内生真菌提高禾草耐重金属胁迫的研究进展
2014-12-24张兴旭南志标李春杰
张兴旭,南志标,李春杰
(草地农业生态系统国家重点实验室兰州大学草地农业科技学院,甘肃兰州730020)
通常把密度大于5 g·cm-3的金属称为重金属,目前自然界中存在的90种元素中有53种是重金属[1],主要包括金(Au)、银(Ag)、铜(Cu)、锌(Zn)、铝(Al)、铅(Pb)和镉(Cd)等。随着全球工业化和地球化学活动的日益频繁,地表和地下水中重金属污染已经成为一个严重的环境问题[2-3]。重金属污染限制作物生产,在食物链中通过生物富集来威胁人类的健康[4-6]。Cd是众多环境污染中对植物有毒害效应的重金属之一,可以影响植物一系列的生物化学和生理代谢过程,包括光合作用、呼吸作用、氮循环、蛋白质新陈代谢以及营养元素的吸收[3,7]。目前一些研究表明,重金属Zn胁迫能够影响植物体内的卡尔文循环[8]和光合作用的活性[9-11]。
禾草内生真菌(Fungal Endophyte or Endophytic Fungi)是指能够在禾草中度过大部分或全部的生命周期,但不使宿主植物显示任何外部症状的一大类真菌[12],包括香柱菌属(Epichloё)及其无性阶段 Neotyphodium属的真菌[13-14]。已经在很多冷季型禾草中发现了Neotyphodium属及其有性阶段Epichloё属的内生真菌[12-13,15],这些内生真菌可以赋予宿主植物很强的竞争能力,主要因为其提高了寄主植物对各种生物胁迫[16-21]和非生物胁迫[15,22-23]的耐受性。
通过一系列物理和化学手段来改善和消除局部地区的土壤重金属污染费时、费力[24],特别是对大面积污染而言更是杯水车薪,而且往往会导致土壤团粒结构、生物区系的改变和土壤功能的退化[25]。植物修复技术是一种对环境友好的、投入高效的选择,但是受到诸多因素的限制[26]。植物能够进行土壤修复的前提条件是该植物对重金属污染具有极强的耐受能力[27]。因此,可以通过提高植物对重金属污染的耐受性从而提高其修复能力[28]。研究已经证实,应用丛枝菌根和植物共生体进行土壤修复是一种提高植物修复的新型而高效的方法[29-32]。与丛枝菌根相类似,Neotyphodium属内生真菌也能够提高寄主禾草对 Al[33]、Zn[34-35]和Cu[15]的耐受性。内生真菌还可以影响宿主禾草对Fe、Zn、Cu、Ca和 P等矿质元素的吸收和运输[33,36]。Ren 等[37]的研究表明,与不带内生真菌的植株相比,带菌多年生黑麦草(Lolium perenne)可以积累更多的Cd元素。
本文综述目前有关重金属胁迫对禾草生长和生理生化的影响、禾草对重金属胁迫的响应以及内生真菌的侵染在禾草耐重金属中调节的作用,以期为耐重金属胁迫禾草品种的选育和植物修复机制的研究提供新的方法手段和理论基础。
1 重金属胁迫对禾草形态学指标的影响
有关重金属离子胁迫主要有金属离子的不足和过量两种情况,在长期的共同进化过程中,植物已经发展了一些策略来应对金属离子的胁迫[38]。
1.1 重金属对禾草种子萌发的影响
重金属离子能够在高浓度条件下抑制多年生黑麦草的种子萌发,其中Cd和Pb的抑制作用较Cu和Zn显著[39]。低浓度的Cd和Pb对多年生黑麦草(Lolium perenne)的种子萌发具有促进作用,而高浓度的Cd和Pb抑制了其萌发[40]。低浓度的Cd对醉马草(Achnatherum inebrians)种子萌发和幼苗生长具有促进作用,而高浓度的Cd明显抑制了其种子萌发,内生真菌的侵染有效缓解了Cd毒害对醉马草种子萌发的影响[41]。随后,在对重金属Cd胁迫下带与不带内生真菌的披碱草(Elymus dahuricus)种子萌发和幼苗生长的研究中也得到了相同的结论[42]。
1.2 重金属对根系活力的影响
