis of many diseases, such as Parkinson’s disease (Wilhelm et al., 2007), diabetes (Yang et al., 2007) and carcinogenesis (Sakurai et al., 2006). The relationship between JNK and apoptosis of retinal ganglion cells in glaucoma is still poorly understood.
This study explores a possible mechanism of action of JNK signaling pathway in glaucomatous optic nerve damage using a mouse model of chronic ocular hypertension and provides a theoretical basis for future research concerning this new target for the treatment of glaucoma.
Materials and Methods
Animals
A total of 44 clean male C57BL/6 mice aged 6 weeks and weighing 18—22 g were provided by the Chongqing Experimental Animal Center (License No. SCXK (Yu) 2007-0001) in China. All mice were fed with solid feed and clean water, and housed at 21°C in a 12-hour light/dark cycle in a specifc pathogen-free environment. The protocols were approved by the Animal Ethics Committee of Kunming Medical University in China.
Establishment of mouse models of chronic ocular hypertension
The right eyes of C57BL/6 mice were used as experimental eyes, and the left eyes as untreated controls. At 30 minutes before surgery, the right eye of each mouse was administered mydriatic (atropine eye drops; Shenyang Xing Qi Pharmaceutical Co., Ltd., Shenyang, Liaoning Province, China) and compound tropicamide eye drops (Santen Pharmaceutical Co., Ltd., Osaka, Japan). After being weighed on an electronic scale (Wuxi Weigher Factory Co., Ltd., Wuxi, Jiangsu Province, China), the mice were injected intraperitoneally (i.p.) with 1.7 mL/kg 3% sodium pentobarbital. Simultaneously, each right eye was administered with three drops of oxybuprocaine hydrochloride (Santen Pharmaceutical Co., Ltd.). The behaviors of the mice were observed closely. When they did not react to a pinch of their dorsal skin, the surgery could begin. Under a slit lamp microscope (Chongqing Kanghua Medical Equipment Co., Ltd., Chongqing, China), a 0.3 mm × 13.0 mm needle punctured the anterior chamber from the corneal limbus below the nose to release aqueous humor. Thus, the anterior chamber became shallow and the anterior chamber angle closed. A 532-diode laser (Zeiss 532 laser machine, Jena, Germany) was directly used to perform right cornea edge 360° photocoagulation. The laser energy, illumination time, and light spot size were 100 mW, 0.05 seconds and 200 µm, respectively. The number of light spots was 93 ± 8. The whole procedure took less than 10 minutes (Aihara et al., 2003; Mabuchi et al., 2003). After the surgery, mice were administered chloramphenicol eye drops (Bausch & Lomb, Jinan, Shandong Province, China) and erythromycin ointment (Nanjing Baijingyu Pharmaceutical Co., Ltd., Nanjing, Jiangsu Province, China). The general condition of the mice and their level of consciousness were monitored. A total of 33 models of ocular hypertension were successfully established. Eleven were excluded, because one mouse died after general anesthesia, two mice suffered from corneal opacity, two mice developed cataracts, one mouse experienced atrophy of the eyeball, one mouse suffered from perforation of the eyeball, and intraocular pressure of a further four mice did not exceed 30% of preoperative intraocular pressure.
Intraocular pressure measurement
We observed whether conjunctival hyperemia, corneal edema, or anterior chamber reaction appeared at 1 and 3 days after the surgery. A TONO-PEN AVIA pen tonometer (Reichert, Inc., Depew, NY, USA) was used to measure intraocular pressure at 3 days after the surgery. Subsequently, intraocular pressure was measured every 4 days. Using a previously accepted standard measurement (Aihara et al., 2003), intraocular pressure after laser photocoagulation increased by more than 30% compared with before laser photocoagulation, which indicated that the models were successful. That is, intraocular pressure (%) = 100% × (intraocular pressureafterphotocoagulation— intraocular pressurebeforephotocoagulation)/ intraocular pressurebeforephotocoagulation≥ 30%. At 3 days after photocoagulation, if intraocular pressure of the experimental eyes exceeded 30% of preoperative intraocular pressure, they were not treated again. If intraocular pressure of experimental eyes was lower than 30% of preoperative intraocular pressure, laser photocoagulation was performed again at 7 days.
