湿热应激诱导小鼠心肌细胞凋亡的机制
2014-11-27王晓武袁彬彬王显悦董文鹏杨永超张卫达
王晓武,袁彬彬,林 曦,王显悦,董文鹏,杨永超,张卫达
(广州军区 广州总医院 心血管外科中心,广东 广州 510010)
湿热应激诱导小鼠心肌细胞凋亡的机制
王晓武,袁彬彬,林 曦,王显悦,董文鹏,杨永超,张卫达*
(广州军区 广州总医院 心血管外科中心,广东 广州 510010)
目的探讨湿热应激诱导心肌细胞凋亡的作用机制。方法构建湿热应激小鼠模型,分为湿热应激组(42 ℃,RH 65%)(H组)和对照组(C组);TUNEL法原位标记凋亡的心肌细胞,EIISA检测Ang Ⅱ的表达水平;体外培养小鼠心肌细胞,分别加入caspase-3抑制剂Z-DEVD-FMK和P38 MAPK抑制剂SB203580共培养24 h,随后加入Ang Ⅱ共培养24 h;Annexin V-FITC凋亡试剂盒检测细胞凋亡率;Western blot检测P38 MAPK及caspase-3的表达水平。结果湿热应激条件导致小鼠心肌细胞凋亡率明显高于常规组,且伴随有大量Ang Ⅱ的生成(Plt;0.05);体外实验证实,Ang Ⅱ能够剂量依赖性的诱导心肌细胞凋亡,同时诱导caspase-3和P38 MAPK的活化;Z-DEVD-FMK预处理明显抑制Ang Ⅱ诱导的心肌细胞凋亡,表明Ang Ⅱ主要通过caspase-3活化途径来诱导心肌细胞凋亡;此外,SB203580可抑制Ang Ⅱ诱导的caspase-3的表达。结论湿热应激诱发心肌细胞凋亡主要是通过Ang Ⅱ诱导的P38 MAPK-caspase-3通路来实现的。
湿热应激;血管紧张素Ⅱ;细胞凋亡;P38 MAPK;caspase-3
高湿热环境对机体各个系统均有不同程度的损害,极端湿热环境可以导致某些蛋白结构和功能改变,导致体能减弱甚至死亡,也是诱发心血管疾病的重要原因[1-2]。研究显示,湿热应激能够导致心肌细胞凋亡,且在温度升高过程中细胞凋亡速率增加[3]。
细胞生长、分裂、衰老、凋亡等改变受多种因素影响,血管紧张素Ⅱ(AngⅡ)在影响细胞改变的因素中占最主要地位。越来越多的证据表明,湿热应激条件可能引发肾素-血管紧张素系统活性被激活,进而导致心肌细胞凋亡[4, 5]。但是,湿热应激导致心肌细胞凋亡的分子机制目前尚不清楚。
本实验通过研究湿热应激诱导小鼠心肌细胞凋亡的机理,为探讨高湿热环境下心肌细胞凋亡分子机制的研究奠定基础,同时为高湿热环境下作业人群心脏功能的防护提供理论依据。
1 材料与方法
1.1 材料
清洁级BALB/c小鼠60只 (5月龄),体质量20~23 g,雌雄不限,由南方医科大学实验动物中心提供 (合格证号:2005A050)。Annexin V-FITC凋亡试剂盒(南京凯基生物有限公司),小鼠Ang Ⅱ EIISA酶联免疫试剂(自上海研卉生物科技有限公司),TUNEL(Roche公司),DMEM培养基(上海高创化学科技有限公司),兔抗鼠P38 MAPK、P-P38 MAPK和caspase-3抗体(Bioworld公司)。
1.2 方法
