浸渍法制备的Pd-MnO x/γ-Al2O3催化剂及不同载体对地表O3降解的影响
2014-10-18任成军周丽娜尚鸿燕陈耀强
任成军 周丽娜 尚鸿燕 陈耀强
(四川大学化学学院,教育部绿色化学重点实验室,成都 610064)
1 Introduction
The researchers have found that ground-level ozone would increase cardiovascular mortality and respiratory disease,decrease lung function.1Ozone is a powerful oxidant.The woody plants including arbor and shrubs had already suffered the harm of ambient ozone.2Karlsson et al.3investigated the negative impacts of ozone on crop yields and forest production.Moreover,ozone can deteriorate valuable materials.4According to OSHA(Occupational Safety and Health Administration)regulations,the threshold level for allowable exposure during an 8 h time period is 1.0×10-7(volume fraction,the same below).5The allowable concentration in the working environment is also 1.0×10-7in Japan.6In March 2012,State Environmental Protection Administration of China promulgated“ambient air quality standard”regulation(GB3095-2012)that ozone average concentration must be lower than 100-160 µg·m-3(4.6×10-8-7.5×10-8)in 8 h and it will be carried out in January 2016.Therefore,the catalytic decomposition of ozone is an important area of research from the point of view of environmental protection and health.7-11
Ground-level ozone was formed from nonlinear reactions between volatile organic compounds(VOCs)and nitrogen oxides(NOx)under ultraviolet light,12-14which are released from power plants and automobile exhaust gases.The numbers of vehicle are dramatically increased with rapid development of economy in recent years.Ozone gradually becomes one of the major air pollutions.Our group8-11focused on improving the activity of catalysts in early work.If the as-prepared catalysts were coated on vehicle radiators,where temperature ranged from 20 to 90°C,ozone would be completely decomposed.However,automobiles often run at high speed,and may jolt and rattle on the rough ground.The washcoat of catalysts could be fallen away.Therefore,it is necessary that the catalysts have not only excellent activity but also better viscosity.
The Al2O3is used as both support and binder due to its large surface area and better viscosity in this paper.MnOxis acted as active species owing to its highly activity.5,11,15,16Moreover,there is high relative humidity on the surface of water tank in automobiles.H2O molecules would compete with ozone for adsorption leading to decrease of MnOxactivity.Therefore,Pd is employed both as a resistant to water vapor and active species.11,16,17The γ-Al2O3support was prepared by peptizing method,and Mn(NO3)2and Pd(NO3)2were impregnated on the γ-Al2O3support,and then,the Pd-MnOx/γ-Al2O3was coated on the cordierite substrate to obtain Pd-MnOx/γ-Al2O3monolith catalysts.In addition,Mn(NO3)2and Pd(NO3)2were impregnated on SiO2,La-Al2O3,and Zr-Al2O3supports,respectively.The performance of catalysts for O3decomposition was investigated under high space velocities(380000,450000,510000,and 580000 h-1)and high relative humidity(RH=85%-90%).The prepared catalysts were characterized by X-ray diffraction(XRD),Brunauer-Emmett-Teller(BET),X-ray photoelectron spectroscopy(XPS),and temperature-programmed reduction(TPR)technologies.And the significant results were obtained.
2 Experimental
2.1 Preparation of catalysts
2.1.1 Supports
A support of γ-Al2O3was prepared by peptizing method.Firstly,concentrated nitric acid(A.R.)and water were added into an appropriate amount of boehmite(A.R.).Subsequently,a clear sol was formed after high-energy ball milling,and aged at 90°C for 6 h in water bath.Then,the precipitates were filtered via vacuum filtration,washed with distilled water,and dried at 110°C overnight.The dried precipitates were calcined at 600°C for 3 h.
La-Al2O3support calcined at 900°C was purchased from Rhodia Corporation.
SiO2support was prepared,for which the SiO2aqueous sol(A.R.)was heated in water bath until vapor was removed,and then,dried at 110°C overnight.The dried powders were calcined at 600°C for 3 h.
Zr-Al2O3support was prepared by co-precipitation method.ZrOCO3·6H2O(A.R.)was dissolved into concentrated nitric acid.Then,the aqueous solutions of ZrOCO3and Al(NO3)3(A.R.)were mixed in a mass ratio of 1:9(ZrO2to Al2O3),and adjusted to pH 8.8 by NH3·H2O(A.R.).The precipitates were filtered off,washed with distilled water,and dried at 110°C overnight.The dried precipitates were calcined at 600°C for 3 h.
2.1.2 Pd-MnOx/γ-Al2O3catalyst powders
MnOx/γ-Al2O3catalyst powders were prepared,for which Mn(NO3)2(A.R.)was impregnated on the γ-Al2O3support,and then,was heated in water bath until vapor was removed,and then dried at 110°C overnight.The dried powders were calcined at 400°C for 6 h.The amount of MnOxwas 8%(mass fraction).
Pd/γ-Al2O3catalyst powders were prepared,for which Pd(NO3)2(ChengDu Guangming Equipment Company,A.R.)was impregnated on the γ-Al2O3support,then was heated in water bath until vapor was removed,and then dried at 110°C overnight.The dried powders were calcined at 400°C for 3 h.The amount of Pd was 2%(mass fraction).