植物暴露于有毒重金属中通常发生一些很明显的外部病症如缺绿病、坏死以及根的生长抑制[43-44]。苏丹草(Sorghum sudanense)和多年生黑麦草的根系活力均随着Cd浓度的升高而逐渐降低[45],黑麦草、高羊茅(Festuca arundinacea)和匍匐剪股颖(Agrostis stolonifera)在重金属Zn胁迫下根系活力降低[46]。但是,多年生黑麦草和匍匐剪股颖的根系活力在低浓度Pb胁迫下比对照有显著提高[47],相同的植物物种对不同种类重金属胁迫的响应不同,可能跟植物的生育期、品种和环境条件等有关。内生真菌可以增加寄主植物的根冠比、须根数目及根毛长度,但是根直径减小[33]。Cd胁迫对胚根生长的影响大于胚芽生长,特别是在高浓度镉胁迫条件下[48-50]。在重金属Cu胁迫条件下,内生真菌侵染的高羊茅具有相对较多须根数[15]。随着Cd胁迫浓度的增加,醉马草[23]、披碱草[42]和野大麦(Hordeum brevisubulatum)[51]的幼苗均呈现出胚芽长度显著降低,叶片失绿黄化、出现红褐色斑点,胚根褐变的现象,这些症状与其他研究报道相同[3,50],伴随着这些症状而来的就是植物干物质产量的减少[50],而内生真菌的侵染可以显著缓解Cd对其幼苗胚根的伤害。很多研究表明,高浓度Cd胁迫对胚根生长的影响大于胚芽生长[48-50]。Cd胁迫对胚根生长的影响相对较大,是因为植物生长过程中根是植物直接接触生长基质中Cd元素的器官,根部可能积累了相对含量较高的Cd元素[52-53]。
1.3 重金属对生物量的影响
一年生黑麦草(L.multiflorum)地上部分的干生物量随着Zn浓度的增加,呈先增后减的变化规律[46]。在5.0 ×10-4mol·L-1的 Cd 胁迫下,多年生黑麦草、高羊茅、匍匐剪股颖生物量均明显下降,且地上部分生物量的下降幅度大于地下部分[47]。与未被侵染植株相比,内生真菌的侵染可以提高细羊茅(F.stapfii)对Al胁迫的耐受性,明显促进宿主植物干物质的积累[54]。内生真菌可以提高紫羊茅(F.rubra)对 Al胁迫的耐受性,增加其生物量[55],也可以提高黑麦草对Zn胁迫的适应能力从而增加植株干质量和分蘖数[11,34]。在重金属Cu胁迫下,内生真菌侵染的高羊茅具有相对较多的地下生物量[15]。Cd胁迫会显著降低高羊茅和草地羊茅(F.pratensis)生物量、分蘖数和绿叶数,内生真菌可以缓解Cd对羊茅属植物的毒害,与不带菌植株相比,带菌植株具有较高的生物量和生长指数[25]。与E-植株相比,E+植株具有较多的分蘖数和生物量,内生真菌的侵染可以提高Cd在高羊茅植株中的积累并且改善 Cd在根部和地上部的运输[56]。醉马草[23,41]、披碱草[42]和野大麦[51]的生物量均随着 Cd胁迫浓度的增加而逐渐减少,内生真菌的侵染可以显著缓解Cd毒害,表现为相对较高的干、鲜质量。随着Mn、Zn和Fe 3种重金属胁迫浓度的增加,醉马草地上生物量积累、株高和分蘖数均显著降低[57](表1)。
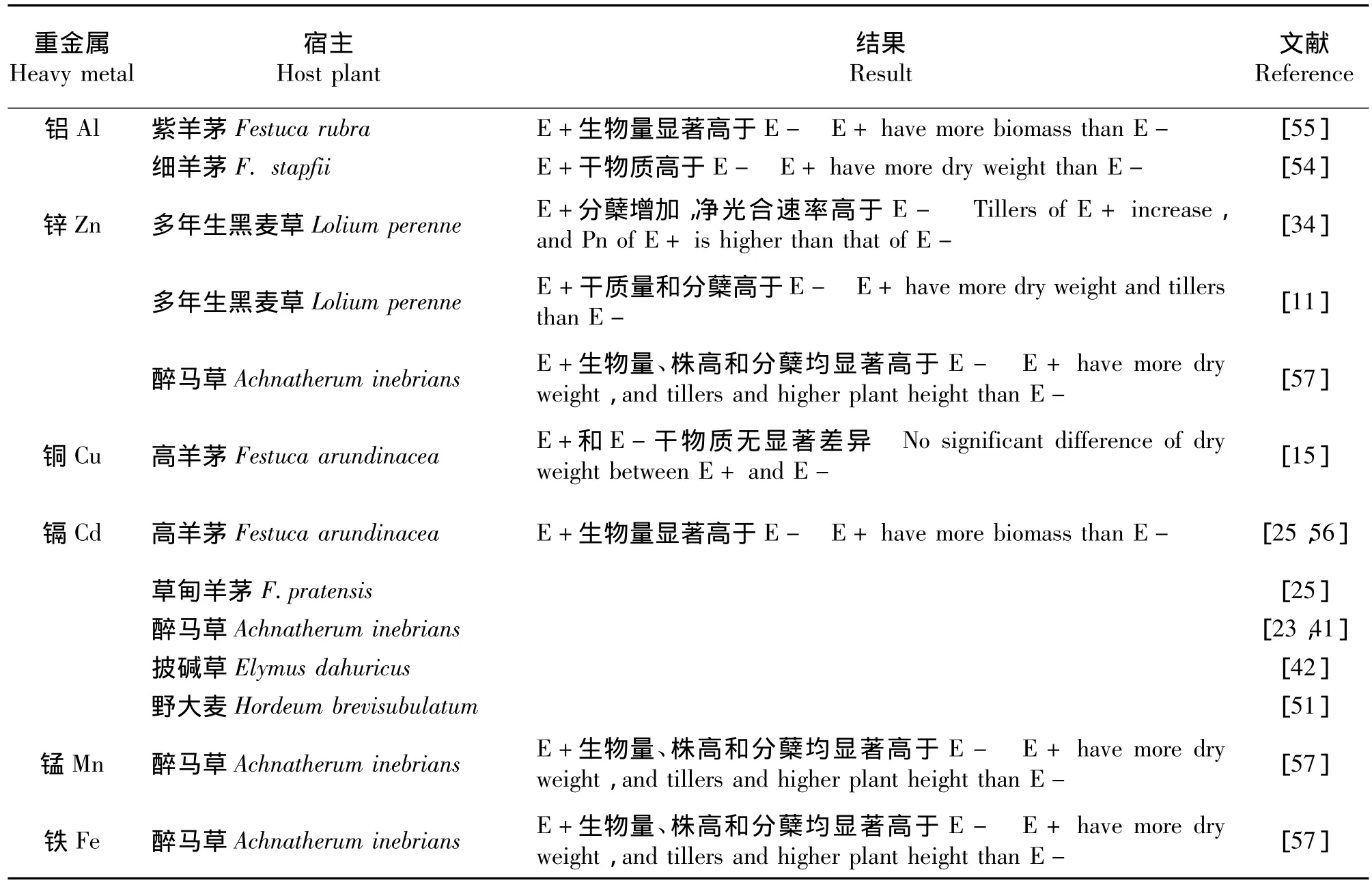
表1 内生真菌对重金属离子胁迫的响应Table 1 Response of endophyte to heavy metal stress
2 禾草对重金属胁迫的生理生化响应
植物在水分胁迫条件下,重金属胁迫往往能够诱导植物一系列的生化反应和生理变化[7]。内生真菌侵染可以提高高羊茅[33]及其他5种羊茅属植物[55]对重金属Al的耐受程度。同时,内生真菌会影响高羊茅对矿质元素(Fe、Zn、Cu、Ca、P)的吸收和体内的转移运输[33]。尽管内生真菌可以增加寄主植物对各种胁迫的抗逆性,但是即便是单一胁迫对植物生理功能的影响也各不相同[11]。如,Cd通过影响植物一系列的生物化学和生理代谢过程,包括光合作用、呼吸作用、氮循环、蛋白质新陈代谢以及营养元素的吸收,进而对植物产生有毒害效应[3,7];而铬(Cr)使植物细胞器的超微结构改变,扰乱代谢活动,抑制生长,加剧细胞膜脂过氧化以及蛋白质和核酸含量和功能的改变[58];Pb可以直接和间接地作用于植物,通过影响叶片的表面形态及结构[59-60]和内在的物质系统、分子基础[61]来改变植物的光合作用,进而影响其生长和代谢[3,58,62]。
2.1 光合作用
目前一些研究表明,重金属Zn胁迫能够影响植物体内的卡尔文循环[8]以及光合作用的活性[9-11];N.lolii内生真菌可以提高多年生黑麦草对重金属Zn胁迫的耐受性,促进地上生物量的积累和分蘖的增加,同时还增强其光合作用[34]。Cd胁迫显著降低了高羊茅和草地羊茅光系统Ⅱ的最大光化学效率(Fv/Fm),但内生真菌侵染的植株下降的幅度要小于不带菌植株[25]。Cd胁迫的毒性可使大麦(H.vulgare)成熟叶片产生红褐色边纹枯死斑[50],从而影响其光合作用。多年生黑麦草的净光合速率和最大光化学效率随着Cd浓度的增加而降低,高浓度Cd胁迫条件下内生真菌可以显著缓解其下降趋势[56]。
