石墨烯氧化程度对Ni(OH)2赝电容性能的影响
2014-10-18贺园园张晋江赵健伟
贺园园 张晋江 赵健伟
(南京大学化学化工学院,生命分析化学国家重点实验室,南京 210008)
1 Introduction
The energy of chemical battery is stored by Faradic charge transfer,which is produced by electrochemical reaction.Chemical battery has many shortcomings,for example,short service life,narrow temperature range,and environmental pollution.1Compared with charge storage capacity of conventional capacitors and rechargeable chemical battery,supercapacitor is higher.It has many characteristics such as high rate and efficiency of charge/discharge,no pollution,long service life,wide temperature range,and high security.2According to the different energy storage mechanisms,supercapacitor can be divided into three categories:(1)electrochemical double-layer capacitors(EDLCs),energy storage by adsorbing anions and cations;(2)pseudocapacitor,energy storage through the rapid surface redox reaction;(3)asymmetric supercapacitor.3
Pseudocapacitor is also known as Faradic supercapacitor.The energy storage is completed by an innovative redox-mediated strategy.The quick reversible redox reaction aroused by redox mediator can efficiently enhance the ionic conductivity and pseudocapacitance.4,5Various materials such as transition metal oxides,metal hydroxides,and polymeric materials have been explored for pseudocapacitor application.6-8Among them,nickel hydroxide is a promising candidate for pseudocapacitors owing to well-defined electrochemical redox activity,high specific capacitance,low cost,and availability of various morphologies.9Yang et al.10reported a electrodeposited α-Ni(OH)2film with a porous-wrinkle structure and ultrahigh capacitance.
Ni(OH)2/graphene has attracted extensive interest as a pseudocapacitor material over the past decade.The stable electrochemical property and large surface area of Ni(OH)2enable it to form layered structure with large interlayer spacing.11-17Oxidation degree of graphene substrates would affect the pseudocapacitance and rate capability of composite materials.18Accordingly,a better conductive system built with well dispersed Ni(OH)2particles and highly conducting graphene sheets can significantly improve supercapacitive behavior.Many researchers19-21considered the large amount of oxygen-containing functional groups on reduced graphene oxide sheet(rGNOs)such as hydroxyl,carbonyl,epoxyl and lactone groups might be attributed to the pseudocapacitance of many metal materials.
However,the reason why oxygen-containing functional groups on rGNOs can improve the characteristics of Ni(OH)2as pseudocapacitor is not yet clear,so we aim to investigate the interaction between oxided defects on rGNOs and Ni(OH)2in atomic scale using density functional theory(DFT)method and analyze the influence of the reduction degree of rGNOs on Ni(OH)2when it is applied as pseudocapacitor material.On the other hand,in order to testify the theoretical results,we also develop a new method to deposite Ni(OH)2on rGNOs substrate and characterize it with modern analytical techniques such as cyclic voltammetry(CV)and electrochemical impedance spectroscopy(EIS).
2 Computational method
Fig.1 shows the models of graphene sheet(GS)and rGNOs with different oxidation degrees.The carbonyl,epoxyl,and hydroxyl groups are anchored on the adjacent C atoms of rGNOs,respectively,in Fig.1(b,c,d).All these geometric structures were optimized at B3LYP level with 6-31G*basic set except Ni at LANL2DZ using the Gaussian 03 software.23,24
3 Experimental
Graphite(Sinopharm Chemicals Reagent Co.,Ltd.,China)was used as received.Na2SO4,Ni(NO3)2,KOH,and H2SO4(95%)(Sinopharm Chemicals Reagent Co.,Ltd.,China)were all of analytical grade purity.Doubly distilled water was used throughout the experiments.Scheme 1 shows the preparation flow of Ni(OH)2nanoparticles/rGNOs.Graphene oxide(GO)was synthesized through a modified Hummersʹmethod.25Graphene oxide sheets(GNOs)were attained by ultrasonically dispersing 50 mg graphitic oxide in 100 mL deionized water for 2 h.GNOs modified electrode(2 cm2in area)was prepared by soaking nickel foam in 0.05%(w)GNOs solution for 4 h through a simple self-assemble process.26-28
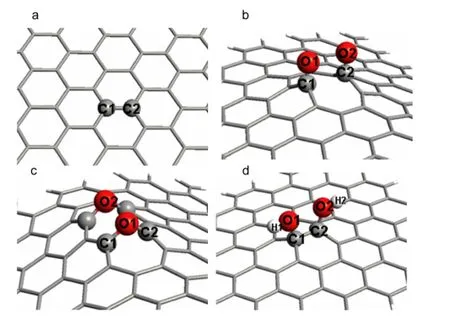
Fig.1 Models of(a)GS,(b)rGNOs with two carbonyl groups,(c)rGNOs with two epoxyl group,and(d)rGNOs with two hydroxyl groups

Scheme 1 Preparation flow of Ni(OH)2nanoparticles/rGNOs
All electrochemical measurements were carried out in a three-electrode experimental setup.Platinum wire electrode and saturated calomel electrode(SCE)were used as the counter and reference electrodes,respectively.All electrochemical measurements were carried out in 3%(w)KOH aqueous electrolyte by using CHI660B(Shanghai Chenhua Apparatus,China)and PGSTAT30(Autolab,EchoChemie,Netherlands)electrochemical workstations.The mass of the deposited Ni(OH)2was measured from the mass difference before and after electrochemical deposition by means of a micro-balance(Sartorius BT125D)with an accuracy of 0.01 mg.The morphology of the sample was observed by a scanning electron microscope Model S-4800(SEM,Hitachi,Japan)and a high-resolution transmission electron microscope Model JEM-2100(HRTEM,Jeol,Japan).
