气相色谱-质谱/选择离子监测法用于苯乙烯不对称环氧化反应的筛选
2014-12-24韦思平
牟 瑶, 韦思平, 徐 敏, 陈 平, 王 钦* , 杜 曦
(1. 泸州医学院药学院,四川 泸州646000;2. 泸州医学院基础医学院,四川 泸州646000;3. 成都理工大学材料与化学化工学院,四川 成都610059)
Widespread concern has arisen on chiral styrene oxide at home and abroad for its use as an important intermediate in the synthesis of pharmaceuticals and fine chemicals,and most of the studies have focused on the synthesis and applications of styrene oxide[1 -5]. At present,chiral styrene oxide is synthesized by asymmetric epoxidation of styrene,in which (1S,2S)-(+)-[1,2-cyclohexanediamino-N,N′-bis (3,5-di-tert-butylsalicylidene)]manganese (Ⅲ)chloride (Salen-Mn (Ⅲ))plays an important role and has been proven to be the most effective catalyst for nonfunctionalized olefin epoxidation [6,7]. Compared with the studies on the synthesis of chiral styrene oxide,the researches on the analytical method of chiral styrene oxide are relatively backward. Moreover,colorimetric method,high performance liquid chromatography (HPLC)and gas chromatography (GC)serve as the three main analytical methods of chiral styrene oxide. Specifically speaking,Tee et al. [8]and Alcalde et al.[9]screened styrene epoxidation using p-nitrothiophenolate and 4-(p-nitrobenzyl)pyridine as the chromogenic agents of styrene oxide respectively,with the result that the enantiomeric excess (ee)was not determined. Rabe et al. [10]used a chiral HPLC to analyze the enantioselectivity of styrene asymmetric epoxidation in their research of the peroxidase activity of CYP119,but the sensitivity was not enough in view of the trace components. Compared with HPLC,the analytical methods of GC and GC-MS enjoy the following advantages:high speed,high sensitivity and high separation efficiency. The method of GC-MS has been widely used in various fields for its good performance in separation and qualitative-quantitative aspects[11 -15]. Till now,there has been only one study simply mentioned the application of GC-MS on determining of styrene epoxidation[16]. The method of GC-MS on determination of styrene and styrene oxide was described in detail only by Sepai et al. [17]and Tornero-Velez et al.[18],who employed the direct and derivatization methods to determine styrene and styrene oxide,but not to analyze the styrene oxide enantiomers.Herein,we explored the use of GC-MS/SIM with CP-Chirasl-Dex CB column to analyze the asymmetric epoxidation mixture of styrene and screen the asymmetric epoxidation of styrene.
1 Experimental
1.1 Chemicals,reagents and materials
Salen-Mn (Ⅲ),styrene,(R)/(S)-styrene oxide with a purity of 99% were supplied by J&K Scientific Ltd. Dichloromethane,acetone,sodium hypochlorite (the chlorine content ≥5%)were purchased from Kelong Chemical Co.,Ltd.Ethylbenzene was obtained from Aladdin. Methanol was supplied by Xilong Chemical Co.,Ltd.
1.2 Solution preparation
The stock standard solution of styrene was prepared in acetone at a mass concentration of 3.11× 103mg/L. The stock standard solutions of(R)/(S)-styrene oxides were also prepared in acetone at a mass concentration of 3.27 ×103mg/L and 3.22 ×103mg/L,respectively. The solution of 1.1 ×104mg/L ethylbenzene prepared in acetone was used as the internal standard.
1.3 Gas chromatography-mass spectrometry
All analyses were performed by using the Thermo Fisher Scientific GC-MS instrument. The chromatographic conditions were as follows:column,CP-Chirasl-Dex CB (30 m×0.25 mm×0.25 μm,Varian,USA);carrier gas,helium at 1.0 mL/min;split ratio,50∶1;injection port temperature,200 ℃;column temperature,initially being set at 80 ℃for 1 min and programmed to rise at 2℃/min to 110 ℃,then hold for 1 min at 110 ℃;ion source temperature,220 ℃;ion mode,electron-impact ionization at 70 eV;full scan mode,m/z 50 - 350;SIM,target ions (m/z 91.00,118.96)for (R)/(S)-styrene oxide,target ions(m/z 78.01,104.00)for styrene,and target ions(m/z 91.01,106.02)for ethylbenzene (internal standard). The total run time for GC was within 17 min.
1.4 Preparation and analysis of mixed standard solution
Reaction scheme for the asymmetric epoxidation of styrene is shown in Fig.1. To simulate styrene epoxidation mixture,three stock standard solutions were mixed and diluted by acetone into a series of concentration gradient solutions,which were mixed standard solutions. After the addition of internal standard solution (ethylbenzene), these mixtures were analyzed by GC-MS.
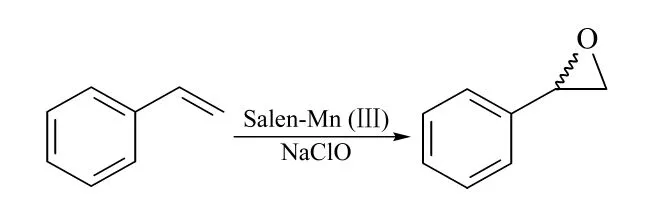
Fig.1 Reaction scheme for the asymmetric epoxidation of styrene
1.5 Epoxidation procedure of styrene
The solution with approximately 0.55 mol/L NaClO and 0.05 mol/L Na2HPO4was prepared,and the pH of the solution was adjusted by the addition of 1 mol/L NaOH solution. The mixed solution was added to a 25 mL round-bottom flask equipped with Salen-Mn (Ⅲ)and styrene in 0.5 mL CH2Cl2. The two-phase mixture was stirred and the reaction progress was monitored by thinlayer chromatography (TLC). After the completion of the reaction,CH2Cl2was added to the mixture and the brown organic phase was separated,then the organic phase was washed twice with saturated NaCl solution and dried with anhydrous Na2SO4. After solvent removal,the residue was dissolved in a certain amount of acetone and determined by GC-MS after filtrating through a 0.45 μm pore membrane.
1.6 Orthogonal experiments
Based on single factor experiment,the following main factors including the amount of the oxidant (NaClO),pH value,reaction time,reaction temperature,solvent and the amount of styrene,were selected for orthogonal experimental design.All samples prepared by orthogonal experiments of styrene epoxidation were analyzed by GC-MS and the optimal reaction conditions was determined according to the yield and ee values of samples (Table 1).

