甜菊糖苷类物质的功能性研究进展
2015-01-03万会达夏咏梅
万会达,李 丹,夏咏梅*
(江南大学 食品科学与技术国家重点实验室,化学与材料工程学院,江苏 无锡 214122)
甜菊糖苷类物质的功能性研究进展
万会达,李 丹,夏咏梅*
(江南大学 食品科学与技术国家重点实验室,化学与材料工程学院,江苏 无锡 214122)
甜菊糖苷是一类从甜叶菊叶子中提取的高甜度、低热值的甜味剂,是一种非常理想的蔗糖替代品。毒理学实验表明,甜菊糖苷不具有致畸、致癌和致突变毒性。除了作为优良的甜味剂应用在食品、饮料中,甜菊糖苷还表现出许多功能活性,如降血压、降血糖、抗肿瘤、抑菌、抗腹泻、增溶等。由于甜菊糖苷的结构可调性,可以对其进行定向修饰,得到更多具有潜在应用价值的衍生物,这方面的研究对于甜菊糖苷的深入开发利用具有重要意义,本文将围绕近几年有关甜菊糖苷功能性的研究展开讨论。
甜菊糖苷;甜菊苷;功能性
甜叶菊为菊科宿根多年生草本植物,数百年前南美洲土著人就已经开始使用这种植物的叶子作为甜味剂。虽然已经发现有多达100多个甜叶菊品种,其中只有Stevia rebaudiana的叶子中含有大量甜菊糖苷[1-2]。甜菊糖苷是一类天然的高甜度低热值甜味剂,被誉为继甘蔗和甜菜之后的“第三糖源”。从2008年美国食品药品监督管理局批准甜菊糖苷中瑞鲍迪苷A(rebaudioside A,Reb A)为“一般认为安全(generally recognized as safe,GRAS)”物质,再到2011年欧盟委员会最终批准甜菊糖苷可以用作食品添加剂,甜菊糖苷已经被广泛地认可和使用,而我国已经成为世界上甜菊糖苷最大的生产国,约占全球80%份额[3],时隔16年,我国重新修订甜菊糖苷国家标准,并于2015年7月28日实施新版GB 8270—2014《食品添加剂 甜菊糖苷》。甜菊糖苷不会引起龋齿[4],毒理学实验表明其不具有致畸、致癌和致突变毒性[5]。与三氯蔗糖、阿巴斯甜等人工合成的高倍甜味剂相比,甜菊糖苷是一种性能优良的天然甜味剂,但其不仅仅是甜味剂,还表现出多种功能活性和药理活性,亦可作为合成其他功能性化合物的前体物质。本文将分别讨论甜菊糖苷类物质的药理功能和理化功能。
1 甜菊糖苷的种类
甜菊糖苷是一类至少由9种甜味成分组成的四环二萜类化合物,包括甜菊苷(stevioside,St)、Reb A、Reb B、Reb C、Reb E、Reb F、甜茶苷、杜克苷、甜菊双糖苷等。它们具有相同的苷元——甜菊醇(steviol)(图1),仅C19和C13位上连接不同数量的葡萄糖基、鼠李糖基或木糖基,从而形成味质、理化性能各异的甜菊糖苷(表1)[6]。其中含量最高的为甜菊苷和Reb A,占甜菊糖苷的80%以上,是甜菊糖苷味质的主成分。除了上述9种甜菊糖苷,从甜叶菊叶子中提取的甜菊糖苷粗品中还有多种含量稀少的甜菊糖苷(表1),而且近几年已有学者开始对这些稀有甜菊糖苷开展研究[7-8]。此外,为了去除甜菊苷不愉快的后苦涩味和促进其功能性质的开发,一些酶催化转糖基甜菊糖苷也被相继合成,但其安全性还需要进一步评估[9-11]。

图1 甜菊醇苷元[6]Fig.1 The aglycone of steviol glycosides[6]
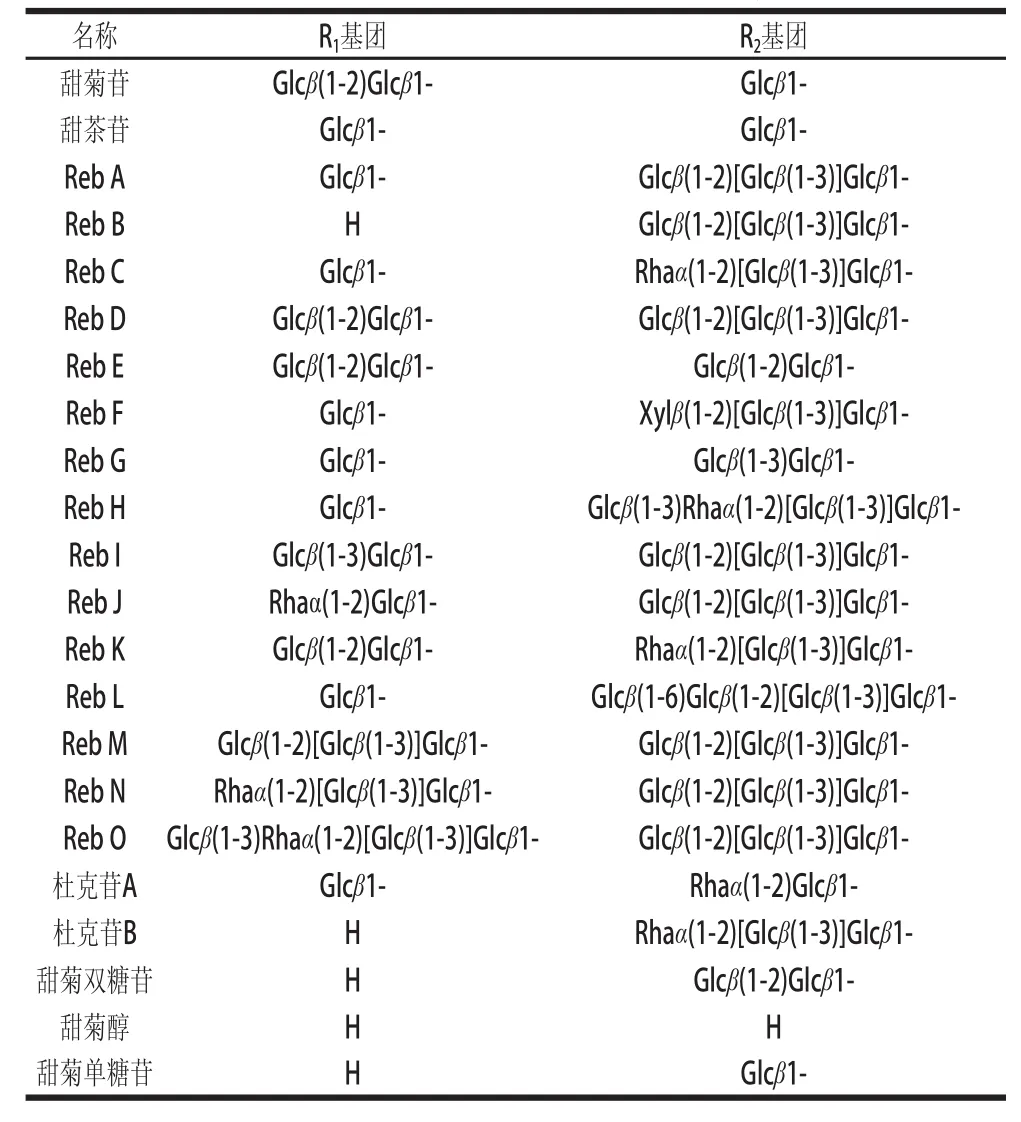