4 Results and discussion
The optimized geometry is shown in Fig.2a.We can see that Ni(OH)2bridges the gap between two adjacent C atoms and the GS remains planar conformation.The distance between Ni(OH)2and the GS surface is 0.248 nm.The adsorption energy is 12.57 kJ·mol-1.From the structure and the small adsorption energy we can predict that there is little chemical adsorption between Ni(OH)2and GS,and GS will have little effect on the electronic properties of Ni(OH)2.
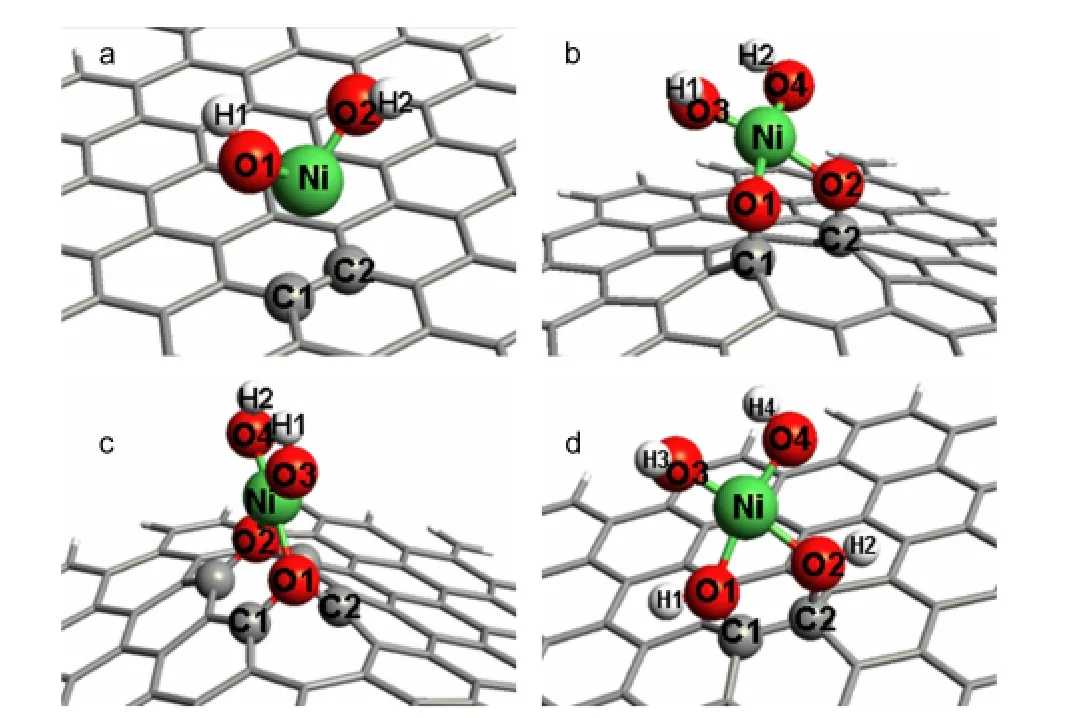
Fig.2 Geometric structures of(a)GS,(b)rGNOs with two carbonyl groups,(c)rGNOs with two epoxyl groups,and(d)rGNOs with two hydroxyl groups after absorbing Ni(OH)2optimized at 6-31G*level except Ni at LANL2DZ,via DFT method
When two carbonyl groups are anchored on the adjacent C atoms in rGNOs,Ni(OH)2bridges the gap between the two adjacent O atoms of the carbonyl groups(Fig.2b).Variations of the atomic distances for rGNOs with two carbonyl groups before and after adsorbing Ni(OH)2are listed in Table 1.The Ni―O and C―O bonds become longer and the distance between Ni(OH)2and the carbonyl group on rGNOs with two carbonyl groups(Ni-O1)is 0.197 nm.It means that Ni(OH)2is closer to rGNOs with two carbonyl groups compared with GS.The adsorption energy is-131.02 kJ·mol-1,which indicates that the adsorption between Ni(OH)2and rGNOs with two carbonyl groups is very strong under the influence of complexation interaction between the Ni atom in Ni(OH)2and O atom in carbonyl group.
When the two carbonyl groups are reduced to be an epoxyl group,the distance between the Ni atom in Ni(OH)2and the O1 atom in epoxyl group is 0.205 nm,a littler longer than that in rGNOs with two carbonyl groups(Fig.2c).The adsorption energy is-131.03 kJ·mol-1.It shows that the complexation interaction exists between the Ni atom in Ni(OH)2and O atom in epoxy group,although it is weaker than that between Ni(OH)2and rGNOs with two carbonyl groups.