Table 1 Factors and levels of orthogonal experiments for styrene epoxidation reaction
2 Results and discussion
2.1 GC-MS analysis
The total ion chromatograms of the mixed standard solution and the mixed styrene epoxidation solution are shown in Fig.2 and Fig.3. The mass spectra of ethylbenzene,styrene,styrene oxide in the mixture are shown in Fig.4. The results indicate that each component of the mixture could be baseline separated and has strong characteristic ions,m/z 91.01,106.02 for ethylbenzene (internal standard), 77.02, 78.01,103.01,104.00 for styrene,89.02,91.00,92.06,118.96,119.98 for styrene oxide.

Fig.2 Total ion chromatogram of mixed standard solution

Fig.3 Total ion chromatogram of mixed solution of styrene epoxidation
2. 2 Linearity,precision and LOQ of the method
Analytical data of the mixed standard solutions at five mass concentrations were used to construct the standard curves,and good linear relationships were obtained for styrene and(R)/(S)-styrene oxide (Table 2). Three groups of mixed standard solutions with different mass concentrations were respectively added into three replicates of a certain sample (No.11 in Table 3),and then analyzed by GC-MS. The recoveries of the three substances ranged from 98.2% to 108.2% with RSDs of 1.2% -5.2% (n =5). The LOQs (S/N =10)of styrene and (R)/(S)-styrene oxide were 1.3,1.1 and 0.7 mg/L,respectively. These results demonstrate that the (GC-MS/SIM)method developed in this study is reliable in the determination of the asymmetric epoxidation mixture of styrene.