Catalyst(MP)was prepared.Firstly,Mn(NO3)2was impregnated on the γ-Al2O3support,then was heated in water bath until vapor was removed,and then dried at 110°C overnight.The dried powders were calcined at 400°C for 6 h.Subsequently,Pd(NO3)2was impregnated on the MnOx/γ-Al2O3support,then was heated in water bath until vapor was removed,and then dried at 110°C overnight.The dried powders were calcined at 400°C for 3 h.The amount of Pd was 2%(mass fraction),and the amount of MnOxwas 8%(mass fraction).
Catalyst(PM)was prepared.At first,Pd(NO3)2was impregnated on the γ-Al2O3support,then was heated in water bath until vapor was removed,and dried at 110°C overnight.The dried powders were calcined at 400°C for 3 h.Subsequently,Mn(NO3)2was impregnated on the Pd/γ-Al2O3support,then was heated in water bath until vapor was removed,and then dried at 110 °C overnight.The dried powders were calcined at 400 °C for 6 h.The amount of Pd was 2%(mass fraction),and the amount of MnOxwas 8%(mass fraction).
Catalyst(CPM)was prepared,which Mn(NO3)2and Pd(NO3)2were co-impregnated on the γ-Al2O3support,then was heated in water bath until vapor was removed,and then dried at 110°C overnight.The dried powders were calcined at 400°C for 6 h.The amount of Pd was 2%(mass fraction),and the amount of MnOxwas 8%(mass fraction).
2.1.3 Pd-MnOx/γ-Al2O3monolith catalyst
The above mentioned powders were ball-milled with distilled water to form slurry.The slurry was coated on cordierite substrate of 0.28 cm3(Coring Corporation in American,400 pore·inch-2,diameter(Φ)=5 mm,length(L)=14 mm)and the excess slurry was blown off.The catalyst was dried at 110°C overnight and calcined in air at 200°C for 3 h to prepare the Pd-MnOx/γ-Al2O3monolith catalyst.The loading of catalyst washcoat was 350 mg·mL-1.
When the support was SiO2,La-Al2O3,or Zr-Al2O3,the monolith catalysts were prepared by the above-mentioned methods,respectively.
2.2 Catalyst characterization
The XRD analysis was conducted on DX-1000 X-ray diffractometer,using Cu Kαradiation(λ=0.15406 nm)at 40 kV and 25 mA.The XRD data were recorded for 2θ values from 10°to 80°at an interval of 0.05(°)·s-1.The specific surface area and pore size of the catalysts were determined by N2adsorption-desorption at-196°C on a QUADRASORB SI,an automated surface area and pore size analyzer(Uuantachrome Instruments).Before the measurements,the samples were degassed in vacuum at 350°C for 1 h.X-ray photoelectron spectra of samples were acquired at room temperature using a Vacuum Generator Scientific XSAM800 system from Kratos Co.in U.K.The spectra were recorded with the Mg Kα(hν=1253.6 eV).X-ray radiation by setting the electron energy analyzer was operated at 180 W(12 kV,15 mA).Temperature programmed reduction of H2was carried out using an automated instrument.In a typical experiment,100 mg of sample was loaded in a U-shaped quartz micro-reactor.The sample was heated from room temperature to 550°C at a heating rate of 10 °C·min-1in a flowing hydrogen mixed gas of 5%(volume fraction)H2and 95%(volume fraction)N2at 30 cm3·min-1.Hydrogen consumption was monitored using a thermal conductivity detector.
2.3 Catalytic tests
The catalyst tests were carried out in a continuous flow tubular quartz reactor(inner diameter:10 mm)placed in a temperature-programmed furnace.The catalyst temperature was controlled by a thermocouple mounted internally.Ozone gases were generated from ozone generator(JY-3 type,Chengdu Qiangui Purification Equipment Company)and were fed from independent mass-flow controller.Air was come into being from air compressor,and then was separated by an oil-water separator and dried by a silica gel,its flow rate was controlled by a rotameter.Measurements over the samples were performed at a RH of 85%-90%using a gas hourly space velocity(GHSV)of 380000,450000,510000,and 580000 h-1,respectively.The feed consists of 6.0×10-7O3,and balance air.The outlet flow of the reactor was analyzed using an ozone analyzer(Nanjing 8Shang Technology Co.,Ltd.).The activity of the catalyst was calculated on the basis of the following equation:

where Cinletand Coutletare inlet concentration of O3and outlet concentration of O3,respectively.
3 Results and discussion
3.1 Activity of catalysts and synergetic effect between Pd and MnO x
As shown in Fig.1,initial O3conversions at 12°C are 53.3%,73.3%,and 30.0%,O3complete conversion temperatures are 56,38,and 70 °C,corresponding to MnOx/γ-Al2O3,Pd-MnOx/γ-Al2O3,and Pd/γ-Al2O3catalysts,respectively.The activity of the Pd/γ-Al2O3catalyst for decomposition of O3is poor,and the activity of MnOx/γ-Al2O3catalyst is better than Pd based catalyst.The performance of the Pd-MnOx/γ-Al2O3catalyst is the best,where both Pd and MnOxspecies are involved.Namely,there was some synergetic effect between Pd and MnOx.For the Pd-MnOx/γ-Al2O3catalyst,Pd is functionalized both as active spe-cies and resistant to humidity,which would suppress deactivation aroused by the adsorption of H2O molecules.16,17It benefits to O3molecules interacting with more MnOxactive species.Therefore,the Pd-MnOx/γ-Al2O3catalyst has an excellent activity under high relative humidity.