2.2 脯氨酸的积累
脯氨酸在植物适应干旱和不良环境中起着重要作用,通过溶质积累达到渗透调节的目的和作用。在重金属胁迫下,植物脯氨酸通常发生积累现象,但是如何产生这种应激反应到目前学术界一直没有达成一致的意见[63-66]。最初的研究发现,植物遭受重金属胁迫后体内产生并积累了大量的脯氨酸[63-64],其实脯氨酸累积并不是植物受重金属损伤的症状[67],相反,脯氨酸被认为是保护植物免受重金属毒害的[65,68],当植物受到各种压力时脂质过氧化和脯氨酸积累之间存在一种关系[69]。Mn、Zn和Fe胁迫下醉马草植株中脯氨酸含量会积累增加[57]。
在田间干旱条件下,E+高羊茅植株中脯氨酸含量的积累低于E-植株,但在室内条件下,200 μmol·L-1CdCl2浓度胁迫条件下,E+植株脯氨酸积累高于E-植株,这可能是由于Cd胁迫压力过大以至于E+植株维持正常的生理代谢过程所致[70]。但是,有关脯氨酸研究结论的不一致,可能归因于不同的逆境胁迫作用于不同的品种和不同环境。纸上芽床发芽试验和盆栽试验的结果均表明,重金属Cd胁迫下,醉马草、披碱草和野大麦3种禾草体内脯氨酸的积累均显著增加,E+禾草体内脯氨酸含量增加要大于 E- 植株[23,41-42,51]。
2.3 抗氧化酶系统
许多Cd胁迫植物中均有H2O2和·O2-积累的报道[71-72],Cd胁迫通过影响质膜上膜脂和膜蛋白的相互作用,伤害细胞膜结构的同时诱导·O2-的产生从而激发质膜上NADPH-氧化酶的活性[73-76]。在水稻(Oryza sativa)根部[77]和叶部[71],Cd 胁迫可以增加·O2-的产生,从而激发了NADPH-氧化酶的活性,被认为是Cd诱导·O2-形成的原因。很多研究表明,植物可以通过诱导抗氧化防御体系来增强对Cd 胁迫的耐受性[3,7,53]。内生真菌的侵染可以提高重金属Cd在高羊茅植株中的积累和抗氧化物酶活性[56]。Zn胁迫下,内生真菌侵染(E+)的多年生黑麦草中抗坏血酸过氧化物酶(Ascorbic acid,APX)的活性低于野生型植株[34]。
目前广泛认为,Cd毒害能够使植物产生氧化应激反应,诱导植物体内产生一些与氧化相关的活性氧自由基[3,53,75]。Cd 胁迫处理的植物中 H2O2积累,可能是由于植物体内H2O2产生和清除之间的动态平衡被打乱的结果[3,53]。超氧化物歧化酶(Superoxide Dismutase,SOD)是植物体内将活性氧自由基清除并将·O2-自由基转化成H2O2的解毒过程中起首要作用的酶。此外,过氧化氢酶(Catalase,CAT)、过氧化物酶(Peroxidas,POD)和 APX也都能够防止体内H2O2的积累。当植物体内H2O2含量增加,如果SOD酶活性被抑制,其他酶将会被激发来分解和清除这些自由基。因此,有理由认为,CAT与APX在配合SOD清除H2O2的过程中发挥着重要作用[3]。内生真菌可以提高醉马草、披碱草和野大麦在重金属Cd胁迫下的抗氧化酶系统,在长期Cd胁迫环境下CAT、SOD、POD和APX的活性都显著增加[23,41-42,51]。王萍等[57]研究表明,CAT、SOD 和POD活性均随着Mn、Zn和Fe 3种重金属胁迫浓度的增加而显著上升。丙二醛(MDA)作为机体受到逆境胁迫后膜脂过氧化的产物,其含量的高低反映了机体细胞受损伤的程度。醉马草[23,41,51,57]、披碱草[42]和野大麦[51]遭受重金属胁迫后体内 MDA 含量均明显升高。
3 植物耐重金属的机理
重金属胁迫与其他各种形式的非生物胁迫相似,也能够导致大量的活性氧自由基产生,这些自由基损伤蛋白质和DNA等遗传物质并且引起膜脂过氧化,而植物自身的抗氧化防卫系统能够清除自由基,从而保护细胞免受伤害[3],这是植物抗重金属胁迫的主要机理之一。
3.1 抗氧化酶