Fig.4 Mass spectra of ethylbenzene,styrene,styrene oxide in the mixture

Table 2 Linear equations,correlation coefficients (r),LOQs,recoveries and RSDs of styrene and (R)/(S)-styrene oxide
2.3 Application in screening for asymmetric epoxidation of styrene
The developed method was applied to screening for asymmetric epoxidation of styrene to be evaluated. From Table 3,it can be observed that there was minor difference between various R′ values,indicating ee not suitable for the core indicator.Therefore,the yield should be taken as the core indicator for screening various factors which affect the reaction. Comparing results of R values indicate that many factors would affect asymmetric epoxidation of styrene. The orderance in descending order was the solvent,the amount of NaClO,the amount of styrene,pH,reaction time and reaction temperature. Taking together with the results of each level,it can easily be concluded that the optimum reaction conditions were E2A3F2B3C2D1,which were 0.01 mmol Salen-Mn(Ⅲ),0.5 mmol styrene,5 mL CH2Cl2-MeOH (1∶2,v/v),and 3 mmol NaClO (pH 11.3).

Table 3 Results of orthogonal experiments
3 Conclusions
GC-MS/SIM method is valid for separating styrene and (R)/(S)-styrene oxides,and it can also be used to screen the enantioselectivity synthesis of styrene oxide. The advantages of the experimental method suggested in this thesis can be briefly concluded as the following several points:excellent precision,good linearity,high recovery and low LOQ. Based on this method,the asymmetry epoxidation of styrene has been screened and some factors seriously affect the reaction have been optimized by orthogonal experiments.The screening method is suitable to analyze the asymmetry epoxidation of styrene and its homologs.
AcknowledgmentWe sincerely express our heartfelt gratitude to Professor CHEN Run (School of Public Health,Luzhou Medical College)for her consistent help.
[1] Harrold N D,Li Y,Chisholm M H. Macromolecules,2013,46:692
[2] Shen S J,Li L,Ding X X,et al. Chem Res Toxicol,2014,27:27
[3] Wasserscheid P,Keim W. Angew Chem Int Ed,2000,39(21):3772
[4] Vassilev K,Stamenova R,Tsvetanov C. React Funct Polym,2000,46:165
[5] Luo Y F,Zou X C,Fu X K,et al. Sci China Chem,2011,41(3):433
[6] Liu Y H,Zhao J Q,Jiao Y J,et al. Petrochemical Technology,2004,33(9):816
[7] Palucki M,McCormick G J,Jacobsen E N. Tetrahedron Lett,1995,36(31):5457
[8] Tee K L,Schwaneberg U,Angew Chem Int Ed,2006,45:5380
[9] Alcalde M,Farinas E T,Arnold F H. J Biomol Screen,2004,9(2):141
[10] Rabe K S,Kiko K,Niemeyer C M. ChemBioChem,2008,9:420
[11] Wang J,Yuan Z M,Kong H W,et al. Chinese Journal of Chromatography,2012,30(1):8
[12] Sichilongo K F,Obuseng V C,Okatch H. Chromatographia,2012,75:1017
[13] Herrington J S. Anal Chem,2013,85(16):7882
[14] Xu X M,He H L,Ruan Y D,et al. Chinese Journal of Chromatography,2013,31(11):1129
[15] Ren Y,Schlager H,Martin D. Chromatographia,2014,77:309
[16] Hu L,Cao X L,Yang J H,et al. Chem Commun,2011,47:1303
[17] Sepai O,Anderson D,Street B,et al. Arch Toxicol,1993,67:28
[18] Tornero-Velez R,Waidyanatha S,Pérez H L,et al. J Chromatogr B,2001,757:59