Fig.1 O3conversion as a function of reaction temperature on Pd/γ-Al2O3,MnO x/γ-Al2O3,and Pd-MnO x/γ-Al2O3catalysts
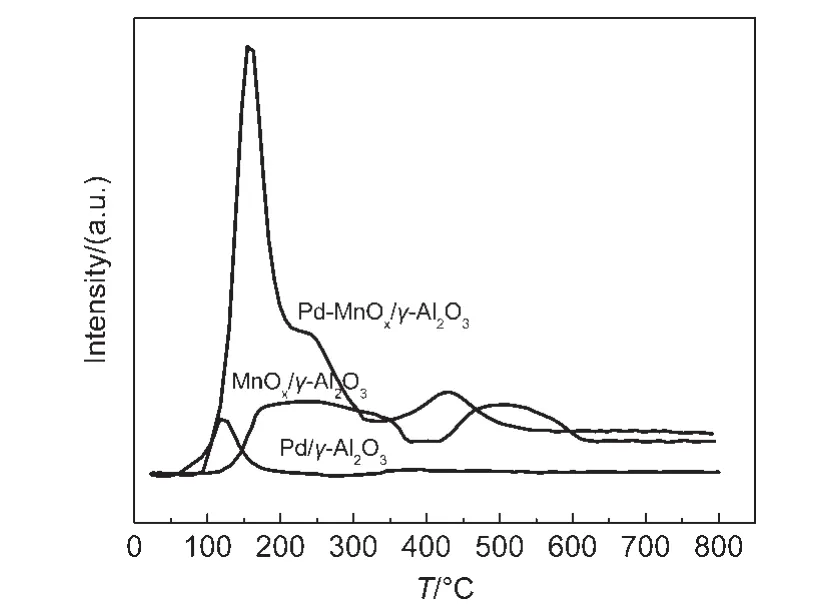
Fig.2 H2-TPR profiles of Pd/γ-Al2O3,MnO x/γ-Al2O3,and Pd-MnO x/γ-Al2O3catalysts
Fig.2 shows H2-TPR profiles of Pd/γ-Al2O3,MnOx/γ-Al2O3,and Pd-MnOx/γ-Al2O3catalysts.It is seen that a weak peak at 121 °C was present in the Pd/γ-Al2O3catalyst,it is attributed to the reduction of PdO species.18,19For the MnOx/γ-Al2O3catalyst,the broad peaks around 150-370 °C and 420-610 °C are observed.The broad peak around 150-370°C can be divided into two peaks at 181 and 258°C,which are ascribed as MnO2and Mn2O3reduction to Mn3O4.19The broad peak around 420-610 °C can be divided into two peaks at 475 and 538 °C,which represent Mn3O4reducing to MnO.19For the Pd-MnOx/γ-Al2O3catalyst,a peak at 155°C is attributed to the reduction of PdO species,18and a peak around 210°C is due to MnO2and Mn2O3reduction to Mn3O4,19and a peak at 430°C is ascribed to Mn3O4reduction to MnO.19The reduction temperature of MnOxis lowered in the presence of Pd,implying that there is an obvious interaction between Pd and MnOx.Xu et al.20reported that the presence of Pd lowered the reduction temperature of MnO2due to hydrogen spillover from Pd to the oxides.This phenomenon can be explained as follows:PdO is easily reduced to Pd by H2.Namely,PdO has changed to Pd before MnO2reduction.The Pd adsorbs H2and dissociates it into H,and then,H spills over onto MnO2to promote its reduction.The interaction of Pd and MnOximproves the reducibility of MnOx.Dhandapani and Oyama5thought that the activity of MnOxis relevant to its reducibility.Therefore,the activity of the Pd-MnOx/γ-Al2O3catalyst is enhanced for decomposition of O3.
3.2 Effect of impregnation orders of Pd and MnO xon performance of catalysts
3.2.1 TPR results of catalysts

Fig.3 H2-TPR profiles of the samples prepared by different impregnation orders of Pd and MnO x
Fig.3 displays the TPR profiles of the catalysts prepared by different impregnation orders of Pd and MnOx.For the catalyst co-impregnated Pd and MnOx,as mentioned in Fig.2,there are three reduction peaks around 155,210,and 430°C.A broad peak arround 60-330 °C and a peak at 426°C were observed in the catalyst impregnated MnOxand then impregnated Pd.The broad peak can be divided into two peaks at 153 and 218°C.For the catalyst impregnated Pd and then MnOx,three peaks at about 151,260,and 437°C were present,the peak at 260 °C shifted towards high temperature.Although there are slight difference of the reduction temperatures of Pd and MnOx,the reduction peak areas of Pd and MnOxare obviously different from the other two samples.The reduction peak area is the largest in the catalyst co-impregnated Pd and MnOx,indicating that the catalyst has the best reducibility.