Sample collection and hematoxylin-eosin staining
Eight mice were separately collected at 1, 2, 4 and 8 weeks after model establishment. All mice were intraperitoneally anesthetized with 3% sodium pentobarbital (1.7 mL/kg). Each eyeball was coronally incised along the pars plana. The anterior segment was discarded. The retina was carefully removed, dried with a filter paper, fixed, and stained with hematoxylin and eosin.
Apoptosis of retinal ganglion cells as detected by TdT-mediated dUTP-biotinnick end labeling (TUNEL)
Frozen sections of mouse retina were fixed with 4% paraformaldehyde/0.01 M PBS (pH 7.4), washed with PBS for 5 minutes × 2, and then fxed with ethanol and acetic acid (2:1) at —20°C for 5 minutes in a coplin jar, followed by PBS washes for 5 minutes × 2. Sections were treated with a balanced solution (pH 7.4) at room temperature for 10 seconds, and incubated with TDT enzyme (Millipore, Boston, MA, USA) at 37°C in a wet box for 1 hour. Sections were shaken in a coplin jar containing a termination solution for 15 seconds, and incubated at room temperature for 10 minutes. The tissue was coated with anti-Dig 65 µL/5 cm2in a wet box at room temperature for 30 minutes, covered with peroxidase substrate at room temperature for 5 minutes, incubated in a series of coplin jars with double-distilled water at room temperature for 5 minutes, counterstained with 0.5% methyl green for 10 minutes, washed three times with double-distilled water and 100% butanol, incubated with xylene for 2 minutes, then mounted with resin and observed under a light microscope (Shanghai Optical Instrument Factory, Shanghai, China). Apoptotic nuclei were stained brown. The tissue was sliced into 5-µm-thick sagittal sections taking the optic axis as the center. Each eyeball was cut into two or three sections. Three felds were randomly selected from each section under a 400× objective lens. TUNEL-positive retinal ganglion cells were observed and quantifed.
JNK3mRNA expression in the mouse retina as measured by real-time quantitative reverse transcription (RT)-PCR
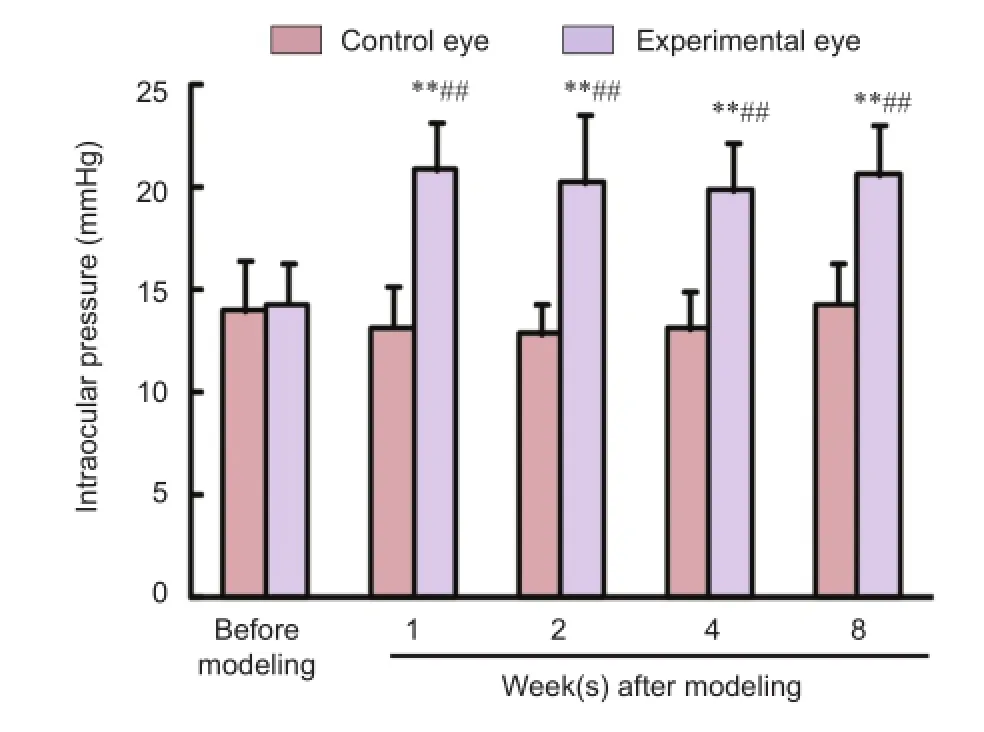
Figure 1 Alterations in intraocular pressure of mouse models of ocular hypertension.