1.2.1 热应激小鼠模型构建:BALB/c小鼠60 只,随机分为高温组(42 ℃,RH 65%)(H组)、常温组(C组),各30只。置动物于模拟气候舱,H组小鼠置于高湿热环境中;C组在室温条件下进行相同强度训练。两组小鼠在培养期间,每天进行高中等训练3次,每次30min,持续14 d,运动后自由进食。
1.2.2 EIISA检测Ang Ⅱ的表达水平: 腹腔注射50 mg/kg戊巴比妥钠(3%)麻醉小鼠后,取出心脏,4 ℃预冷的0.9%氯化钠注射液逆行灌洗主动脉,然后用滤纸吸干。切取左室心尖部分心肌组织3块。其中2块分别编号放入-70 ℃冰箱保存待测,另外一块在处理干净后快速冷冻,切碎,然后加入冰乙酸(1 mol/L)静置过夜,4 ℃,15 000r/min离心40 min,取上清。加入乙醚振荡混匀后继续蒸干,加入等量PBS完全溶解后,用EIISA试剂盒测定Ang Ⅱ浓度。具体操作步骤按照操作说明,简述为甲醇洗脱,蒸发后,用100 μL EIA稀释两遍,加入96孔板37 ℃孵育1 h,弃去上清,洗涤后加入IgG避光孵育6~8 h,再次弃去上清,洗涤后加入Ellman氏试剂,避光静置1 h,用分光光度仪405 nm波长测定Ang Ⅱ含量,1式3份。
1.2.3 TUNEL染色:取样操作同1.2.2,用4%多聚甲醛室温固定40 min,PBS清洗后70%乙醇在-20 ℃条件下静置1 h,PBS清洗后加入3% H2O2的甲醇室温放10 min,PBS清洗后置于1% Triton X-100+0.1%柠檬酸钠4 ℃静置3 min,PBS洗涤后加入TUNEL反应混合物,37 ℃放置90 min,PBS洗后加入POD 37 ℃放置40 min,PBS清洗后DAB 100 μL室温静置10 min,加入苏木精进行对比染色2 min,得到棕色沉淀, 100倍显微镜下分析结果。
1.2.4 心肌细胞培养及处理:取出生半天的小鼠,无菌操作取出心脏,迅速放入D-Hanks溶液中,剪取心尖部分的心室组织,用预冷的0.9%氯化钠注射液清洗,除去血污。剪碎后加入消化液(0.1%分散酶、0.05%胰蛋白酶、0.1% Ⅱ型胶原蛋白酶),37 ℃振荡培养消化15 min。弃上清重复3次。离心弃上清,沉淀细胞重悬计数后,培养于含血清的培养液(DMEM培养基10%马血清,5%的胎牛血清),差速贴壁的方法富集心肌细胞。计数后按照细胞1×105个/mL浓度,每皿2 L细胞悬液培养于培养皿。原代培养心肌细胞,每3孔为一组接种于24孔板中,每孔细胞约为1×108个/mL。将细胞随机分为3组:A组,用分别含有1×10-6、1×10-7和1×10-8mol/L浓度梯度 Ang Ⅱ的DNEM培养液培养;B组,1×10-6mol/L Ang Ⅱ和SB203580处理组;C组,1×10-6mol/L Ang Ⅱ和Z-DEVD-FMK处理组;DNEM培养液加DMSO作为对照。均培养24 h后观察结果。
1.2.5 AnnexinV-FITC检测细胞凋亡,按照说明书操作。
1.2.6 Western blot:提取各组细胞总蛋白:弃培养基后,用4 ℃预冷的PBS缓冲液洗涤细胞,洗2次后加入200 μL预冷的裂解液,冰上放置20 min,4 ℃,12 000r/min离心10 min,取上清测即为总蛋白。200 μg总蛋白加入上样缓冲液总体积至30 μL,样品处理后进行SDS-PAGE电泳。电泳结束后,采用半干转法将蛋白转移至PVDF膜上,用5%脱脂奶粉4 ℃过夜孵育,TBST洗涤3次后加一抗37 ℃孵育1 h,再次用TBST洗3次,加入二抗。ECL底物显色,红外线灯照射显影。
1.3 统计学分析

2 结果
2.1湿热应激对心肌细胞凋亡率及小鼠AngⅡ蛋白表达水平的影响
与对照组相比,H组诱导检测到大量棕色沉淀信号(图1)。湿热应激条件下Ang Ⅱ蛋白含量约为3 500 pg/mL,对照组约为1 400 pg/mL。
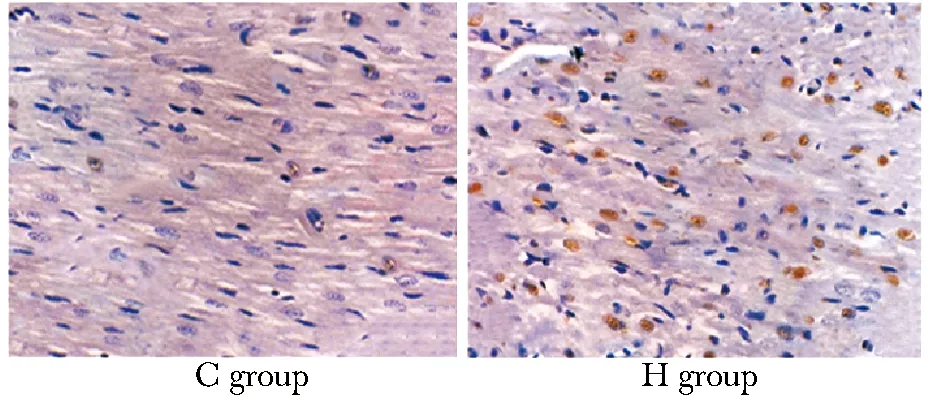
图1 湿热应激对小鼠心肌细胞凋亡的影响Fig 1 The role of damp-heat on the myocardial apoptosis (×100)
2.2 Ang Ⅱ对心肌细胞凋亡的影响
Ang Ⅱ能够诱导大量阳性信号的产生(图2A)。定量分析数据显示,Ang Ⅱ 浓度为1×10-7和1×10-6mol/L时诱导的心肌细胞凋亡率显著高于对照组 (Plt;0.05)(图2B)。

A.apoptosis of myocardial cell detected by Annexin V; B.quantitative analysis of myocardial cell apoptosis rate; *Plt;0.05, **Plt;0.01 compared with control图2 Ang Ⅱ预处理对心肌细胞的影响Fig 2 The role of Ang Ⅱ on apoptosis of myocardial cell
2.3AngⅡ通过活化caspase-3诱导心肌细胞凋亡
Ang Ⅱ诱导心肌细胞caspase-3蛋白大量活化(图3A)。Caspase-3抑制剂Z-DEVD-FMK预处理后Ang Ⅱ诱导的细胞凋亡率明显低于对照组(图3B)。
2.4AngⅡ通过P38MAPK信号通路诱导caspase-3的活化
Ang Ⅱ明显诱导心肌细胞P38 MAPK的活化(图4A),Ang Ⅱ蛋白表达量与对照组相比约为1.7倍(图4B)。此外,P38 MAPK抑制剂SB203580预处理后,caspase-3的表达下降(图4C),约为对照组的0.3倍(图4D)。
3 讨论
高湿热能诱导多种细胞凋亡,对于在高湿热环境工作人员健康造成极大威胁。本实验通过构建湿热应激小鼠模型,检测心肌细胞凋亡率,发现湿热应激诱导小鼠心肌细胞大量凋亡,同时伴有Ang Ⅱ蛋白的大量表达。Ang Ⅱ在细胞血管平滑肌细胞(VSMC)生长、凋亡、细胞迁移、炎性反应和纤维化等多种变化中占主要地位[6-7],但是, Ang Ⅱ是否参与湿热应激诱导的心肌细胞凋亡及其分子机制尚不清楚。
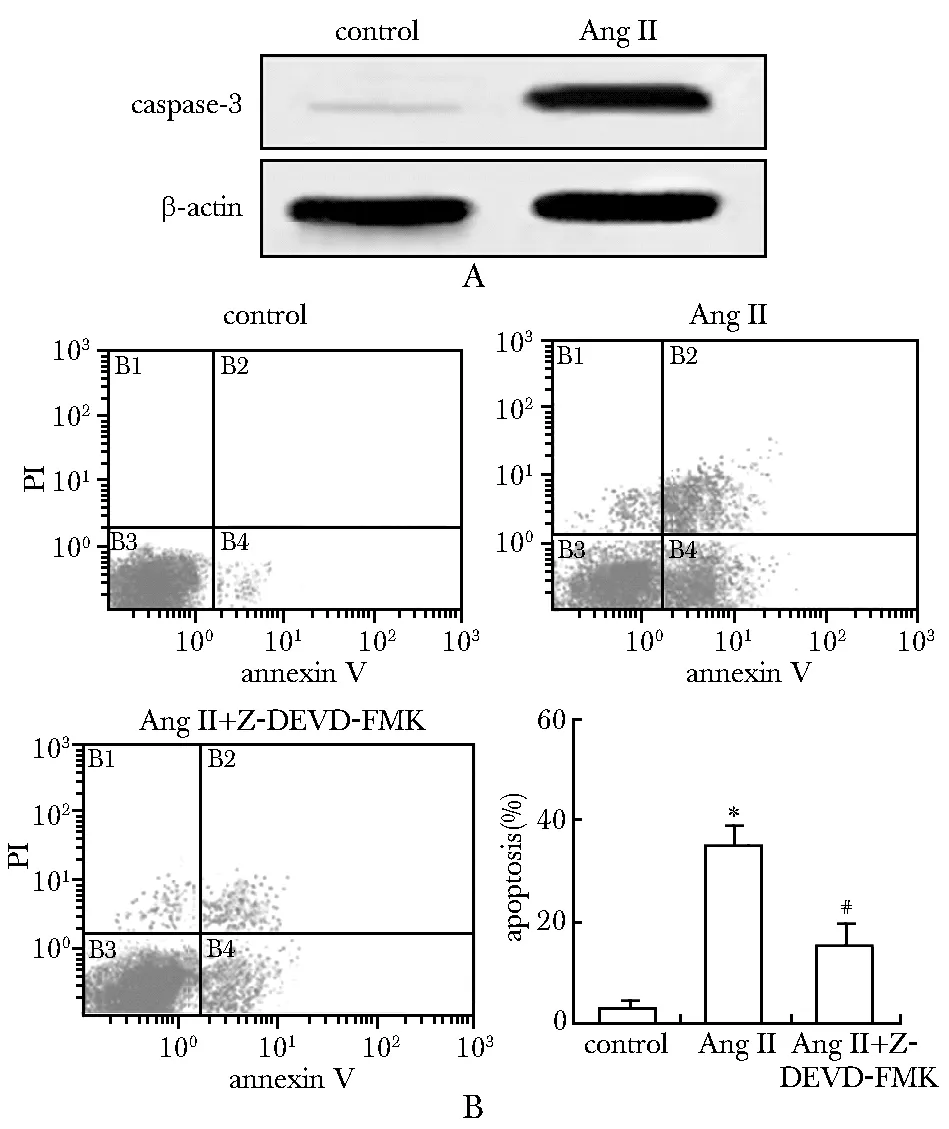
*Plt;0.05 compared with control; #Plt;0.05 compared with Ang Ⅱ图3 Ang Ⅱ通过活化caspase-3诱导心肌细胞凋亡Fig 3 Ang Ⅱ induced cardiomyocytes apoptosis by activating caspase-3
进一步体外实验证实,Ang Ⅱ能够剂量依赖的诱导小鼠心肌细胞大量凋亡。Caspase-3是多种细胞凋亡途径中重要的效应蛋白酶,是细胞凋亡的主要执行者[8]。Caspase-3与染色体凝聚和DNA片断化等细胞凋亡的特征性标志均密切相关,同时是caspase家族介导的细胞凋亡的线粒体通路和死亡受体通路的交汇点[8]。本研究发现,心肌细胞与AngⅡ共培养可诱导caspase-3蛋白的活化,caspase-3抑制剂Z-DEVD-FMK预处理能够显著降低AngⅡ诱导的细胞凋亡,表明AngⅡ可通过诱导caspase-3蛋白的活化介导心肌细胞凋亡。P38 MAPK通路是MAPK信号通路的一种,在细胞多种信号传导通路中起重要作,用参与应激介导的细胞凋亡反应[9-10]。进一步机制分析表明,Ang Ⅱ 可诱导P38 MAPK通路的活化,加入P38 MAPK抑制剂SB203580后caspase-3的表达明显降低,表明湿热应激诱导心肌细胞凋亡主要是通过Ang Ⅱ 诱导的P38 MAPK-caspase-3通路来实现的,提示Ang Ⅱ 可作为预防和治疗湿热环境诱导的心血管疾病的潜在靶标,对高湿热环境作业的人群心脏功能的防护具有重要意义。
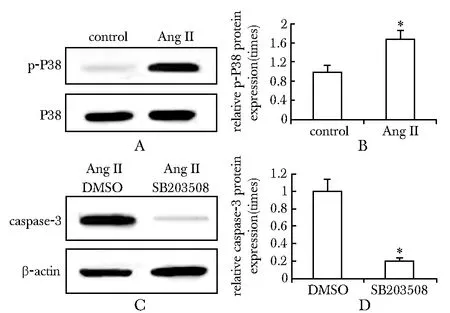
A.the role of Ang Ⅱ on the expression of P38; B.the role of SB203580 on the expression of caspase-3; *Plt;0.05 compared with control图4 Ang Ⅱ通过P38MAPK信号通路诱导caspase-3的活化Fig 4 Ang Ⅱ activate caspase-3 by way of P38 MAPK
此外,有研究表明Ang Ⅱ可通过NADPH诱导的活性氧(ROS)的生成诱导多种血管效应[11]。一方面ROS可通过改变细胞内氧化还原状态和对蛋白质的氧化修饰影响甚至损害细胞膜、线粒体膜和DNA等的结构和功能,激发 P38MAPK等信号通路活化促使细胞凋亡;另一方面过量的ROS可作为信号分子,并通过信号级联反应,参与并调控细胞凋亡程序的启动[12-13]。湿热应激条件下,Ang Ⅱ是否通过诱导ROS产生来调节P38 MAPK介导的细胞凋亡还有待于进一步研究。
[1] Febbraio M, Snow R, Stathis C,etal. Effect of heat stress on muscle energy metabolism during exercise[J]. J App Physiol, 1994, 77, 2827-2831.
[2] Périard JD, Ruell P, Caillaud C,etal. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity[J]. Cell Stress Chaperon, 2012, 17, 375-383.
[3] Zhao Y, Wang W, Qian L,etal. Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis[J]. Cell stress chaperon, 2007, 12, 83.
[4] McCurley A, Jaffe I Z. Mineralocorticoid receptors in vascular function and disease[J]. Mol Cell Endocrinol, 2012, 350, 256-265.
[5] Coutinho DC, Foureaux G, Rodrigues KD,etal. Cardiovascular effects of angiotensin A: A novel peptide of the renin-angiotensin system[J]. J Renin-Angio-Aldo S, 2013: 1160-1167.
[6] Wolf G, Wenzel UO. Angiotensin Ⅱ and cell cycle regulation[J]. Hypertension, 2004, 43, 693-698.
[7] Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin Ⅱ in vascular smooth muscle cells[J]. Pharmacol Rev, 2000, 52, 639-672.
[8] Fiandalo M, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis[J]. Exp Oncol, 2012, 34, 165-175.
[9] 梁先敏, 杨克敌. Caspase和JNK/SAPK, p38 MAPK与细胞凋亡[J]. 国外医学: 卫生学分册, 2008, 35, 5-10.
[10] Chou CT, He S, Jan CR,etal. Paroxetine-induced apoptosis in human osteosarcoma cells: Activation of p38 MAP kinase and caspase-3 pathways without involvement of Casup2sup sub sub elevation[J]. Toxicol Appl Pharm, 2007, 218, 265-273.
[11] Nguyen Dinh Cat A, Montezano AC, Burger D,etal. Angiotensin Ⅱ, NADPH oxidase, and redox signaling in the vasculature[J]. Antioxid Redox Sign, 2012, 52: 639-672.
[12] 魏燕, 辛晓燕. 活性氧调控的细胞凋亡信号[J]. 现代肿瘤医学, 2011, 19, 371-373.
[13] Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis[J]. Free Radical Bio Med, 2010, 48, 749-762.
Mechanism involved in damp-heat induced mice myocardial cell apoptosis
WANG Xiao-wu, YUAN Bin-bin, LIN Xi, WANG Xian-yue, DONG Wen-peng,YANG Yong-chao, ZHANG Wei-da*
( Centre of Cardiovascular Surgery, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou 510010, China)
ObjectiveTo explore the mechanism of damp-heat induced mice myocardial cell apoptosis.MethodsIn this study, mice was exposed to damp-heat (42 ℃, RH 65%) (H group) environment or room temperature (C group). The myocardial cell apoptosis rates in tissues were analyzed by TUNEL staining. The expression levels of Ang Ⅱ were detected by EIISA assay. Cardiomyocytes of rats with 1 day were pretreated with caspase-3 inhibitor Z-DEVD-FMK, or P38 MAPK inhibitor SB203580, for 24 h, followed by culturing with the indicated dose of Ang Ⅱ. AnnexinV-FITC was used to analyze cell apoptosis ratio. In addition, the expression of caspase-3 and P38 MAPK was assessed by Western blot.ResultsThe rates of cell apoptosis and Ang Ⅱ expression levels in H group were significantly higher than those in C group (Plt;0.05).Invitro, Ang Ⅱ dose-dependently induced cardiomyocytes apoptosis, accompany with the up-regulation of caspase-3 and P38 MAPK expression. When preconditioning with Z-DEVD-FMK, the apoptotic ratio of cardiomyocytes was significantly attenuated in Ang Ⅱ-treated group, implying that Ang Ⅱ triggered cell apoptosis in caspase-3-depedent manner. Additionally, pretreatment with SB203580 dramatically abrogated caspase-3 expression induced by Ang Ⅱ.ConclusionsDamp-heat environment induced cardiomyocytes apoptosis by Ang Ⅱ-activated P38 MAPK-caspase-3 pathway. Consequently, Ang Ⅱ may be a potential target for innovative strategies against cardiovascular diseases induced by Damp-heat environment.
damp-heat stress; angiotensin Ⅱ; apoptosis; P38 MAPK; caspase-3
2013-08-21
2013-12-23
*通信作者(correspondingauthor): weidazhanggz@163.com
1001-6325(2014)04-0531-05
研究论文
R 852.51
A