表1 甜菊糖苷类物质的分子结构Table1 Chemical structures of steviol glycosides
除了表1列出的多种甜菊糖苷,新的甜菊糖苷仍然不断被分离、纯化和鉴定出来,如Ibrahim等[12]除了分离得到Reb E、Reb M、Reb N、Reb O和甜菊苷以外,还发现了两种新的甜菊糖苷(图2)。

图2 两种新型甜菊糖苷的结构式[12]Fig.2 Chemical structures of two novel steviol glycosides[12]
2 药理功能
2.1 降血压降血糖
人们最先了解甜菊糖苷的两种药理功能是降血压和血糖。早在数 百年前南美洲土著人使用甜菊糖苷作为甜味剂之初,就意识到了甜菊糖苷的这两种神奇功效。研究表明其降血压原理可归结于三方面的作用:降低细胞外Ca2+的内流、减少Na+再吸收和刺激血管舒张剂前列腺素的生成[13]。临床实验表明,服用甜菊苷1a后轻中度高血压患者的收缩压和舒张压明显下降且未见副作用(收缩压从(166.0±9.4)mmHg降至(152.6±6.8)mmHg、舒张压从(104.7±5.2)mmHg降至(90.3±3.6)mmHg(P<0.05))[14];服用甜菊苷2a的高血压患者血压显著降低,身体质量指数及血液生理生化指标等均未产生重大变化(收缩压从(150.0±7.3)mmHg降至(140.0±6.8)mmHg、舒张压从(95.0±4.2)mmHg降至(89.0±3.2)mmHg(P<0.05))[15]。同时甜菊糖苷这种降血压作用对于血压正常人群并无影响,所以甜菊糖苷适合于各类人群食用[16-17]。
前文已叙述,甜菊糖苷具有高甜度、低热值的特点,甜菊糖苷的甜度是蔗糖甜度的200~350倍,而热量仅为蔗糖的1/300。这能满足健康人群、爱美人士、肥胖患者、尤其是糖尿病人对甜味的需求,同时还具有辅助治疗糖尿病的作用。体内、体外实验均表明甜菊苷及其相关化合物通过以下几个途径调节血糖平衡:刺激胰岛素的分泌和周围组织对胰岛素的敏感性,促进血液中葡萄糖的代谢[18];抑制肠道中葡萄糖的吸收和肝脏中葡萄糖的生成,通过抑制葡萄糖合成关键酶来减少血糖输入(图3)[19]。连续给糖尿病小鼠喂食甜叶菊提取物2.5 g/(kg·d),1周后小鼠血糖水平明显降低(给药前为(21.5±3.0)mmol/L,给药后为(6.5±3.3)mmol/L)[20]。Toskulkao等[21]进行体外实验(使用空肠环组织和外翻囊)研究甜菊苷和甜菊醇对葡萄糖吸收的影响,甜菊苷在5 mmol/L时没有对葡萄糖的吸收出现抑制作用,但是1 mmol/L的甜菊醇却抑制了约40%葡萄糖的吸收。甜菊糖苷可刺激胰岛素分泌和增强胰岛素敏感性,甜菊醇可通过改善血脂含量调节血糖平衡[22-23]。近期研究发现,甜菊糖苷降血糖的主要机理是其能够激活μ-阿片受体和促进肝糖原的形成[24]。此外,甜菊糖苷只在高血糖情况下才发挥降血糖的功效,因而对健康人而言,食用甜菊糖苷是安全的[23]。

图3 甜菊苷及其类似物可能的降血糖原理[19]Fig.3 Possible anti-hyperglycemic actions of stevioside and its analogs[19]