3.2.2 Textural property of catalysts
As shown in Table 1,the surface areas are similar in three samples.However,the pore volume and average pore diameter of the catalyst co-impregnated Pd and MnOxare slightly larger than those of the catalysts impregnated Pd and MnOxsequentially.Large pore volume is favorable of more O3molecules activated by the active species on the surface of catalyst.Large pore diameter is in favor of O3coming into pores rapidly under high space velocity.Therefore,better textural property of the co-impregnated catalyst is beneficial to decomposition of O3.
3.2.3 Activity of catalysts
Fig.4 indicates the activity of catalysts impregnated Pd and MnOxby different impregnation orders.It is seen that initial O3conversions at 12°C are 73.3%,69.7%,and 66.7%,complete conversion temperatures of O3are 38,46,and 48°C,corresponding to the catalyst co-impregnated Pd and MnOx,the catalyst impregnated MnOxand then Pd,and the catalyst impregnated Pd and then MnOx,respectively.The impregnation order has some impacts on the activity of catalysts,the activity of the co-impregnated catalyst is the highest in the three samples.For the co-impregnated catalyst,Mn4+and Pd2+were uniformly mixed in ionic form before impregnation,in favorable of MnOxand Pd dispersion evenly onto the surface of γ-Al2O3support.It is possible that more active species were exposed on the surface of the co-impregnated catalyst.In general,the more the active species exposed(such as Pd or MnOx),the better the activity of the catalyst.Moreover,when Pd or MnOxwas loaded on the surface of γ-Al2O3support,the negative charges of Al2O3would be transferred into PdO or MnOxdue to strong interaction of metal and support.For the co-impregnated catalyst,the interaction could be more obvious,resulting in better reducibility of PdO and MnOx,which is helpful to O3decomposition.In addition,the activity of the catalyst impregnated MnOxand then Pd is slightly better than that of the catalyst impregnated Pd and then MnOx.It can be ascribed to good reducibility of MnOxin the catalyst(see Fig.3,H2-TPR).Furtherover,a little Pd was possibly covered by MnOxon the surface of the catalyst impregnated Pd and then MnOx,the amount of Pd exposed decreased more or less.

Table 1 Textural property of the samples prepared by different impregnation orders of Pd and MnO x

Fig.4 O3catalytic performance of samples prepared by different impregnation orders of Pd and MnO x
3.3 Effect of supports on the performance of the catalyst co-impregnated Pd and MnO x
3.3.1 Activity of catalysts
Fig.5 reveals the activity of the catalyst co-impregnated Pd and MnOxon various supports under different space velocities,respectively.For space velocity of 380000 h-1,O3conversions,at 14°C are 82%,82%,77%,and 68%,O3complete conversion temperatures are 36,36,38,and 48°C corresponding to La-Al2O3,SiO2,γ-Al2O3,and Zr-Al2O3supports,respectively.For space velocity of 450000 h-1,O3conversions at 14°C are 72%,68%,64%,and 58%,O3complete conversion temperatures are 50,56,59,and 65 °C corresponding to La-Al2O3,SiO2,γ-Al2O3,and Zr-Al2O3supports,respectively.For space velocity of 510000 h-1,O3conversions at 14°C are 60%,52%,50%,and 40%,O3complete conversion temperatures are 66,78,80,and 88 °C corresponding to La-Al2O3,SiO2,γ-Al2O3,and Zr-Al2O3supports,respectively.For space velocity of 580000 h-1,O3conversions at 14°C are 43%,34%,36%,and 28%,O3complete conversion temperatures are 86,100,94,and 112°C corresponding to La-Al2O3,SiO2,γ-Al2O3,and Zr-Al2O3supports,respectively.It can be seen that the Pd-MnOx/La-Al2O3catalyst has the best activity;the Pd-MnOx/SiO2catalyst has better activity when the space velocity varied from 380000 to 510000 h-1,however,its activity is slightly weaker than that of the Pd-MnOx/γ-Al2O3catalyst at the space velocity of 580000 h-1;the activity of the Pd-MnOx/Zr-Al2O3catalyst is the worst in all the catalysts.