Figure 2 Changes in the anterior chamber angle and retinal morphology of experimental eyes of mouse models of ocular hypertension (hematoxylin-eosin staining, × 400).
Eight mice were separately collected at 1, 2, 4 and 8 weeks after model establishment. After general anesthesia, each eyeball was coronally incised along the pars plana. The anterior segment was discarded. The retina was carefully removed, and dried with a filter paper. Total RNA was extracted in accordance with Trizol’s method (Li et al., 2008). GeneQuantRNA/DNA analyzer (GE (General Electric), Coventry, UK) was used to measure the ratio of absorbance at 260 nm to absorbance at 280 nm of RNA samples. Simultaneously, samples were electrophoresed on a 1% agarose gel. The extent of RNA degradation was measured. RNA was converted into cDNA using a cDNA synthesis kit (Fermentas, Pittsburgh, PA, USA). JNK3 primers were designed using Primer 5.0 software (PREMIER Biosoft, Palo Alto, CA, USA). The products of the above primers were correct after detection using Blast software (National Center for Biotechnology Information, Bethesda, MD, USA). An internal reference gene GAPDH was designed and produced by Fermentas. JNK3 primer sequence: upstream 5′-GAT GAC TCC GTA TGT GGT G-3’, downstream 5′-GCT GGC TTT AAG TTT ATT GT-3′, product of 334 bp. Internal reference gene GAPDH sequence: upstream 5′-CAA GGT CAT CCA TGA CAA CTT TG-3′, downstream 5′-GTC CAC CAC CCT GTT GCT GTA G-3′, the product of 496 bp. The TaqMan probe was designed and synthesized by ABI (Grand Island, NY, USA). RT-PCR was performed in a 20-µL reaction system with a 7300 Real-Time PCR System (ABI). Amplifcation conditions are as follows: 50°C for 2 minutes, one cycle; 95°C for 10 minutes, one cycle; 95°C for 15 seconds, one cycle; 60°C for 1 minute, 40 cycles. RT-PCR results could be revealed by their Ct values. The difference in gene expression was analyzed using the 2—ΔΔCtmethod (Livak and Schmittgen, 2001).
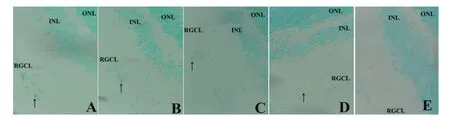
Figure 3 Apoptosis of retinal ganglial cells in mouse models of ocular hypertension.