But when the epoxyl group is reduced to two hydroxyl groups,the Ni―O and C―O bonds become longer and the distance between the Ni atom in Ni(OH)2and O atom in one of the hydroxyl groups changes to be 0.197 nm that is equivalent to that in rGNOs with an carbonyl group(Fig.2d).The adsorption energy has dropped to be-131.11 kJ·mol-1and it is the lowest value in the systems containing rGNOs with different extents of oxidation.We can get the conclusion that the complexation interaction between Ni(OH)2and oxidation defects on rGNOs becomes stronger whenthe reduction degree of the oxidation defects on rGNOs increases.The electron transfer between Ni(OH)2and rGNOs is promoted by the strong complexation interaction.Hence the interfacial resistance of Ni(OH)2/rGNOs decreases and Ni(OH)2shows more pseudocapacitance characteristics.
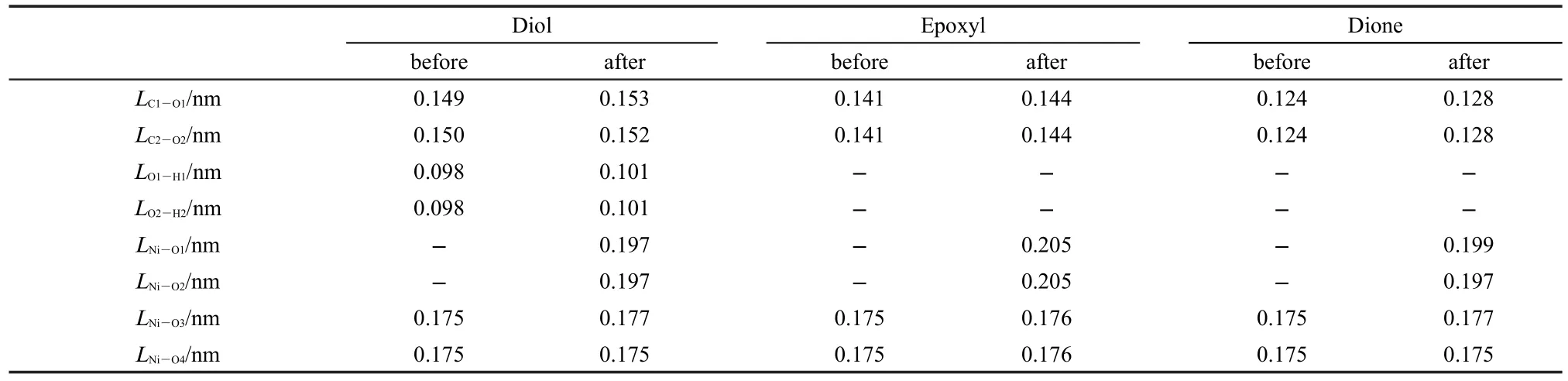
Table 1 Variations of the atomic distances for rGNOs before and after adsorbing Ni(OH)2
We further analyze the charge distribution over GS and rGNOs before and after adsorbing Ni(OH)2.Charge distribution over GS changes little after adsorbing Ni(OH)2.The net charge of Ni(OH)2is-0.205e,which means that electrons transfer from Ni(OH)2to graphene are not effective.Then we list the charge variations on every atom in rGNOs and Ni(OH)2in Table 2 in detail.When Ni(OH)2is adsorbed with the two carbonyl or epoxy groups on rGNOs,the net charges of Ni(OH)2are-0.380e and-0.388e,respectively.However,when Ni(OH)2is adsorbed with the two hydroxyl groups on rGNOs,the positive charge of C atoms in rGNOs increases and the net charge on Ni(OH)2is-0.462e.Electrons transfer from rGNOs substrate to Ni(OH)2through hydroxyl groups,which makes Ni(OH)2negative and even increases the oxidation activity of Ni(OH)2.
The quantum chemical calculations above indicate that Ni(OH)2takes negative charges when it is adsorbed on rGNOs.The asymmetric electron transfer pathway from rGNOs to Ni(OH)2promotes the rectifying effect of Ni(OH)2.The negative charge on Ni(OH)2makes it easier for Ni(OH)2to be oxidized to NiOOH with higher reactivity by improving the efficiency of electron transfer.The discharge potential of Ni(OH)2/rGNOs will shift positive and the charge/discharge rate tends to be consistent.As a result,the oxygen-containing groups on rGNOs help to enhance the pseudocapacitance characteristic of Ni(OH)2.
In order to verify this mechanism supposed above,we study the morphology of Ni(OH)2particles electrochemically deposited on rGNOs with different reduction degrees(Fig.3).Ni(OH)2particles are deposited at-0.7 V in 0.1 mol·L-1Ni(NO3)2solution for 1000 s with potentiostatic method on rGNOs that have been reduced in 0.5 mol·L-1Na2SO4solution at-0.9 V for 3000 and 6000 s,respectively,in advance.When rGNOs are electrochemically reduced for different time,functional group densities on surface of them also change.Morphology of Ni(OH)2deposited on GNOs is greatly different from that deposited on rGNOs.Ni(OH)2particles with larger diameter on GNOs are readily aggregated.In contrast,the Ni(OH)2particles with smaller diameter are homogeneously dispersed on surface of rGNOs.Furthermore,the Ni(OH)2particles have a smaller diameter and more uniform dispersion when the reduction time of rGNOs becomes longer.