与干旱胁迫相似,重金属胁迫也能够通过诱导植物体内的一系列生理生化变化来缓解胁迫程度或者提高植物适应胁迫的能力[7]。重金属(如Cd)能诱导产生自由基和活性氧(Reactive Oxygen Species,ROS),从而破坏植物主要细胞大分子,如蛋白质、脂质和脱氧核糖核酸(DNA)。重金属胁迫能够激发或诱导植物抗氧化酶体系的表达,具体表现在SOD、CAT和POD活性有不同程度的变化[3]。内生真菌可以提高醉马草、披碱草和野大麦在重金属Cd胁迫下的抗氧化酶系统,与不带菌植株相比,带菌植株的 CAT、SOD、POD和 APX活性均有显著增加[23,41-42,51],然而,Cd 胁迫诱导植株 ROS 含量的分子基础以及内生真菌的调控作用机制,仍然需要通过将来的研究来进一步阐明。
3.2 抗氧化物质
谷胱甘肽(Glutathione,GSH)参与植物抗氧化代谢途径中活性氧的产生、调节和清除,寄主植物可以通过APX-谷胱甘肽循环来参与活性氧解毒[78-80]。当植物遭受重金属胁迫时,液泡膜内积累的金属离子与谷胱甘肽发生螯合反应并形成复合体隐含于液泡中[81]。由于谷胱甘肽在植物抗活性氧反应中具有重要作用,目前已经通过转基因手段等分子生物学的方法来调控谷胱甘肽的表达,从而提高目标植物的耐重金属能力[82]。
3.3 重金属螯合物
当植物遭受重金属胁迫时,植物螯合肽及其复合物将金属离子螯合并且诱发相关酶活性的启动,从而在植物应对金属胁迫的过程中发挥功能性作用[2,83]。目前,仅见有关拟南芥(Arabidopsis thaliana)中植物螯合物与重金属Cd螯合成重金属复合体并将其隔离在液泡中[84]。此外,植物螯合态还可以作用于谷胱甘肽,通过对Pb的螯合及形成Pb的螯合物来降低重金属毒害[85]。内生真菌也可能通过与寄主互作分泌一系列的酚类化合物使其结合可溶性的Al、Fe和Mn来降低金属离子的胁迫[86-87]。
3.4 植物自我修复
内生真菌侵染的高羊茅在温室[88]和田间[89]试验中均积累了较低浓度的Cu含量。Neotyphodium属内生真菌的侵染提高了高羊茅和草地羊茅植株地上和地下部分对重金属镉Cd的积累和体内运输从而减轻了其对宿主的伤害,内生真菌侵染的植株具有相对较高的干物质,植株地上部和根部积累重金属Cd的潜能也要高于不带菌植株。这两种草类植物对Cd具有超积累效应,而内生真菌的侵染增强了这一效应[25]。与E-植株相比,E+植株具有较多的分蘖数和生物量,内生真菌的侵染可以提高重金属Cd在高羊茅植株中的积累并且改善Cd在根部和地上部的运输,带菌植株对重金属的提取和吸收能力强于不带菌植株的[56]。
4 展望
综上所述,微量的重金属作为植物生长的有益元素,能够促进植物正常的生长和生理代谢,但是重金属浓度过高会严重影响禾草类植物的生长、生理和生化代谢途径。通过微生物接种和植物组织培养等方法,能够成功转接内生真菌从而获得一些新的带菌禾草新品系。用内生真菌侵染禾草来增加寄主植物对Cd元素的耐受性,可以筛选一些具有优良耐受性、更加有效的菌株和禾草新品种(系)用于重金属污染环境中的植物修复。但是,各种重金属在诱导植物自由基产生过程中的角色和地位以及内生真菌在禾草耐重金属中的调控作用及其机理仍然需要进一步研究。
[1]Myriam SZ,Mari D G,Maria L T,Maria P B.Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana[J].Plant Science,2007,173:190-197.
[2]张芳,方溪,张丽静.草类对重金属胁迫的生理生化响应机制[J].草业科学,2012,29(4):534-541.
[3]Zhang F Q,Zhang H X,Wang G P,Xu L L,Shen Z G.Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes[J].Journal of Hazardous Materials,2009,168:76-84.
[4]Wong M H.Ecological restoration of mine degraded soils,with emphasis on metal contaminated soils[J].Chemosphere,2003,50:775-800.