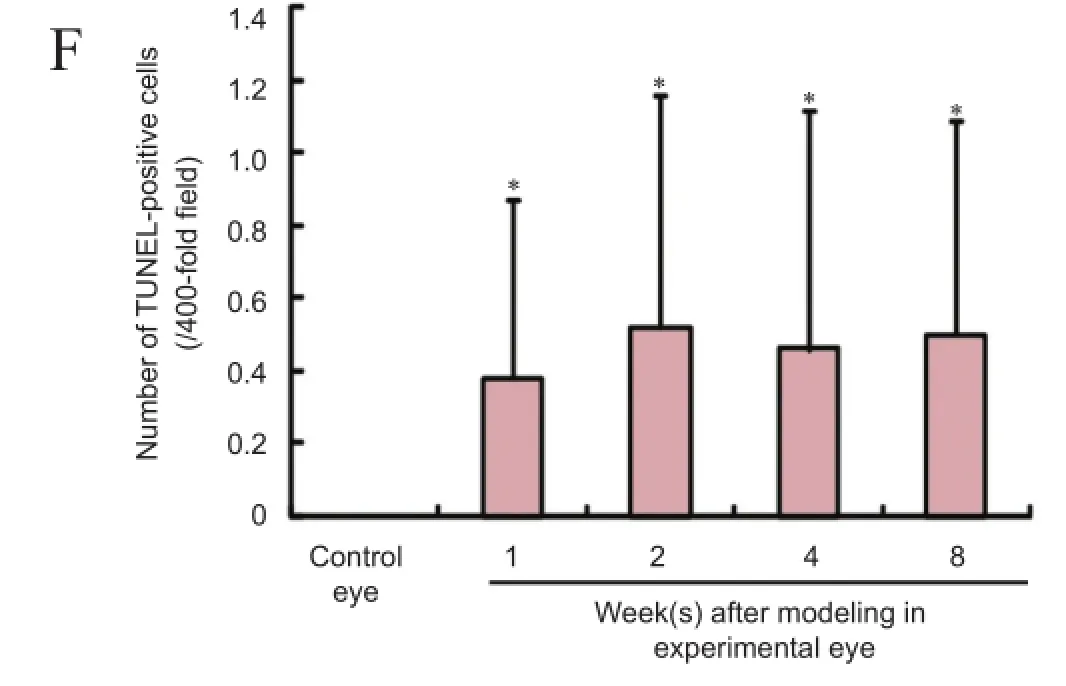
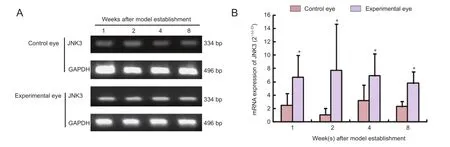
Figure 4JNK3mRNA expression in the retina of mouse models of ocular hypertension.
Statistical analysis
The data are analyzed using SPSS 11.5 statistics software (SPSS, Chicago, IL, USA), and expressed as the mean ± SD. The difference was compared using one-way analysis of variance followed by the least signifcant difference test. A value ofP< 0.05 was considered statistically signifcant.
Results
General conditions of mouse models of ocular hypertension
At 1 day after the surgery, the experimental eyes of all mice suffered from conjunctival hyperemia and corneal edema. Slightly turbid aqueous humor was visible in the anterior chamber of some eyes. These manifestations disappeared at 3 days, when the conjunctiva, cornea, and anterior chamber of control eyes were all normal.
Changes in intraocular pressure of mouse models of ocular hypertension
The intraocular pressures were similar between the left and right eyes of mice before model establishment (P> 0.05). The intraocular pressures of the right eyes were signifcantly increased after model establishment (P< 0.01) and were higher than the paired control left eyes (P< 0.01); however, they didnot increase with prolonged time after modeling (P> 0.05). The intraocular pressures of the control eyes did not alter signifcantly (P> 0.05; Figure 1).
Alterations in retinal morphology in mouse models of ocular hypertension
Hematoxylin-eosin staining demonstrated that the chamber angle in control eyes was open. Retinal tissue was even and the structure of each layer was clear. The photoreceptors and pigment epithelium cells were tightly connected. The inner and outer nuclear layers were in clear bands, stained uniformly and the cells were of regular shape. The retinal ganglion cells were arranged neatly. At 1, 2, 4 and 8 weeks after model establishment, the experimental eyes displayed a narrow and closed chamber angle. Vacuoles were observed in the ganglion cell layer and inner nuclear layer. The interstitial tissue, nerve fber layer and inner plexiform layer were more loosely arranged. Cells in the inner nuclear layer were disordered (Figure 2).
Apoptosis of retinal ganglion cells in mouse models of ocular hypertension
TUNEL assay displayed that brown, TUNEL-positive cells were seen in the ganglion cell layer of the experimental eyes but were not detected in the control eyes of the mice. The number of TUNEL-positive cells was signifcantly higher in the experimental eyes than in control eyes (P< 0.05) but did not increase signifcantly after 1 day (P> 0.05; Figure 3).