Fig.4(a,c,e)shows the SEM,TEM,and HRTEM images of Ni(OH)2electrochemically deposited on bare nickel foam in 0.1 mol·L-1Ni(NO3)2solution at-0.7 V for 1000 s.They depict the disorderly aggregated morphology of Ni(OH)2that is loose and porous.Fig.4(b,d,f)shows the SEM,TEM,and HRTEM images of Ni(OH)2electrochemically deposited on rGNOs that have been reduced for 6000 s at the same condition as above.It can be observed that the plate-like Ni(OH)2particles are densely anchored on the surface of rGNOs.More-over,Ni(OH)2particles deposited on rGNOs have smaller diameter and their distribution is more uniform than that deposited on graphene.29They provide larger specific surface area and higher capacity utilization.
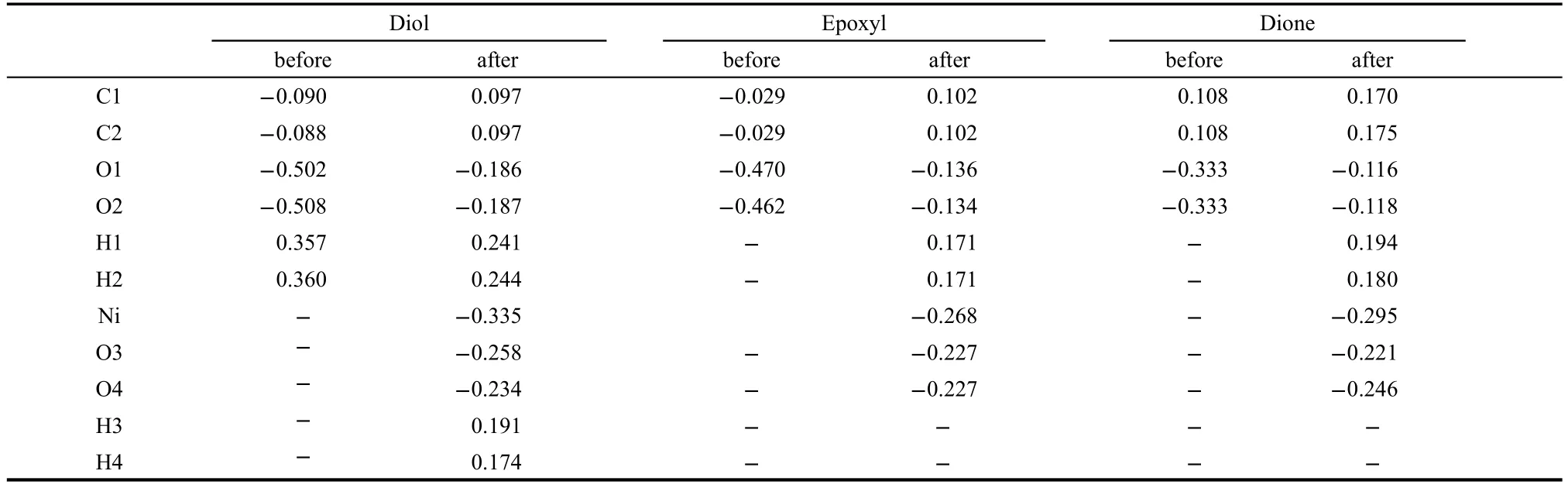
Table 2 Charge changes(e)of rGNOs after adsorbing Ni(OH)2

Fig.3 Transmission electron microscope(TEM)images of Ni(OH)2particles electrodeposited on GNOs(a),rGNOs electrochemical reduced for 3000 s(b),and 6000s(c)

Fig.4 Scanning electron microscope(SEM)(a),TEM(c),high-resolution transmission electron microscope(HRTEM)(e)images of Ni(OH)2clusters deposited on bare nickel foam and SEM(b),TEM(d),HRTEM(f)images of Ni(OH)2nanoparticles deposited on rGNOs surface
The electrodeposition process of Ni(OH)2film can be expressed as follows:30,31
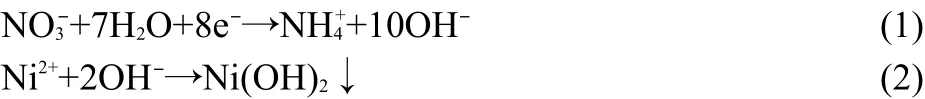
When rGNOs are applied as electrochemical deposition substrates,the Ni2+ions are absorbed on rGNOs surface and probably combine with the oxidation defects on rGNOs under the influence of strong chemical adsorption.Ni(OH)2particles are slowly formed on rGNOs during electrochemical reduction.The strong adsorption between Ni(OH)2and oxidation defects on rGNOs makes Ni(OH)2particles be anchored on rGNOs firmly.