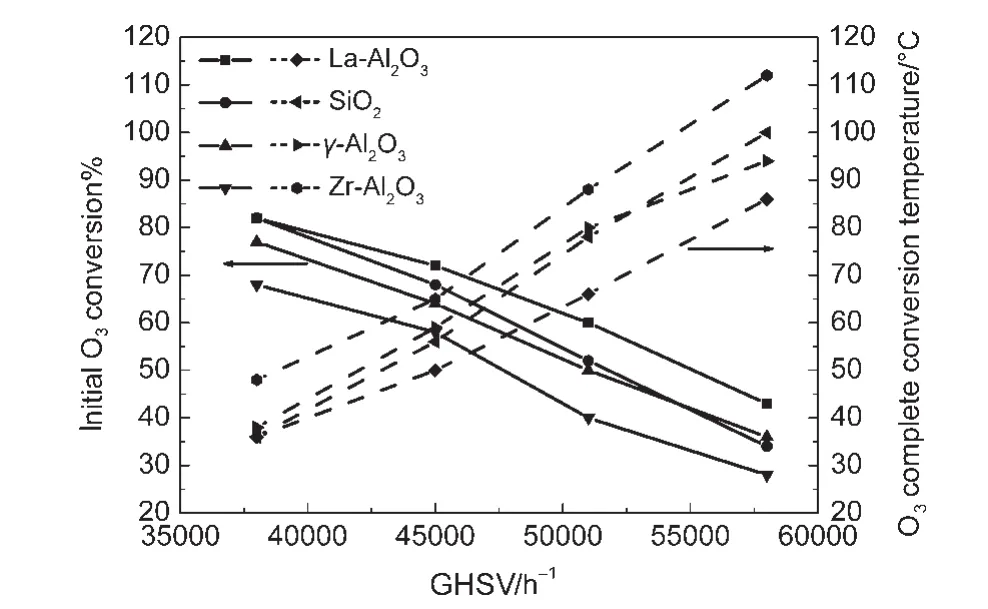
Fig.5 Effect of supports on the catalytic performance of samples co-impregnated Pd and MnO xunder different space velocities
3.3.2 XRD characterization of catalysts
Fig.6 shows XRD patterns of samples co-impregnated Pd and MnOxon different supports.The diffraction peaks of supports were observed in XRD patterns for all of the samples.For example,SiO2was observed in the Pd-MnOx/SiO2catalyst.The γ-Al2O3was present in the Al2O3-based catalysts.The weak diffraction peaks of MnO2were appeared,indicating that MnO2was in the presence of microcrystalline due to low MnO2content and high dispersion on the surface of supports.In addition,a weak diffraction peak of PdO was also emerged,implying that PdO was evenly dispersed on the surface of supports,and was a microcrystalline.
3.3.3 Textural property of catalysts
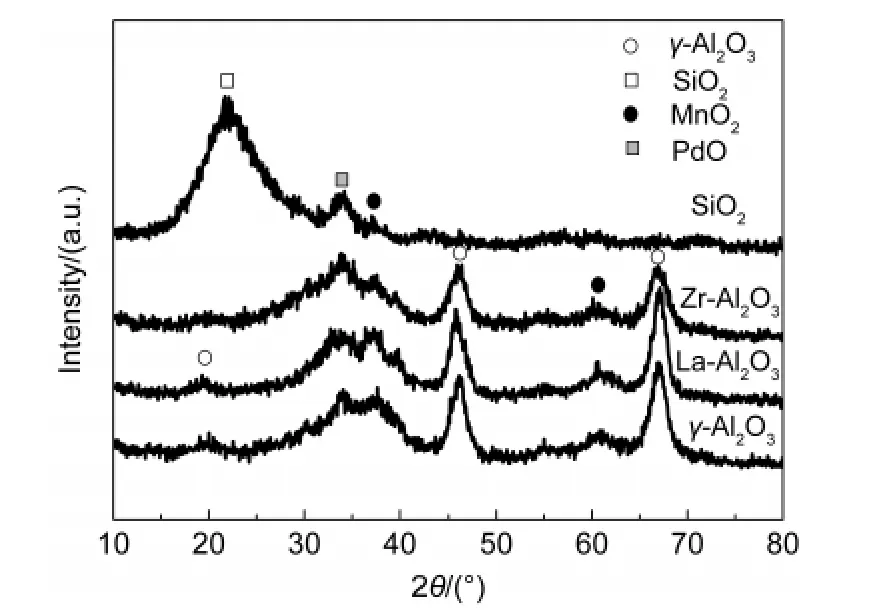
Fig.6 XRD patterns of samples co-impregnated Pd and MnO xon different supports

Table 2 Textural properties of the samples co-impregnated Pd and MnO xon different supports
As shown in Table 2,the surface areas of the Pd-MnO2/SiO2,Pd-MnO2/γ-Al2O3,and Pd-MnO2/Zr-Al2O3are large,whereas the surface area of the Pd-MnO2/La-Al2O3is the least.Total pore volume is almost the same for the Pd-MnO2/γ-Al2O3,Pd-MnO2/La-Al2O3,and Pd-MnO2/Zr-Al2O3,while pore volume of the Pd-MnO2/SiO2is the least.The average pore diameter of the Pd-MnO2/La-Al2O3is the largest,in favor of O3mass transfer under high space velocity.The average pore diameter of the Pd-MnO2/SiO2catalyst is the least.The textural property of the Pd-MnO2/SiO2catalyst is related to its support,which has large surface area and small average pore diameter.21,22Large surface area is in favor of O3adsorption on the SiO2support.However,its small pore volume and small average pore diameter make against the mass transfer of O3molecules,especially,under high space velocity.Therefore,the activity of the Pd-MnO2/SiO2catalyst sharply declined with the increase of space velocity.