JNK3 mRNA expression in the retina of mouse models of ocular hypertension
JNK3 mRNA could be detected in the retina of normal mice and mouse models of ocular hypertension. Compared with control eyes, JNK3 mRNA expression was signifcantly higher in the retina of mouse models of ocular hypertension (P<0.05), but JNK3 mRNA expression did not increase further with time after modeling (P> 0.05; Figure 4).
discussion
Glaucoma is the second leading cause of blindness, following cataract. The difference is that glaucoma-induced decreased vision cannot be restored using current technology and therefore is particularly prevalent. The most dangerous factor for glaucoma-induced decreased vision is an increase in intraocular pressure. Current treatment is mainly preventative, aimed at reducing and stabilizing the intraocular pressure. Thus, it is important to establish a simple, economical and practical animal model of ocular hypertension for studying pathogenesis and the treatment of glaucoma and protection of the optic nerve. The anatomical structure of the mouse eyeball is similar to that of a human. The development cycle is relatively short and it is easy to perform transgenic manipulation in the mouse eyeball. It has been an ideal model for studying the molecular mechanisms of glaucoma pathogenesis, genetics and drug treatment. Mouse models of laser-induced ocular hypertension are most commonly used at present. Aihara et al. (2003) frst used glass microtubules to remove some aqueous humor from mice eyes, causing the anterior chamber to become shallower. A 532-diode laser (capability 200 mW, time 0.05 seconds, spot size 200 µm, and number of spots 64 ± 6) was then used for photocoagulation of mouse corneal limbus to induce an increase in intraocular pressure (Aihara et al., 2003). Among 22 NIH black Swiss mice, the intraocular pressure increased by more than 30% in 15 mice at 1 week after surgery, in nine mice at 4 weeks, and fve mice at 12 weeks. The highest intraocular pressure reached 39.6 mmHg. Mabuchi et al. (2003) designed mouse models of ocular hypertension, and their intraocular pressure was mainly below 30 mmHg. Grozdanic et al. (2003) established mouse models of ocular hypertension by injecting indocyanine green in the anterior chamber and by photocoagulation of the trabecular meshwork using a diode laser, thus increasing the intraocular pressure of the experimental eyes. The highest intraocular pressure reached was 45 mmHg. The intraocular pressure persisted for 60 days. Shepard et al. (2007) confrmed human glucocorticoid-induced overexpression of the trabecular meshwork reactive protein mutants in mouse eyes using a viral vector, resulting in an evident increase in intraocular pressure. This study established mouse models of ocular hypertension by laser photocoagulation of the mouse limbus. The intraocular pressure increased by ≥ 30% in 75% of the experimental mice. Moreover, no obvious severe complications occurred for the duration of the experiment.
JNK is strongly associated with the occurrence and development of neurodegenerative diseases (Pan et al., 2007; Wang et al., 2013). Glaucoma is also a neurodegenerative disease, therefore the role of JNK is worth investigating to avoid the apoptosis of retinal ganglion cells. JNK3 mainly presents in the nervous system, and involves neuronal cell death (Bogoyevitch, 2006). The JNK signaling pathway mechanisms remain poorly understood, but numerous studies have shown their important effects. Initial studies found that γ-radiation can activate JNK1 and induce apoptosis (Chen et al., 1996). γ-Radiation can continuously stimulate JNK1 synthesis for a long time, and induces apoptosis of Jurkat cells (Chen et al., 1996). In the present study, a TaqMan probe of RT-PCR was applied to determine JNK3 mRNA expression. Our results verifed that as long as the intraocular pressure remained high, there was an increase in JNK mRNA expression. Over time, there was an increased tendency for JNK3 mRNA expression. Ganglion cell apoptosis appeared and JNK3 mRNA expression increased in experimental eyes, but the above phenomena were not detectable in control eyes. These data suggested that JNK3 activation was directly or indirectly correlated with the apoptosis of retinal ganglion cells.
In summary, in the state of ocular hypertension, JNK3 was activated, which was directly or indirectly associated with the apoptosis of retinal ganglion cells. Our follow-up experiments will investigate whether retinal ganglion cells survive the state of ocular hypertension after inhibiting or knocking out JNK3.