[5]Gisbert C,Clemente R,Navarro-Avino J,Carlos B,Alfonso G,Ramon S,David J W,Bernal M P.Tolerance and accumulation of heavy metals by Brassicaceae species grown in contaminated soils from Mediterranean regions of Spain[J].Environmental and Experimental Botany,2006,56(1):19-27.
[6]Muhammad D,Chen F,Zhao J,Zhang G P,Wu F B.Comparison of EDTA and citric acidenhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia[J].International Journal of Phytoremediation,2009,11(6):558-574.
[7]Barcelo J,Poschenrieder C.Plant water relations as affected by heavy metal stress:A review[J].Joural of Plant Nutrition,1990,13:1-37.
[8]Chaney R L.Zn toxicity[A].Robson A D.Zn in Soils and Plants[M].Dordrecht:Kluwer,1993.
[9]Van Assche F,Clijsters H.Inhibition of photosynthesis by treatment of Phaseolus vulgaris with toxic concentration of Zn:Effects on electron transport and photophosphorylation[J].Plant Physiology,1986,66:717-721.
[10]Van Assche F,Clijsters H.Inhibition of photosynthesis in Phaseolus ulgaris by treatment with toxic concentration of Zn:Effects on ribulose-1,5-bisphosphate carboxylase/oxygenase[J].Journal of Plant Physiology,1986,125:355-360.
[11]Monnet F,Vaillant N,Hitmi A,Coudret A,Sallanao H.Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne[J].Physiologia Plantarum,2001,113:557-563.
[12]南志标,李春杰.禾草-内生真菌共生体在草地农业系统中的作用[J].生态学报,2004,24(3):605-616.
[13]Schardl CL,Leuchtmann A.The Epichloёendophytes of grasses and the symbiotic continnuum[A].Dighton J,White JF,Oudemans P.The Fungal Community[M].3rd edtion.Boca Raton:CRCPress,2004:475-503.
[14]Kuldau G,Bacon C.Clavicipitaceous endophytes:Their ability to enhance resistance of grasses to multiple stresses[J].Biological Control,2008,46:57-71.
[15]Malinowski D P,Belesky D P,Lewis G C.Abiotic stresses in endophyte grasses[A].Craig A R,Charles PW,Donald E S.Neotyphodium in Cool-season Grasses[C].Current Research and Application[M].Oxford:Blackwell Scientifie Publicatious,2005:187-200.