3.3.4 XPS of catalysts
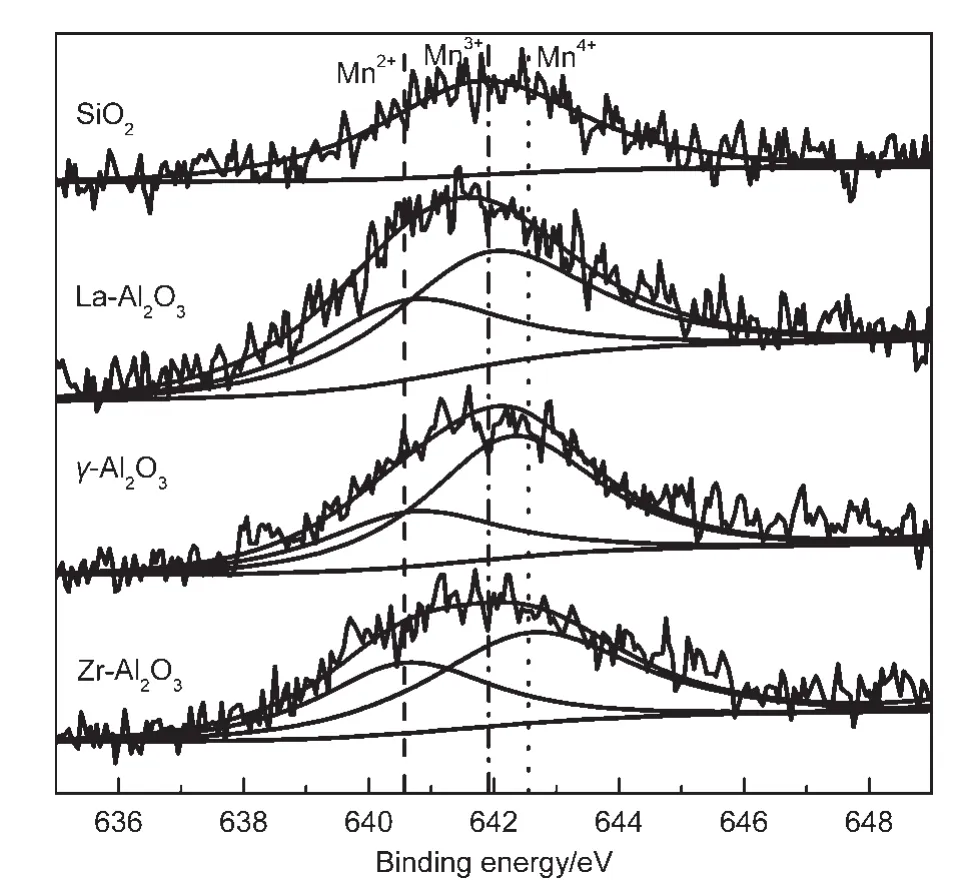
Fig.7 Mn 2p3/2XPS spectra for the samples co-impregnated Pd and MnO xon different supports

Table 3 XPS results of Mn 2p3/2for the samples co-impregnated Pd and MnO xon different supports
Fig.7 shows Mn 2p3/2XPS spectra for the samples co-impregnated Pd and MnOxon different supports.According to literature,23,24the binding energies of Mn 2p3/2were 640.8,641.8,and 642.6 eV for Mn(II),Mn(III),and Mn(IV),respectively.24,25As shown in Fig.7,valence state of Mn is+3 on the surface of the Pd-MnOx/SiO2catalyst.Although XRD pattern(Fig.6)shows MnO2microcrystalline,it is possible that negative charge in SiO2support was transferred into Mn4+due to strong interaction between MnOxand SiO2support,resulting in the formation of Mn2O3on the surface of the catalyst.Valence states of Mn are+2 and+3 on the surface of the Pd-MnOx/La-Al2O3catalyst.It indicates that MnOxis in the presence of MnO and Mn2O3on the surface of the catalyst,and there is a strong interaction between MnOxand La-Al2O3support.Valence states of Mn are+2 and+4 on the surfaces of the Pd-MnOx/γ-Al2O3catalyst and the Pd-MnOx/Zr-Al2O3catalyst.Namely,MnO,Mn3O4,or Mn5O8formed by Mn4+got electrons from the support(γ-Al2O3or Zr-Al2O3)on the surface of the catalysts.The relative amount of Mn2+,Mn3+,and Mn4+species on the surface of samples is listed in Table 3.
3.3.5 TPR of catalysts
Fig.8 exhibits H2-TPR profiles of samples co-impregnated Pd and MnOxon different supports.It can be seen that support obviously affects the reducibility of PdO and MnOx.For the Pd-MnOx/SiO2catalyst,a large reduction peak around 66-160°C can be divided into two peaks at 102 and 132°C,which represent the reduction of PdO and MnOx,respectively.The reduction peak of MnOxobviously shifted towards low temperature due to strong interaction between MnOxand SiO2support,in agreement with the result of XPS analysis.Namely,MnOxspecies is highly active and is easily reduced for the Pd-MnOx/SiO2catalyst.For the Pd-MnOx/La-Al2O3catalyst,a large reduction peak around 58-200°C can be divided into two peaks at 116 and 145°C,which are ascribed to the reduction of PdO and MnOx,respectively.18The reduction temperatures of PdO and MnOxare low for Pd-MnOx/SiO2and Pd-MnOx/La-Al2O3,implying high reducibility and better activity of PdO and MnOx.The peak at 155°C is due to the reduction of PdO,18the peak at 210°C is ascribed as MnO2reduction to Mn3O4,the peak at 430°C belongs to Mn3O4reducing to MnO.19The reduction peak areas of PdO and MnOxare large for the Pd-MnOx/γ-Al2O3catalyst,leading to better catalytic performance for O3decomposition.A peak at 120°C is attributed to the reduction of PdO,small peaks around 180 and 260°C are ascribed to the reduction of MnOxfor the Pd-MnOx/Zr-Al2O3catalyst.The catalyst has less activity,which is related to poor reducibility of MnOx.