For exploring its potential applications,Ni(OH)2/rGNOs composite is used as the electroactive material for pseudocapacitor electrode and characterized by cyclic voltammetry(CV).Fig.5 shows the CV curves of Ni(OH)2composited on GS and rGNOs reduced in 0.5 mol·L-1Na2SO4solution at-0.9 V for 3000 and 6000 s,respectively,in advance.The potential window is 0-0.55 V32and the scan rate is 10 mV·s-1in 3%(w)KOH solution.The anodic peak is due to the oxidation of Ni(OH)2to NiOOH,and the cathodic peak is for the reverse process.Oxidation peaks of Ni(OH)2/GS and Ni(OH)2/rGNOs are both observed from 0.30 V.When the reduction time increases,the reduction degree of rGNOs also increases.The redox peaks of Ni(OH)2/rGNOs reduced for 6000 s are smoother and more rectangular shaped than that reduced for 3000 s,which illustrates the lower contact resistance of Ni(OH)2/rGNOs that have been reduced for 6000 s.33,34
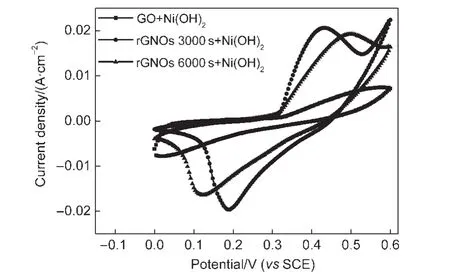
Fig.5 CV curves of Ni(OH)2composited on GS and rGNOs reduced for 3000 and 6000 s in 3%(w)KOH solution
Fig.6 shows the CV curves of Ni(OH)2electrode at different scan rates on different substrates in 3%(w)KOH solution.The CV slope of Ni(OH)2/rGNOs is larger than that of Ni(OH)2at 0.30-0.35 V,which indicates that the Ni(OH)2/rGNOs has faster oxidation rate and smaller interfacial resistance.They are caused by the strong chemical interaction between Ni(OH)2and the functional groups on rGNOs and the electron transfer between nanoparticles is more efficient.
It is also observed that the redox peaks of Ni(OH)2/rGNOs are smoother and more rectangular shaped than Ni(OH)2on bare nickel foam,which illustrates the lower contact resistance of Ni(OH)2/rGNOs.33,34The wider redox peaks and higher peak current density of Ni(OH)2/rGNOs are due to the excellent dispersion of Ni(OH)2nanoparticles on rGNOs,which promotes the diffusion of reactant molecules in bulk solution and thus improves the material utilization.When rGNOs is applied as electrochemical disposition substrate,the redox peaks shift lit-tle with the increase of scan rate,which demonstrates the higher reactivity of Ni(OH)2/rGNOs.It is caused by the increase of electric double layer(EDL),since the EDL of rGNOs is much larger than that of bare nickel foam.

Fig.6 CV curves of Ni(OH)2electrode at different scan rates in 3%(w)KOH solution
The specific mass capacitances(SC)at different scan rates can be calculated according to the Eq.(3):
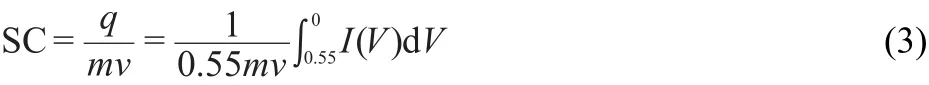
where m is the mass of the electroactive materials Ni(OH)2and ν is the scan rate.The capacitances under different scan rates are shown in Fig.7.SC of Ni(OH)2/rGNOs is 1591 F·g-1at 5 mV·s-1,while the value of Ni(OH)2on bare nickel foam is 656 F·g-1.Besides,at a higher scan rate of 100 mV·s-1,Ni(OH)2/rGNOs has a specific capacitance retaining 49%of that at 5 mV·s-1,whereas the value is 41%for bare nickel substrate.It proves the higher utilization and electrochemical activity of Ni(OH)2on rGNOs.Buglione et al.35observed that graphite microparticles showed a capacitance of 0.88 F·g-1and electrochemical reduction of GO leaded to a capacitance to 4.99 F·g-1.Although SC of rGNOs has increased dramatically,it is still so small to be neglected comparing to that of Ni(OH)2/rGNOs.The electric double-layer capacitance is influenced by the chemical adsorption between Ni(OH)2and rGNOs,it is impossible to measure the electric double-layer capacitance of rGNOs separately through experimental measurements.Therefore,the contribution from rGNOs can not be quantitatively assessed in the specific capacitance calculation.It is believed that the layered structure of composites with high surface area and the positive synergy effect between Ni(OH)2and rGNOs might enhance the capacitive performance.36As discussed above,the Ni(OH)2/rGNOs electrode exhibits good capacity retention and capacitive property,indicating that Ni(OH)2/rGNOs has the potential of being used as the electrode material of pseudocapacitor.