2.2抗炎抗肿瘤
甜菊苷及甜菊醇对结肠癌细胞Caco-2、乳腺炎细胞(S. aureus-infected mouse mammary gland)具有抗炎和免疫调节活性,它们经由IκBα/NF-κB途径影响细胞因子表达,从而削弱脂多糖(lipopolysaccharides,LPS)诱导的促炎因子的生成[25-27]。通过加速Bax蛋白和细胞色素c的表达和释放到细胞质中,甜菊苷能够加速去血清所诱导的PC12细胞凋亡[28]。佛波酯(12-O-tetrade canoylphorbol-13-acetate,TPA)能够引起局部炎症和皮肤癌,而甜菊苷和甜菊醇能够有效抑制TPA的生成[29]。异甜菊醇(甜菊醇酸性重排产物)同样对TPA诱发的炎症具有抑制作用;此外异甜菊醇能够阻碍3种人体癌细胞的生长,通过抑制DNA聚合酶和DNA拓扑异构酶Ⅱ来治疗炎症和癌症[30]。Akihisa等[31]通过微生物发酵法得到了5种异甜菊醇衍生物,然后考察了包括甜菊苷和异甜菊醇在内的其中糖苷对肿瘤的抑制活性,选取的模型为Raji细胞中由TPA诱导的EPSTEIN-BARR病毒早期抗原(EBV-EA)。
2.3 抗腹泻
Tomita等[32]首先报道甜叶菊提取物能够杀灭包括肠出血型大肠杆菌(enterohemorrhagicEsherichia coli)在内的食物污染菌,这种菌能够导致严重的出血性腹泻,因此甜菊糖苷具有治疗腹泻的潜质。轮状病毒(rotavirus,RV)是一种双链核糖核酸病毒,它是婴幼儿腹泻的单一主因,除了对人类健康的影响之外,轮状病毒也会感染动物,是家畜的病原体之一。体内(猪仔)和体外(porcine rotavirus G5[P7] strain)实验表明,甜菊苷和苦参提取物复配使用具有抑制轮状病毒的功效,但单一使用其中任意一种会使抑制效果降低[33]。Wang等[34]研究发现给断奶仔猪喂食甜菊苷和Reb A可以明显降低仔猪的腹泻发病率,最优平均日摄取量分别为250 mg/kg和191~213 mg/kg。
2.4 治疗失忆
Sharma等[35]给大鼠服用东莨菪碱(0.5 mg/kg)使之产生记忆障碍,然后进行经典的Morris水迷宫测试用于评估老鼠的学习和记忆能力。通过测定大鼠脑部乙酰胆碱酯酶(acetylcholinestrase enzyme,AChE)活性来评估中枢胆碱能的活性,同时对大鼠脑部硫代巴比妥酸反应性物质(thiobarbituric acid-reactive species,TBARS)及还原型谷胱甘肽(reduced glutathione,GSH)的水平进行测定,以评估大鼠发生氧化应激的程度。结果表明,东莨菪碱诱发大鼠学习和记忆发生显著障碍,通过Morris水迷宫的能力显著下降。东莨菪碱也显著增强了大鼠脑部AChE活性和氧化应激水平(增加TBARS含量和减少GSH含量)。给予大鼠口服甜菊苷(250 mg/kg)可以降低东莨菪碱诱发的大鼠脑部AChE活性和脑氧化应激水平升高,显著逆转东莨菪碱诱发的学习和记忆障碍。
3 理化功能
3.1 增溶剂