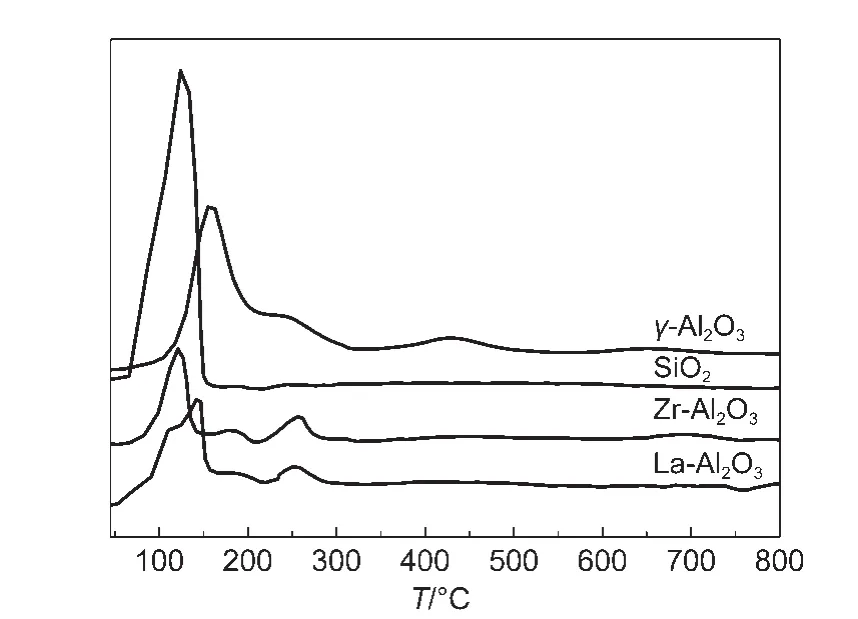
Fig.8 H2-TPR profiles of samples co-impregnated Pd and MnO xon different supports
Lin et al.26supposed an interaction of metal with catalyst support,and adsorption ability of support affected the activity of catalyst.According to the result of H2-TPR,MnOxhas high reducibility due to strong interaction between MnOxand SiO2support,in favor of Mnn+participating in O3decomposition.27SiO2is a good adsorbent for O3,and H2O molecules were weakly adsorbed on the surface of SiO2,hardly compete with O3for adsorption under high humidity.26Moreover,mild acidic SiO2support promotes the formation of oxygen species intermediates(e.g.,O3-and O-),which would enhance the adsorption and decomposition of O3.28These factors are in favor of O3decomposition.Therefore,the Pd-MnOx/SiO2catalyst has excellent catalytic activity.However,when the space velocity increased a lot,the activity of the Pd-MnOx/SiO2catalyst was drastically declined due to small pore diameter going against mass transfer of O3molecules and its products.For the Pd-MnOx/La-Al2O3catalyst,both high reducibility of PdO and MnOxand large average pore diameter are in favor of O3decomposition under high space velocity.Therefore,the catalyst shows the best catalytic performance.
3.4 Durability of catalyst
O3(6.0 ×10-7)was continuously decomposed at 90°C for 10 days on the Pd-MnOx/γ-Al2O3catalyst under space velocity of 580000 h-1and RH of 85%-90%.The activity of the catalyst hold steady,and the washcoat of catalyst did not desquamate,which are related to stable physicochemical properties of the γ-Al2O3support,active species of Pd and MnOx,and better viscidity of the support.
4 Conclusions
When active Pd and MnOxcoexist in the Pd-MnOx/γ-Al2O3catalyst,its activity is higher than that of the Pd or MnOxcatalyst(e.g.,Pd/γ-Al2O3or MnOx/γ-Al2O3).The catalyst co-impregnated Pd and MnOxhas better activity than the catalyst impregnated Pd or MnOxsequentially.The supports have significant impacts on catalytic activity for O3decomposition.The Pd-MnOx/La-Al2O3catalyst has the best activity.Next is the catalyst using SiO2as a support.Again is the catalyst using γ-Al2O3as a support.Finally,the Pd-MnOx/Zr-Al2O3sample has the worst catalytic performance in all of the catalysts.The activity of catalysts prepared on different supports is nearly in agreement with the reducibility of Pd and MnOx.Ground-level ozone would be completely decomposed if the Pd-MnOx/La-Al2O3catalyst was coated on vehicle radiators,in which their temperature ranged from 20 to 90°C.Therefore,the as-prepared catalyst has a potential applicable value.
(1)Gryparis,A.;Forsberg,B.;Katsouyanni,K.;Analitis,A.;Touloumi,G.;Schwartz,J.;Samoli,E.;Medina,S.;Anderson,H.R.;Niciu,E.M.American Journal of Respiratory and Critical Care Medicine 2004,170,1080.doi:10.1164/rccm.200403-333OC
(2)Wan,W.X.;Xia,Y.J.;Zhang,H.X.;Wang,J.;Wang,X.K.Acta Ecologica Sinica 2013,33(4),1098. [万五星,夏亚军,张红星,王 娇,王效科.生态学报,2013,33(4),1098.]doi:10.5846/stxb
(3)Karlsson,P.E.;Pleijel,H.;Belhaj,M.;Danielsson,H.;Dahlin,B.;Andersson,M.;Haneeon,M.;Munthe,J.;Grennfelt,P.AMBIO 2005,34,32.