Fig.7 Specific capacitances of Ni(OH)2electrode at different scan rates on different substrates in 3%(w)KOH solution
To further investigate the influence of such rGNOs substrate on electrolyte diffusion,electrochemical impedance spectroscopy(EIS)of the prepared Ni(OH)2/rGNOs is explored.Fig.8 shows the Nyquist plots of the prepared Ni(OH)2and Ni(OH)2/rGNOs at the potential of 0.0 V(a)and 0.5 V(b)with a frequency range from 105to 0.01 Hz in 3%(w)KOH aqueous.The equivalent series resistance(ESR),which measures the conductivity of an electrode material,has been obtained from the tangential intersection of the corresponding Nyquist plots on Zʹ-axis.37
In generally,a sharp increase of the imaginary part of EIS at lower frequency is due to capacitive behavior of the cell,where a semicircular loop at higher frequencies is due to charge-transfer resistance.The semicircle diameter of Ni(OH)2at high frequency has been reduced notably after modifying by rGNOs,indicating that the electron transport is greatly accelerated by the strong adsorption between Ni(OH)2and rGNOs.38In the inset of Fig.8a,ESR of Ni(OH)2and Ni(OH)2/rGNOs are found to be ~1.63 and 1.40 Ω,respectively,clearly suggesting the higher conductivity of Ni(OH)2/rGNOs.However,there is no straight lines on both of the EIS of Ni(OH)2and Ni(OH)2/rGNOs at lower frequency region at 0.0 V and the EIS of Ni(OH)2/rGNOs shows remarkable linear at low frequency region at 0.5 V.The linear part represents Warburg impedance resistance controlled by proton diffusion,finally reflecting concentration polarization impedance.The results demonstrate that at the potential of 0.0 V,the material rGNOs has only limited benefit to Ni(OH)2in improving its pseudocapacitor performance,whereas impedance spectroscopy of Ni(OH)2/rGNOs performed a typical capacitance characteristic.39
In the low frequency region,the capacitance(C)can be defined as the combination of real(C')and imaginary(C'')parts of the capacitance.40,41
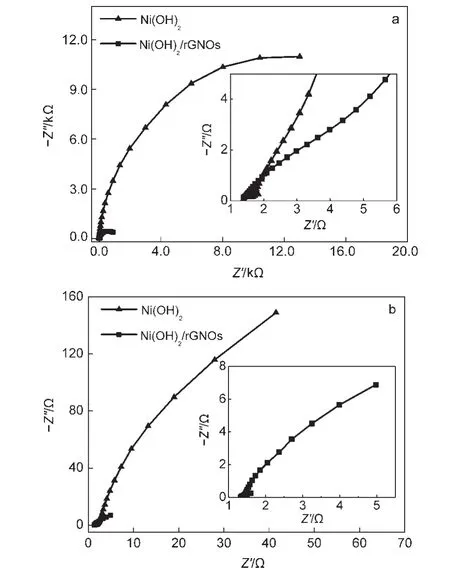
Fig.8 Nyquist plots for Ni(OH)2and Ni(OH)2/rGNOs at potentials of 0.0 V(a)and 0.5 V(b)
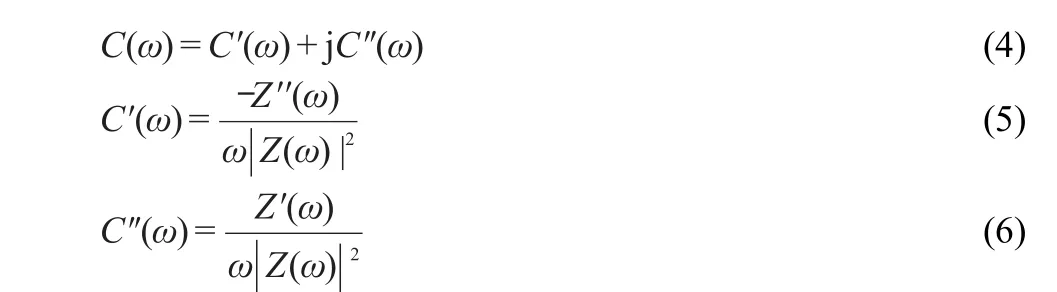
where C'corresponds to the capacitance measured under low frequency alternating current conditions and C''is directly proportional to resistance and corresponds to losses in the form of energy dispersion.The real capacitance(C′)decreases as the frequency increases,and at high frequency,the capacitance value is nearly to zero.C'of the capacitance at 0.5 V is over 20 times of that at 0.0 V,which further illustrates the pseudocapacitance of Ni(OH)2(Fig.9).Capacitance of Ni(OH)2electrode increases by nearly 10 times when it is deposited on rGNOs substrate.This incremental capacitance is mainly the electric double-layer capacitance of Ni(OH)2/rGNOs,which is mostly attributed by large surface area of rGNOs substrate.
There are two reasons for rGNOs substrate′s effect upon the pseudocapacitance enhancement of Ni(OH)2.The unique structure of Ni(OH)2/rGNOs can increase the utilization of Ni(OH)2greatly.As shown in TEM images,Ni(OH)2nanoparticles dispersed on rGNOs can increase surface area of the electrode and further contact more electrolyte solution.In addition,the theoretical result that the strong adsorption energy between rGNOs and Ni(OH)2calculated above can be applied to explain the higher capacitance and lower resistance of Ni(OH)2/rGNOs,as it can make the electron transfer between rGNOs and Ni(OH)2easier.The variations of the atomic distances and charge distributions for rGNOs after adsorbing Ni(OH)2also contribute to decreasing the interface resistance between Ni(OH)2and rGNOs.