Reb A和甜茶苷是优良的增溶剂:质量分数大于12.5%的Reb A可以提高甜菊苷的稳定性和溶解性[36];甜茶苷可以用于增加疏水性抗癌物质如紫杉醇、姜黄素、依托泊苷、辣椒素的水溶性,同时保持甚至增加化合物的药理活性[37-41]。为增强白藜芦醇(resveratrol,RES)这种天然抗氧化剂在食品系统中的的功效,利用甜菊苷(St)的增溶性质提高RES的溶解性,并考察St-RES复合物对大豆分离蛋白(soybean protein isolate,SPI)乳状液稳定性的影响,评价了其物理性质及抗氧化性能(图4)。当甜菊苷浓度达到其临界胶束浓度时,RES的水溶性增加,推测RES增溶在St自组装胶束中。St胶束与SPI竞争吸附在油-水界面上,由此降低了SPI乳状液颗粒尺寸,并提高了SPI乳状液的物理稳定性。当形成St-RES复合物后,减少了脂质过氧化物和挥发性醛的生成,SPI乳状液表现出抗氧化稳定性增强。这种增强效应主要归因于甜菊苷对RES的增溶作用[42]。Wan Zhili等[43]进一步研究了甜菊苷与SPI的协同作用,来解释乳状液稳定性的成因。
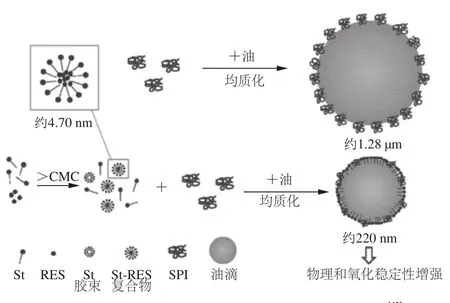
图4 St-RES复合物对大豆分离蛋白乳状液稳定性的影响[42]Fig.4 Schematic illustration of the formation of O/W emulsion stabilized by SPI and St-RES[42]
3.2 抑菌剂
目前关于甜菊糖苷抑菌性的报道,主要集中于甜叶菊的提取物方面。甜叶菊的乙醇和丙酮提取物表现出最佳的抗食源性致病菌性能,其中包括粘质沙雷氏菌(Serratia marcescens)、肺炎克雷伯菌(Klebsiella pneumonia)、蜡状芽孢杆菌(Bacillus cereus)、铜绿假单胞菌(Pseudomonas aeruginosa)、枯草芽孢杆菌(B. subtilis)、脱氮产碱杆菌(Alcaligenes denitrificans)、鼠伤寒沙门氏菌(Salmonella typhimurium)等,甜菊苷能够抑制枯草芽孢杆菌(B. subtilis)、肺炎克雷伯菌(K. pneumonia)和鼠伤寒沙门氏菌(S. typhimurium)等细菌的生长,这有利于延长食品的货架期[44]。Jayaraman等[45]也指出甜叶菊丙酮提取物的抗菌活性较其他提取物更高。甜叶菊丙酮提取物对革兰氏阳性菌的抗菌活性强于对革兰氏阴性菌的抗菌活性。可能是由于甜叶菊提取物在这些有机溶剂中溶解度更大,丙酮和乙醇提取物有更高抗菌活性。甜叶菊乙酸乙酯提取物对毛癣菌和白色念珠菌表现出高抑制活性,这可能是由于活性物质在溶剂中可长时间保持良好的稳定性。也有一些结论相反的报道称,甜叶菊氯仿提取物对芽孢杆菌、伤寒杆菌、大肠杆菌均没有明显抑制活性,对葡萄球菌也不存在抑制活性,甜叶菊水提取物对所测试的微生物均无抗菌效果[46-49]。但由于溶剂提取液是一个复杂的混合物,其中包括甜菊糖苷类物质、黄酮、尼克酸、核黄素、生物碱、单宁等,究竟是哪一种或几种成分起主导抗菌效果还需进一步逐一研究。
甜菊苷还可以作为一种保护剂,使山梨酸钾这种高安全性、普遍应用的防腐剂在含水酸性体系中免遭破坏从而达到提高抑菌效果的目的,并且在一定条件下,甜菊糖苷类物质具有抑制非酶促褐变反应的功效[50]。
3.3 前体物质
甜菊糖苷类物质具有贝壳杉烯的苷元结构,通过简单的水解即可得到甜菊醇和异甜菊醇,以此为先导化合物,进一步衍生化可以得到具有保护心脑细胞、抑菌、抗炎、抗肿瘤等生物活性的物质[9]。因而借助生物催化和化学催化手段,对甜菊醇和异甜菊醇进行修饰与改造,已成为甜菊糖苷类化合物应用研究方向之一。目前,主要方法是在甜菊糖苷骨架结构的C13位羟基、C19位羧基连接已知活性分子,在C15、C16和C17位引入氮或氧杂环、含活泼氢的羟基或氨基、exo-亚甲基环戊酮及α-亚甲基内酯等活性片段[51]。异甜菊醇衍生物(NC-8,图5a)具有抵抗乙型肝炎病毒(hepatitis B virus,HBV)的活性,似乎是通过干扰复制和HBV的基因表达以及抑制宿主TLR2/NF-κB信号通路来介导的[52]。从甜菊苷和Reb A出发,水解制备得到12种异甜菊醇的甲基甲氧醚衍生物。通过四甲基偶氮唑蓝实验发现其中两种物质(图5b、5c)对人肺癌细胞H1299具有较强的抑制活性,IC50值分别为14、21μmol/L。同时这两种物质对人正常肺细胞NL-20的毒性较小,同时能诱导人肺癌细胞H1299更快凋亡[53]。

图5 3 种异甜菊醇衍生物的分子结构[53]Fig.5 Chemical structures of three isosteviol derivatives[53]
4 结 语
本文综述了甜菊糖苷类化合物除作为甜味剂以外的功能性质的研究进展,包括药理活性、增溶剂、作为前体物质等。但仍然有些问题制约着甜菊糖苷的发展和应用,如甜菊糖苷在人体内代谢过程及机理的研究还处于数据积累阶段,而这一问题实际上关系到甜菊糖苷类物质的安全性;各种研究所用的甜菊糖苷纯度各异,导致所得到的结论有一定差异;由于甜菊苷类物质经由肠道细菌代谢之后,主要产生甜菊醇及甜菊醇葡萄糖醛酸,而关于这方面的研究还比较缺乏。我国是世界上最大的甜菊糖苷生产国,但4/5的甜菊糖苷用于出口,国内消费者对甜菊糖苷知之甚少,如何扩大甜菊糖苷内需、提高国内消费者的认知度,也是国内学者需要考虑的问题之一。
参考文献:
[1] GONZ☒LEZ C, TAPIA M, P REZ E, et al. Main properties of steviol glycosides and their potential in the food industry∶ a review[J]. Fruits, 2014, 69(2)∶ 127-141.
[2] WÖLWER-RIECK U. The leaves of Stevia rebaudiana (bertoni), their constituents and the analyses thereof∶ a review[J]. Journal of Agricultural and Food Chemistry, 2012, 60(4)∶ 886-895.
[3] GUPTA E, PURWAR S, SUNDARAM S, et al. Nutritional and therapeutic values of Stevia rebaudiana∶ a review[J]. Journal of Medicinal Plants Research, 2013, 7(46)∶ 3343-3353.
[4] BRAMBILLA E, CAGETTI M G, IONESCU A, et al. An in vitro and in vivo comparison of the effect of Stevia rebaudiana extracts on different caries-related variables∶ a randomized controlled trial pilot study[J]. Caries Research, 2013, 48(1)∶ 19-23.
[5] URBAN J D, CARAKOSTAS M C, BRUSICK D J. Steviol glycoside safety∶ is the genotoxicity database sufficient?[J]. Food and Chemical Toxicology, 2013, 51∶ 386-390.
[6] XU Zhongwei, LI Yuqiang, WANG Yonghua, et al. Production of β-fructofuranosidase by Arthrobacter sp. and its application in the modification of stevioside and rebaudioside A[J]. Food Technology and Biotechnology, 2009, 47(2)∶ 137-143.
[7] NIKIFOROV A I, RIHNER M O, EAPEN A K, et al. Metabolism and toxicity studies supporting the safety of rebaudioside D[J]. International Journal of Toxicology, 2013, 32(4)∶ 261-273.
[8] PURKAYASTHA S, PUGH G, Jr, LYNCH B, et al. In vitro metabolism of rebaudioside B, D, and M under anaerobic conditions∶comparison with rebaudioside A[J]. Regulatory Toxicology and Pharmacology, 2014, 68(2)∶ 259-268.
[9] MOONS N, de BORGGRAEVE W, DEHAEN W. Stevioside and steviol as starting materials in organic synthesis[J]. Current Organic Chemistry, 2012, 16(17)∶ 1986-1995.
[10] LU Tong, XIA Yongmei. Transglycosylation specificity of glycosyl donors in transglycosylation of stevioside catalysed by cyclodextrin glucanotransferase[J]. Food Chemistry, 2014, 159∶ 151-156.
[11] MUSA A, MIAO Ming, ZHANG Tao, et al. Biotransformation of stevioside by Leuconostoc citreum SK24.002 alternansucrase acceptor reaction[J]. Food Chemistry, 2014, 146∶ 23-29.
[12] IBRAHIM M A, RODENBURG D L, ALVES K, et al. Minor diterpene glycosides from the leaves of Stevia rebaudiana[J]. Journal of Natural Products, 2014, 77(5)∶ 1231-1235.
[13] TIRAPELLI C R, AMBROSIO S R, de OLIVEIRA A M, et al. Hypotensive action of naturally occurring diterpenes∶ a therapeutic promise for the treatment of hypertension[J]. Fitoterapia, 2010, 81(7)∶690-702.
[14] CHAN P, TOMLINSON B, CHEN Y J, et al. A double-blind placebocontrolled study of the effectiveness and tolerability of oral stevioside in human hypertension[J]. British Journal of Clinical Pharmacology, 2000, 50(3)∶ 215-220.
[15] HSIEH M H, CHAN P, SUE Y M, et al. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension∶ a two-year, randomized, placebo-controlled study[J]. Clinical Therapeutics, 2003, 25(11)∶ 2797-2808.
[16] BARRIOCANAL L A, PALACIOS M, BENITEZ G, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in type 1 and type 2 diabetics[J]. Regulatory Toxicology and Pharmacology, 2008, 51(1)∶ 37-41.
[17] MAKI K C, CURRY L L, CARAKOSTAS M C, et al. The hemodynamic effects of rebaudioside A in healthy adults with normal and low-normal blood pressure[J]. Food and Chemical Toxicology, 2008, 46(7)∶ 40-46.
[18] JEPPESEN P B, GREGERSEN S, POULSEN C R, et al. Stevioside acts directly on pancreatic β cells to secrete insulin∶ actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitivie K+-channel activity[J]. Metabolism-Clinical and Experimental, 2000, 49(2)∶ 208-214.
[19] CHATSUDTHIPONG V, MUANPRASAT C. Stevioside and related compounds∶ therapeutic benefits beyond sweetness[J]. Pharmacology & Therapeutics, 2009, 121(1)∶ 41-54.
[20] 曹芳, 冯文静, 陈明, 等. 甜菊糖苷降血糖作用研究[J]. 中国药物与临床, 2009, 9(2)∶ 127.
[21] T O S K U L K A O C, S U T H E E R A W A T T A N A N O N M, PIYACHATURAWAT P. Inhibitory effect of steviol, a metabolite of stevioside, on glucose-absorption in everted hamster intestine in vitro[J]. Toxicology Letters, 1995, 80(1/3)∶ 153-159.
[22] NORDENTOFT I, JEPPESEN P B, HONG J, et al. Isosteviol increases insulin sensitivity and changes gene expression of key insulin regulatory genes and transcription factors in islets of the diabetic KKAy mouse[J]. Diabetes Obesity & Metabolism, 2008, 10(10)∶939-949.
[23] LAILERD N, SAENGSIRISUWAN V, SLONIGER J A, et al. Effects of stevioside on glucose transport activity in insulin-sensitive and insulin-resistant rat skeletal muscle[J]. Metabolism-Clinical and Experimental, 2004, 53(1)∶ 101-107.
[24] YANG P S, LEE J J, TSAO C W, et al. Stimulatory effect of stevioside on peripheral mu opioid receptors in animals[J]. Neuroscience Letters, 2009, 454(1)∶ 72-75.
[25] BOONKAEWWAN C, BURODOM A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells[J]. Journal of the Science of Food and Agriculture, 2013, 93(15)∶ 3820-3825.
[26] YINGKUN N, ZHENYU W, JING L, et al. Stevioside protects LPS-induced acute lung injury in mice[J]. Inflammation, 2013, 36(1)∶ 242-250.
[27] WANG Tiancheng, GUO Mengyao, SONG Xiaojing, et al. Stevioside plays an anti-inflammatory role by regulating the NF-κB and MAPK pathways in S. aureus-infected mouse mammary glands[J]. Inflammation, 2014, 37(5)∶ 1837-1846.
[28] TAKAHASHI K, SUN Y, YANAGIUCHI I, et al. Stevioside enhances apoptosis induced by serum deprivation in PC12 cells[J]. Toxicology Mechanisms and Methods, 2012, 22(4)∶ 243-249.
[29] YASUKAWA K, KITANAKA S, SEO S. Inhibitory effect of stevioside on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin[J]. Biological & Pharmaceutical Bulletin, 2002, 25(11)∶ 1488-1490.
[30] MIZUSHINA Y, AKIHISA T, UKIYA M, et al. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors[J]. Life Sciences, 2005, 77(17)∶ 2127-2140.
[31] AKIHISA T, HAMASAKI Y, TOKUDA H, et al. Microbial transformation of isosteviol and inhibitory effects on epstein∶ barr virus activation of the transformation products[J]. Journal of Natural Products, 2004, 67(3)∶ 407-410.
[32] TOMITA T, SATO N, ARAI T, et al. Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157∶H7 and other foodborne pathogenic bacteria[J]. Microbiology and Immunology, 1997, 41(12)∶1005-1009.
[33] ALFAJARO M M, RHO M C, KIM H J, et al. Anti-rotavirus effects by combination therapy of stevioside and Sophora fl avescens extract[J]. Research in Veterinary Science, 2014, 96(3)∶ 567-575.
[34] WANG L S, SHI Z, SHI B M, et al. Effects of dietary stevioside/ rebaudioside A on the growth performance and diarrhea incidence of weaned piglets[J]. Animal Feed Science and Technology, 2014, 187∶104-109.
[35] SHARMA D, PURI M, TIWARY A K, et al. Antiamnesic effect of stevioside in scopolamine-treated rats[J]. Indian Journal of Pharmacology, 2010, 42(3)∶ 164-167.
[36] GOTO A, CLEMENTE E. Rebaudioside A influence on the flavor and solubility of stevioside[J]. Food Science and Technology, 1998, 18(1)∶3-6.
[37] JEANSONNE D P, KOH G Y, ZHANG F, et al. Paclitaxel-induced apoptosis is blocked by camptothecin in human breast and pancreatic cancer cells[J]. Oncology Reports, 2011, 25(5)∶ 1473-1480.
[38] ZHANG F, KOH G Y, JEANSONNE D P, et al. A novel solubilityenhanced curcumin formulation showing stability and maintenance of anticancer activity[J]. Journal of Pharmaceutical Sciences, 2011, 100(7)∶ 2778-2789.
[39] ZHANG F, KOH G Y, HOLLINGSWORTH J, et al. Reformulation of etoposide with solubility-enhancing rubusoside[J]. International Journal of Pharmaceutics, 2012, 434(1/2)∶ 453-459.
[40] LIU Dongwu, CHEN Zhiwei. The effect of curcumin on breast cancer cells[J]. Journal of Breast Cancer, 2013, 16(2)∶ 133-137.
[41] NGUYEN T T H, JUNG S J, KANG H K, et al. Production of rubusoside from stevioside by using a thermostable lactase from Thermus thermophilus and solubility enhancement of liquiritin and teniposide[J]. Enzyme and Microbial Technology, 2014, 64/65∶ 38-43.
[42] WAN Zhili, WANG Jinmei, WANG Liying, et al. Enhanced physical and oxidative stabilities of soy protein-based emulsions by incorporation of a water-soluble stevioside∶ resveratrol complex[J]. Journal of Agricultural and Food Chemistry, 2013, 61(18)∶ 4433-4440.
[43] WAN Zhili, WANG Liying, WANG Jinmei, et al. Synergistic interfacial properties of soy protein∶ stevioside mixtures∶ relationship to emulsion stability[J]. Food Hydrocolloids, 2014, 39∶ 127-135.
[44] PURI M, SHARMA D. Antibacterial activity of stevioside towards food-borne pathogenic bacteria[J]. Engineering in Life Sciences, 2011, 11(3)∶ 326-329.
[45] JAYARAMAN S, MANOHARAN M S, ILLANCHEZIAN S. Invitro antimicrobial and antitumor activities of Stevia rebaudiana (Asteraceae) leaf extracts[J]. Tropical Journal of Pharmaceutical Research, 2008, 7(4)∶ 1143-1149.
[46] ALONSO PAZ E, CERDEIRAS M, FERNANDEZ J, et al. Screening of uruguayan medicinal plants for antimicrobial activity[J]. Journal of Ethnopharmacology, 1995, 45(1)∶ 67-70.
[47] VLIETINCK A J, van HOOF L, TOTT J, et al. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties[J]. Journal of Ethnopharmacology, 1995, 46(1)∶ 31-47.
[48] LIN J, OPOKU A R, GEHEEB-KELLER M, et al. Preliminary screening of some traditional zulu medicinal plants for antiinflammatory and anti-microbial activities[J]. Journal of Ethnopharmacology, 1999, 68(1/2/3)∶ 267-274.
[49] TADHANI M B, SUBHASH R. In vitro antimicrobial activity of Stevia rebaudiana Bertoni leaves[J]. Tropical Journal of Pharmaceutical Research, 2007, 5(1)∶ 557-560.
[50] HRACEK V M, GLIEMMO M F, CAMPOS C A. Effect of steviosides and system composition on stability and antimicrobial action of sorbates in acidified model aqueous systems[J]. Food Research International, 2010, 43(8)∶ 2171-2175.
[51] 王婷婷, 陈莉. 异甜菊醇衍生物的生物活性及构效关系研究进展[J].药学进展, 2013(4)∶ 152-160.
[52] HUANG T J, CHOU B H, LIN C W, et al. Synthesis and antiviral effects of isosteviol-derived analogues against the hepatitis B virus[J]. Phytochemistry, 2014, 99∶ 107-114.
[53] MALKI A, LAHA R, BERGMEIER S C. Synthesis and cytotoxic activity of MOM-ether analogs of isosteviol[J]. Bioorganic & Medicinal Chemistry Letters, 2014, 24(4)∶ 1184-1187.
Progress in Functions of Steviol Glycosides
WAN Huida, LI Dan, XIA Yongmei*
(School of Chemical and Material Engineering, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China)
Steviol glycosides are high-potency and low-calorie sweeteners extracted from the leaves ofStevia rebaudiana. They are considered as the most ideal altern ative to sucrose. In toxicological tests, steviol glycosides do not exert serious acute toxic, genotoxic, or carcinogenic effects. In addition to being an excellent sweetener, steviol glycosides possess a lot of functional attributes such as anti-hypertensive, anti-hyperglycaemic, anti-tumour, antibacterial, anti-inflammatory, etc. Owing to their highly adjustable structu res, steviol glycosides have been used as precursors for the synthesis of the derivatives which have many potential applications. Recent progress in understanding the func tions of steviol glycosides is discussed in this review to guide future exploitation of these bioactive compounds.
steviol glycosides; stevioside; functionality
Q946.3
1002-6630(2015)17-0264-06
10.7506/spkx1002-6630-201517049
2014-11-05
国家自然科学基金面上项目(31171752;31371837);江南大学青年自主科研课题(1042050205141410)
万会达(1984—),男,副教授,博士,研究方向为生物催化。E-mail:huidawan@jiangnan.edu.cn
*通信作者:夏咏梅(1965—),女,教授,博士,研究方向为生物催化,精细化学品合成。E-mail:ymxia@jiangnan.edu.cn