Author contributions:YSY and YL designed the study. YH and SGZ implemented the study. JC participated in data analysis, statistics and image processing. HBL and JHG proofread this paper. YH wrote the paper and obtained the funding. All authors approved the final version of the paper.
Conficts of interest:None declared.
Aihara M, Lindsey JD, Weinreb RN (2003) Experimental mouse ocular hypertension: establishment of the model. Invest Ophth Vis Sci 44:4314-4320.
Bogoyevitch MA (2006) The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays 28:923-934.
Chen YR, Meyer CF, Tan TH (1996) Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J Biol Chem 271:631-634.
Chung HS, Harris A, Evans DW, Kagemann L, Garzozi HJ, Martin B (1999) Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol 43 Suppl 1:S43-50.
Emre M, Orgül S, Gugleta K, Flammer J (2004) Ocular blood fow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol 88:662-666.
Flammer J, Gasser P, Prünte C, Yao K (1992) The probable involvement of factors other than intraocular pressure in the pathogenesis of glaucoma. In: Pharmacology of Glaucoma (Drance SM, Van Buskirk EM, Neufeld AH, eds), pp 273-283. Baltimore: Williams & Wilkins.
Grieshaber MC, Flammer J (2005) Blood fow in glaucoma. Curr Opin Ophthalmol 16:79-83.
Grozdanic SD, Betts DM, Sakaguchi DS, Allbaugh RA, Kwon YH, Kardon RH (2003) Laser-induced mouse model of chronic ocular hypertension. Invest Ophth Vis Sci 44:4337-4346.
Halpern DL, Grosskreutz CL (2002) Glaucomatous optic neuropathy: mechanisms of disease. Ophthalmol Clin North Am 15:61-68.
Hatt S, Wormald R, Burr J (2006) Screening for prevention of optic nerve damage due to chronic open angle glaucoma. Cochrane Database Syst Rev:CD006129.
Johnson GL, Nakamura K (2007) The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta 1773:1341-1348.
Li HY, Zhao JL, Zhang H (2008) Transfection of brain-derived neurotrophic factor gene by recombinant adeno-associated virus vector in retinal ganglion cells in vitro. Zhonghua Yan Ke Za Zhi 44:354-360.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408.
Mabuchi F, Aihara M, Mackey MR, Lindsey JD, Weinreb RN (2003) Optic nerve damage in experimental mouse ocular hypertension. Invest Ophth Vis Sci 44:4321-4330.
Pan J, Zhao YX, Wang ZQ, Jin L, Sun ZK, Chen SD (2007) Expression of FasL and its interaction with Fas are mediated by c-Jun N-terminal kinase (JNK) pathway in 6-OHDA-induced rat model of Parkinson disease. Neurosci Lett 428:82-87.
Sakurai T, Maeda S, Chang L, Karin M (2006) Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A 103:10544-10551.
Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF (2007) Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet 16:609-617.
Wang D, Fu Q, Zhou Y, Xu B, Shi Q, Igwe B, Matt L, Hell JW, Wisely EV, Oddo S, Xiang YK (2013) 2 adrenergic receptor, protein kinase A (PKA) and c-Jun N-terminal kinase (JNK) signaling pathways mediate tau pathology in Alzheimer disease models. J Biol Chem 288:10298-10307.
Wilhelm M, Xu Z, Kukekov NV, Gire S, Greene LA (2007) Proapoptotic Nix activates the JNK pathway by interacting with POSH and mediates death in a Parkinson disease model. J Biol Chem 282:1288-1295.
Yang R, Wilcox DM, Haasch DL, Jung PM, Nguyen PT, Voorbach MJ, Doktor S, Brodjian S, Bush EN, Lin E, Jacobson PB, Collins CA, Landschulz KT, Trevillyan JM, Rondinone CM, Surowy TK (2007) Liver-specifc knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J Biol Chem 282:22765-22774.
Copyedited by Dawes EA, Wysong S, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*
#These authors contributed equally to this work.
10.4103/1673-5374.153692
http://www.nrronline.org/
Accepted: 2015-01-19
杂志排行
中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters