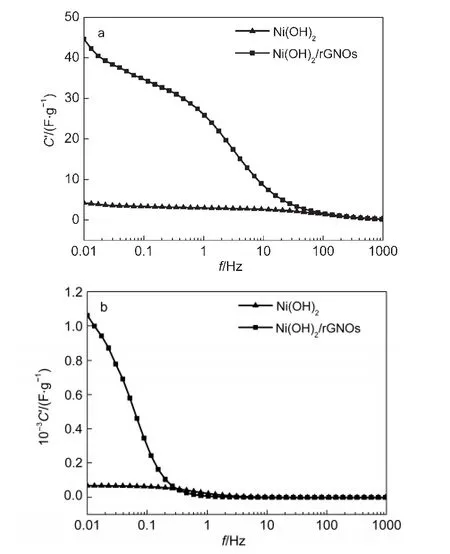
Fig.9 Real(C')part variation of the capacitance for Ni(OH)2and Ni(OH)2/rGNOs with the frequency at 0.0 V(a)and 0.5 V(b)
5 Conclusions
In conclusion,the adsorption energy between rGNOs with hydroxyl groups and Ni(OH)2is the highest in the rGNOs with all oxidation degrees and the atomic distance between rGNOs with hydroxyl groups and Ni(OH)2is also the shortest.The charge distribution demonstrates that rGNOs with different oxidation degrees can improve the efficiency of electron transfer between rGNOs and Ni(OH)2.All these factors can enhance the pseudocapacitor characteristic of Ni(OH)2.We further synthesized Ni(OH)2nanoparticles about 5 nm in size and dispersed them on rGNOs substrate uniformly using the potentiostatic electrodeposition method.The specific mass capacitance of Ni(OH)2/rGNOs is 1591 F·g-1at 5 mV·s-1,2.5 times of that of the Ni(OH)2on bare nickel foam.The higher conductivity of Ni(OH)2/rGNOs also indicates that Ni(OH)2/rGNOs had the potential of being used as the electrode material of pseudocapacitor.
(1)Levi,E.;Gofer,Y.;Aurbach,D.Chem.Mater.2010,22,860.doi:10.1021/cm9016497
(2)Yuan,Y.F.;Xia,X.H.;Wu,J.B.;Yang,J.L.;Chen,Y.B.;Guo,S.Y.Electrochim.Acta 2011,56,2627.doi:10.1016/j.electacta.2010.12.001
(3)Pang,S.C.;Anderson,M.A.;Chapman,T.W.J.Electrochem.Soc.2000,147,444.doi:10.1149/1.1393216
(4)Aricò,A.S.;Bruce,P.;Scrosati,B.;Tarascon,J.M.;Van Schalkwijk,W.Nature Materials 2005,4,366.doi:10.1038/nmat1368
(5)Choi,B.G.;Yang,M.;Jung,S.C.;Lee,K.G.;Kim,J.G.;Park,H.;Park,T.J.;Lee,S.B.;Han,Y.K.;Huh,Y.S.ACS Nano 2013,7,2453.doi:10.1021/nn305750s
(6)Yang,X.F.;Wang,G.C.;Wang,R.Y.;Li,X.W.Electrochim.Acta 2010,55,5414.doi:10.1016/j.electacta.2010.04.067
(7)Pico,F.;Morales,E.;Fernandez,J.A.;Centeno,T.A.;Ibañez,J.;Rojas,R.M.;Amarilla,J.M.;Rojo,J.M.Electrochim.Acta 2009,54,2239.doi:10.1016/j.electacta.2008.10.028
(8)Zhao,D.D.;Bao,S.J.;Zhou,W.J.;Li,H.L.Electrochem.Commun.2007,9,869.doi:10.1016/j.elecom.2006.11.030
(9)Zhang,L.L.;Xiong,Z.G.;Zhao,X.S.J.Power Sources 2013,222,326.doi:10.1016/j.jpowsour.2012.09.016
(10)Yang,G.W.;Xu,C.L.;Li,H.L.Chem.Commun.2008,6537.