(5)Dhandapani,B.;Oyama,S.T.Appl.Catal.B 1997,11,129.doi:10.1016/S0926-3373(96)00044-6
(6)Japan Air Cleaning Association.Air Cleaning Handbook;Ohm Press:Tokyo,1981;p178
(7)Zhang,B.;Shi,R.;Zhang,P.Y.;Xu,J.H.Rare Metal Mat.Eng.2010,39(4),692.[张 博,史 蕊,张彭义,徐九华. 稀有金属材料与工程,2010,39(4),692.]
(8)Zhang,B.;Zhang,P.Y.;Shi,R.;Wang,H.J.Chin.J.Catal.2009,30(3),235.[张 博,张彭义,史 蕊,王化军.催化学报,2009,30(3),235.]
(9)Yu,Q.W.;Zhao,M.;Liu,Z.M.;Zhang,X.Y.;Zheng,L.M.;Chen,Y.Q.;Gong,M.C.Chin.J.Catal.2009,30(1),1.[余全伟,赵 明,刘志敏,张晓玉,郑灵敏,陈耀强,龚茂初.催化学报,2009,30(1),1.]doi:10.1016/S1872-2067(08)60082-0
(10)Pan,H.;Zhou,L.N.;Zhu,Y.;Peng,N.;Gong,M.C.;Chen,Y.Q.Chin.J.Catal.2011,32(6),1040.[潘 浩,周丽娜,朱艺,彭 娜,龚茂初,陈耀强.催化学报,2011,32(6),1040.]
(11)Zhou,L.N.;Chen,Y.Q.;Ren,C.J.;Gong,M.C.Chin.J.Inorg.Chem.2013,29(11),2363.[周丽娜,陈耀强,任成军,龚茂初.无机化学学报,2013,29(11),2363.]
(12)Thompson,A.M.Science 1992,256,1157.doi:10.1126/science.256.5060.1157
(13)Russell,A.;Milford.J.;Bergin,M.S.;McBride,S.;McNair,L.;Yang,Y.;Stockwell,W.R.;Croes,B.Science 1995,269,491.doi:10.1126/science.269.5223.491
(14)Yu,L.P.;Jia,J.J.J.Shandong Univ.Sci.Technol.Nat.Sci.2001,20(4),111.[于林平,贾建军.山东科技大学学报(自然科学版),2001,20(4),111.]
(15)Sadao Terui,H.;Yoshiyuki Yokota,S.Catalyst and Method of Preparing the Catalyst.US Patent,5187137,1993-02-16.
(16)Kameya,T.;Urano,K.J.Environ.Eng.2002,128,286.doi:10.1061/(ASCE)0733-9372(2002)128:3(286)
(17)Wu,M.C.;Kelly,N.A.Appl.Catal.B 1998,18,93.doi:10.1016/S0926-3373(98)00028-9
(18)Yao,Y.L.;Fang,R.M.;Shi,Z.H.;Gong,M.C.;Chen,Y.Q.Chin.J.Catal.2011,32(4),589.[姚艳玲,方瑞梅,史忠华,龚茂初,陈耀强.催化学报,2011,32(4),589.]
(19)Rezaei,E.;Soltan,J.;Chen,N.;Lin,J.R.Chem.Eng.J.2013,214,219.doi:10.1016/j.cej.2012.10.044
(20)Xu,G.P.;Zhu,Y.X.;Ma,J.;Yan,H.J.;Xie,Y.C.Stud.Surf.Sci.Catal.1997,11,333.
(21)Ren,C.J.;Qiu,W.;Chen,Y.Q.Sep.Purif.Technol.2013,107,264.doi:10.1016/j.seppur.2013.01.037
(22)Qiu,W.;Ren,C.J.;Gong,M.C.;Hou,Y.Z.;Chen,Y.Q.Acta Phys.-Chim.Sin.2011,27,1487.[仇 伟,任成军,龚茂初,侯云泽,陈耀强.物理化学学报,2011,27,1487.]doi:10.3866/PKU.WHXB20110621
(23)Santos,V.P.;Pereira,M.F.R.;Órfaˇo,J.J.M.;Figueiredo,J.L.Appl.Catal.B 2010,99,353.doi:10.1016/j.apcatb.2010.07.007
(24)Wei,Y.J.;Yan,L.Y.;Wang,C.Z.;Xu,X.G.;Wu,F.;Chen,G.J.Phys.Chem.B 2004,108,18547.doi:10.1021/jp0479522
(25)O′Shea,V.A.D.P.;Álvarez-Galván,M.C.;Fierro,J.L.G.;Arias,P.L.Appl.Catal.B 2005,57,191.doi:10.1016/j.apcatb.2004.11.001
(26)Lin,J.J.;Kawai,A.;Nakajima,T.Appl.Catal.B 2002,39,157.doi:10.1016/S0926-3373(02)00081-4
(27)Einaga,H.;Harada,M.;Futamura,S.Chem.Phys.Lett.2005,408,377.doi:10.1016/j.cplett.2005.04.061
(28)Kumar,N.;Konova,P.;Naydenov,A.;Salmi,T.;Murzin,D.Y.;Heikillä,T.;Lehto,V.P.Catal.Today 2007,119,342.doi:10.1016/j.cattod.2006.08.048