[16]Siegel M R,Latch GCM,Bush L P,Fannin FF,Rowan D D,Tapper B A,Bacon CW,Johnson M C.Fungal endophyte-infected grasses:Alkaloid accumulation and aphid response[J].Journal of Chemistry Ecology,1990,16:3301-3315.
[17]West CP,Izekor E,Robbins R T,Gergerich R,Mahmood T.Acremonium coenophialum effects on infestations of barley yellow dwarf virus and soil-borne nematodes and insects in tall fescue[A].Proceedings of the International Symposium on Acremonium/Grass Interactions[C].Louisinana,USA,1990:196-198.
[18]Eerens J P J,Visker M H PW,Lucas R J,white JG H.Influence of the ryegrass endophyte(Neotyphodium lolii)on morphology,physiology,and alkaloid synthesis of perennial ryegrass during high temperature and water stress[J].New Zealand Journal of Agricultural Research,1998,41:219-226.
[19]Conover M R.Impact of the consumption of endophyte-infected perennial ryegrass by meadow voles[J].Agriculture,Ecosystems and Environment,2003,97:199-203.
[20]Conover M R.Impact of consuming tall fescue seeds infected with the endophytic fungus,Neotyphodium coenophialum,on reproduction of chickens[J].Theriogenology,2003,59:1313-1323.
[21]Li CJ,Gao JH,Nan Z B.Interactions of Neotyphodium gansuense,Achnatherum inebrians and plant-pathogenic fungi[J].Mycology Research,2007,111:1220-1227.
[22]Bacon CW.Abiotic stress tolerances(moisture,nutrients)and photosynthesis in endophyte-infected tall fescue[J].Agriculture,Ecosystems and Environment,1993,44:123-142.
[23]Zhang X X,Li CJ,Nan Z B.Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense[J].Journal of Hazardous Materials,2010,175:703-709.
[24]Abe T,Fukami M,Ichizen N,Ogasawara M.Susceptibility of weed species to cadmium evaluated in a sand culture[J].Weed Biology and Management,2006,6:107-114.
[25]Soleimani M,Hajabbasi M A,Afyuni M,Mirlohi A,Borggaard O K,Holm P E.Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca arundinacea and Festuca pratensis[J].International Journal of Phytoremediation,2010,12:535-549.
[26]Pilon-Smits E.Phytoremediation[J].Annual Review of Plant Biology,2005,56:15-39.
[27]铁梅,张朝红,董厚德,徐锐,邢志强.草坪草对土壤中镉的吸收与富集作用的研究[J].辽宁大学学报(自然科学版),2001,28(2):189-192.
[28]Alkorta I,Becerril H A,Amezaga I.Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc,cadmium,lead,and arsenic[J].Review of Environment and Science Biotechnology,2004,3:71-90.
[29]Orlowska E,Ryszka P,Jurkiewicz A.Effectiveness of arbuscular mycorrhizal fungal(AMF)strains in colonisation of plants involved in phytostabilization of zinc wastes[J].Geoderma,2005,129:92-98.
[30]Khan A G.Mycorrhizoremediation——An enhanced form of phytoremediation[J].Journal of Zhejiang University Science,2006,7:503-514.
[31]Göhre V,Paszkowski U.Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation[J].Planta,2006,223:1115-1122.
[32]Jankong P,Visoottiviseth P.Effects of arbus-cular mycorrhizal inoculation on plants growing on ar-senic contaminated soil[J].Chemosphere,2008,72:1092-1097.
[33]Malinowski D P,Belesky D P.Adaptation of endophyte-infected cool-season grasses to environment stresses:Mechanisms of drought and mineral stress tolerance[J].Crop Science,2000,40:923-940.
[34]Bonnet M,Camares O,Veisseire P.Effects of zinc and influence of Acremonium lolii on growth parameters,chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass(Lolium perenne L.cv.Apollo)[J].Journal of Experimental Botany,2000,51:945-953.
[35]Monnet F,Vaillant N,Hitmi A,Coudret A,Sallanao H.Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne[J].Plant Physiology,2001,113:557-563.
[36]Rahman M H,Saiga S.Endophytic fungi(Neotyphodium coenophialum)affect the growth and mineral uptake,transport and efficiency ratios in tall fescue(Festuca arundinacea)[J].Plant and Soil,2005,272:163-171.
[37]Ren A Z,Gao Y B,Zhang L,Xie F X.Effects of cadmium on growth parameters of endophyte-infected and endophyte-free ryegrass[J].Journal of Plant Nutrition and Soil Science,2006,169:857-860.
[38]Marschner H.Mineral Nutrition of Higher Plants[M].Second edition.London:Academic Press,1995:889.
[39]多立安,高玉葆,赵树兰.重金属递进胁迫对黑麦草初期生长的影响[J].植物研究,2006,26(1):117-122.
[40]David E S,Michael B,Nanda P B A K,Viatcheslav D,Burt D E,Ilan C,Ilya R.Phytoremediation:A novel strategy for the removal of toxic metals from the environment using plants[J].Biotechnology,1995,13:468-475.
[41]Zhang X X,Fan X M,Li C J,Nan Z B.Effects of cadmium stress on seed germination,seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte[J].Plant Growth Regulation,2010,60(2):91-97.
[42]Zhang X X,Li C J,Nan Z B.Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte[J].Science China Life Sciences,2012,55(9):793-799.
[43]Carrier P,Baryla A,Havaux M.Cadmium distribution and microlocalization in oilseed rape(Brassica napus)after long term growth on cadmium contaminated soil[J].Planta,2003,216:939-950.
[44]Sandalio L M,Dalurzo H C,Gomez M,Romero-Puertas M C,del Rio L A.Cadmium-induced changes in the growth and oxidative metabolism of pea plants[J].Journal of Experimental Botany,2001,52:2115-2126.
[45]陆秀君,关欣,李常猛,王国娟.镉对草类幼苗生长的影响[J].北方园艺,2007(4):56-58.
[46]徐卫红,熊治庭,李文一.4品种黑麦草对重金属Zn的耐性及Zn积累研究[J].西南农业大学学报(自然科学版),2005,27(6):785-790.
[47]王慧忠,何翠屏.重金属离子胁迫对草坪草根系生长及其活力的影响[J].中国草地,2002,24(3):55-63.
[48]Ouzounidou G,Moustakas M,Eleftheriou E P.Physiologial and ultrastructural effects of cadmium on wheat(Triticum aestivum)leaves[J].Archives of Environmental Contamination and Toxicology,1997,32:154-160.
[49]Vitoria A P,Lea P J,Azevedo R A.Antioxidant enzymes responses to cadmium in radish tissues[J].Phytochemistry,2001,57:701-710.
[50]Melis T,Selim E,Faruk O,Soren H,Ismail C.Antioxidant defense system and cadmium uptake in barely genotypes differing in cadmium tolerance[J].Journal of Trace Elements in Medicine and Biology,2006,20:181-189.
[51]张兴旭.醉马草——内生真菌共生体对胁迫的响应及其次生代谢产物的活性研究[D].兰州:兰州大学,2012.
[52]Grant C A,Buckley W T,Bailey L D,Selles F.Cadmium accumulation in crops[J].Canadian Journal of Plant Science,1998,78:1-17.
[53]Hegedusü A,Erdei S,Horvath G.Comparative studies of H2O2detoxifying enzymes in green and greening barley seedlings under cadmium stress[J].Plant Science,2001,160:1085-1093.
[54]Liu H,Heckman JR,Murphy JA.Screening fine fescues for aluminum tolerance[J].Journal of Plant Nutrition,1996,19:677-688.
[55]Zaurov D E,Bonos S,Murphy J A,Richardson M,Belanger F C.Endophyte infection can contribute to aluminum tolerance in fine fescues[J].Crop Science,2001,41:1981-1984.
[56]Ren A Z,Li C,Gao Y B.Endophytic fungus improves growth and metal uptake of Lolium arundinaceum Darbyshire Ex.Schreb[J].International Journal of Phytoremediation,2011,13:233-243.
[57]王萍,张兴旭,赵晓静,李春杰.3种重金属元素胁迫对醉马草生长及生理生化指标的影响[J].草业科学,2014.31(6):1080-1086.
[58]Sinha S,Rai U N,Tripathi R D,Chandra P.Chromium and manganese uptake by Hydrilla verticillata(l.f.)Royle:amelioration of chromium toxicity by manganese[J].Journal of Environmental Science and Health,Part A.Toxic/Hazardous Substances and Environmental Engineering,1993,28:1545-1552.
[59]Krupa Z,Baszyński T.Some aspects of heavy metals toxicity towards photosynthetic apparatus-direct and indirect effects on light and dark reactions[J].Acta Physiologiae Plantarum,1995,17:177-190.
[60]Parys E,Romanowska E,Siedlecka M,Poskuta J W.The ffect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum[J].Acta Physiologiae Plantarum,1998,20:13-322.
[61]Patra M,Sharma A.Mercury toxicity in plants[J].Botanical Review,2000,66:379-422.
[62]Tomar M,KaurNeelu I,Bhatnagar A K.Tomar M,Kaur I,Effect of enhanced lead in soil on growth and development of Vigna radiata(L).Wilczek[J].Indian Journal of Plant Physiology,2000,5:13-18.
[63]Schat H,Sharma SS,Vooijs R.Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris[J].Plant Physiology,1997,101:477-482.
[64]Shah K,Dubey R S.Effect of cadmium on proline accumulation and ribonuclease activity in rice seedlings:Role of proline as a possible enzyme protectant[J].Biology Plantarum,1998,40:121-130.
[65]Mehta SK,Gaur JP.Heavy metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris[J].New Phytologist,1999,143:253-259.