(11)Yang,D.N.;Wang,R.M.;He,M.S.;Zhang,J.;Liu,Z.F.J.Phys.Chem.B 2005,109,7654.doi:10.1021/jp050083b
(12)Xu,L.P.;Ding,Y.S.;Chen,C.H.;Zhao,L.L.;Rimkus,C.Chem.Mater.2008,20,308.doi:10.1021/cm702207w
(13)Wang,D.B.;Song,C.X.;Hu,Z.S.;Fu,X.J.Phys.Chem.B 2005,109,1125.doi:10.1021/jp046797o
(14)Chen,X.;Chen,X.H.;Zhang,F.Q.;Yang,Z.;Huang,S.M.J.Power Sources 2013,243,555.doi:10.1016/j.jpowsour.2013.04.076
(15)Zhao,D.D.;Xu,M.W.;Zhou,W.J.;Zhang,J.;Li,H.L.Electrochim.Acta 2008,53,2699.doi:10.1016/j.electacta.2007.07.053
(16)Kottegoda,I.R.M.;Idris,N.H.;Lu,L.;Wang,J.Z.;Liu,H.K.Electrochim.Acta 2011,56,5815.doi:10.1016/j.electacta.2011.03.143
(17)Li,S.M.;Wang,B.;Liu,J.H.;Yu,M.;An,J.W.Acta Phys.-Chim.Sin.2012,28,2754. [李松梅,王 博,刘建华,于 美,安军伟.物理化学学报,2012,28,2754.]doi:10.3866/PKU.WHXB201208292
(18)Wang,H.L.;Casalongue,H.S.;Liang,Y.Y.;Dai,H.J.J.Am.Chem.Soc.2010,132,7472.doi:10.1021/ja102267j
(20)Xu,H.B.;Fan,X.Z.;Lu,Y.H.;Zhong,L.A.;Kong,X.F.;Wang,J.Carbon 2010,48,3300.doi:10.1016/j.carbon.2010.04.051
(21)Fan,X.Z.;Lu,Y.H.;Xu,H.B.;Kong,X.F.;Wang,J.J.Mater.Chem.2011,21,18753.doi:10.1039/c1jm13214h
(22)Sun,Z.P.;Lu,X.M.Ind.Eng.Chem.Res.2012,51,9973.doi:10.1021/ie202706h
(23)Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03,Revision A.01;Gaussian Inc.:Pittsburgh,PA,2003.
(24)Zhao,J.W.;Liu,H.M.;Ni,W.B.;Guo,Y.;Yin,X.Acta Phys.-Chim.Sin.2009,25,1472.[赵健伟,刘洪梅,倪文彬,郭 彦,尹 星.物理化学学报,2009,25,1472.]doi:10.3866/PKU.WHXB20090744
(25)Hummers,W.S.;Offeman,R.E.J.Am.Chem.Soc.1958,80,1339.doi:10.1021/ja01539a017
(26)Ramesha,G.K.;Sampath,S.J.Phys.Chem.C 2009,113,7985.doi:10.1021/jp811377n
(27)Guo,H.L.;Wang,X.F.;Qian,Q.Y.;Wang,F.B.;Xia,X.H.ACS Nano 2009,3,2653.doi:10.1021/nn900227d
(28)Gao,F.;Qi,X.W.;Cai,X.L.;Wang,Q.X.;Gao,F.;Sun,W.Thin Solid Films 2012,520,5064.doi:10.1016/j.tsf.2012.03.002
(29)Zhao,C.M.;Wang,X.;Wang,S.M.;Wang,Y.Y.;Zhao,Y.X.;Zheng,W.T.Int.J.Hydrog.Energy 2012,37,11846.doi:10.1016/j.ijhydene.2012.05.138
(30)Wang,D.H.;Choi,D.W.;Li,J.;Yang,Z.G.;Nie,Z.M.;Kou,R.;Hu,D.H.;Wang,C.M.;Saraf,L.V.;Zhang,J.G.;Aksay,I.A.;Liu,J.ACS Nano 2009,3,907.doi:10.1021/nn900150y
(31)Corrigan,D.A.;Bendert,R.M.J.Electrochem.Soc.1989,136,723.doi:10.1149/1.2096717
(32)Kim,S.J.;Park,G.J.;Kim,B.C.;Chung,J.K.;Wallace,G.G.;Park,S.Y.Synthetic Metals 2012,161,2641.
(33)Gomez,J.;Kalu,E.E.J.Power Sources 2013,230,218.doi:10.1016/j.jpowsour.2012.12.069
(34)Zhang,W.K.;Wang,L.;Huang,H.;Gan,Y.P.;Wang,C.T.;Tao,X.Y.Electrochim.Acta 2009,54,4760.doi:10.1016/j.electacta.2009.04.008
(35)Buglione,L.;Chng,E.L.K.;Ambrosi,A.;Sofer,Z.;Pumera,M.Electrochem.Commun.2012,14,5.doi:10.1016/j.elecom.2011.09.013
(36)Li,L.;He,Y.Q.;Chu,X.F.;Li,Y.M.;Sun,F.F.;Huang,H.Z.Acta Phys.-Chim.Sin.2013,29,1681. [李 乐,贺蕴秋,储晓菲,李一鸣,孙芳芳,黄河洲.物理化学学报,2013,29,1681.]doi:10.3866/PKU.WHXB201305223
(37)Zhang,J.T.;Jiang,J.W.;Zhao,X.S.J.Phys.Chem.C 2011,115,6448.doi:10.1021/jp200724h
(38)Jagadale,A.D.;Kumbhar,V.S.;Dhawale,D.S.;Lokhande,C.D.Electrochim.Acta 2013,98,32.doi:10.1016/j.electacta.2013.02.094
(39)Grden,M.;Alsabet,M.;Jerkiewicz,G.ACS Appl.Mater.Interfaces 2012,4,3012.doi:10.1021/am300380m
(40)Taberna,P.L.;Simon,P.;Fauvarque,J.F.J.Electrochem.Soc.2003,150,A292.
(41)Chmiola,J.;Yushin,G.;Dash,R.;Gogotsi,Y.J.Power Sources 2006,158,765.doi:10.1016/j.jpowsour.2005.09.008