[66]Zengin F K,Munzuroglu O.Effect of some heavy metals on content of chlorophyll,proline and some antioxidant chemicals in bean seedling[J].Acta Biologica Cracoviensia Series Botanica,2005,47:157-164.
[67]Lutts S,Kinet J M,Bouharmont J.Effects of various salts and of mannitol on ion and proline accumulation in relation to osmotic adjustment in rice(Oryza sativa L.)callus cultures[J].Journal of Plant Physiology,1996,149:186-195.
[68]Siripornadulsil S,Train S,Verma D PS,Sayre R T.Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae[J].The Plant Cell,2002,14:2837-2847.
[69]Molinari H B C,Marur CJ,Daros E,De Campos M K F,De Carvalho JF R P,Filho JCB,Pereira L F P,Vieira L GE.Evaluation of the stress-inducible production of proline in transgenic sugarcane(Saccharum spp.):Osmotic adjustment chlorophyll fluorescence and oxidative stress[J].Plant Physiology,2007,130:218-229.
[70]Elbersen H W,West C P.Growth and water relations of field-grown tall fescue as influenced by drought and endophyte[J].Grass and Forage Science,1996,51:333-342.
[71]Yeh CM,Chien PS,Huang H J.Distinct signalling pathways for induction of MAPkinase activities by cadmium and copper in rice roots[J].Journal of Experimental Botany,2007,58:659-671.
[72]Maksymiec W,Krupa Z.The effects of short-term exposition to Cd,excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana[J].Environmental and Experimental Botany,2006,57:187-194.
[73]Wahid A,Perveen M,Gelani S,Shahzad M A B.Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins[J].Journal of Plant Physiology,2007,164:283-294.
[74]Doussiere J,Gaillard J,Vignais P V.The heme component of the neutrophil NADPH oxidase complex is a target for aryliodonium compounds[J].Biochemistry,1999,38:3694-3703.
[75]Olmos E,Martinez-Solano J R,Piqueras A,Hellin E.Early steps in the oxidative burst induced by cadmium in cultured tobacco cells(BY-2 line)[J].Journal of Experimental Botany,2003,54:291-301.
[76]Rodríguez-Serrano M,Romero-Puertas M C,Zabalza A,Corpas F J,Gomez M,Delrio L,Sandalio L.Cadmium effect on oxidative metabolism of pea(Pisum sativum L.)roots.Imaging of reactive oxygen species and nitric oxide accumulation in vivo[J].Plant,Cell and Environment,2006,29:1532-1544.
[77]Hsu Y T,Kao CH.Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation[J].Plant and Soil,2007,298:231-241.
[78]Foyer C H,Noctor G.Redox homeostasis and antioxidant signaling:A metabolic interface between stress perception and physiological responses[J].Plant Cell,2005,17:1866-1875.
[79]Shao H B,Liang Z S,Shao M A,Sun SM,Hu Z M.Investigation on dynamic changes of photosynthetic characteristics of 10 wheat(Triticum aestivum L.)genotypes during two vegetative-growth stages at water deficits[J].Colloids and Surfaces B:Biointerfaces,2005,43:221-227.
[80]Freeman JL,Persans M W,Nieman K.Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators[J].The Plant Cell,2004,16:2176-2191.
[81]Sharma SS,Dietz K J.The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress[J].Journal of Experimental Botany,2006,57:711-726.
[82]Yadav SK.Heavy metals toxicity in plants:An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants[J].South African Journal of Botany,2010,76:167-179.
[83]Memon A R,Chino M,Yatazawa M.Microdistribution of aluminum and manganese in the tea leaf tissue as revealed by X-ray microanalyzer[J].Soil Science and Plant Nutrition,1981,27:317-328.
[84]Rauser W E.Phytochelatins and related peptides,structure,biosynthesis,and function[J].Plant Physiology,1995,109:1141-1149.
[85]Piechalak A,Tomaszewska B,Baralkiewicz D,Malecka A.Accumulation and detoxification of lead ions in legumes[J].Phytochemistry,2002,60:153-162.
[86]Howden R,Goldsbrough PB,Andersen CR,Cobbett CS.Cadmium sensitive,cad1 mutants of Arabidopsis thaliana are phytochelatin deficient[J].Plant Physiology,1995,107:1059-1066.
[87]Schmöger M E,Oven M,Grill E.Detoxification of arsenic by phytochelatins in plants[J].Plant Physiology,2000,122:793-801.
[88]Dennis SB,Allen V G,Saker K E,Fontenot JP,Ayad JY,Brown CP.Influence of Neotyphodium coenophialum on copper concentration in tall fescue[J].Journal of Animal Science,1998,76:2687-2693.
[89]Allen V G,Fontenot J P,Bagley C P.Effects of seaweed treatment of tall fescue on grazing steers[A].In Williams M J.Proceeding of the 1997 Animal and Forage Grassland Council Conference[C].Fort Worth,TX,1997:13-15.
