Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
2014-10-14QINShanShanYUZhiWu
QIN Shan-Shan YU Zhi-Wu
(Key Laboratory of Bioorganic Phosphorous Chemistry&Chemical Biology,Ministry of Education,Department of Chemistry,Tsinghua University,Beijing 100084,P.R.China)
Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
QIN Shan-Shan YU Zhi-Wu*
(Key Laboratory of Bioorganic Phosphorous Chemistry&Chemical Biology,Ministry of Education,Department of Chemistry,Tsinghua University,Beijing 100084,P.R.China)
Abstract:Molecular dynamics simulations ofα-tocopherol in a number of saturated phospholipid bilayers were performed at 280,310,and 350 K.The phospholipids contained either short acyl tails,i.e.,dimyristoylphosphatidylcholine and dimyristoylphosphatidylethanolamine orlong acyltails,namely distearoylphosphatidylcholine and distearoylphosphatidylethanolamine. The preferential position,hydrogen bonding,orientation,and dynamic properties ofα-tocopherol in the bilayers were examined in detail and several conclusions were made.First,the hydroxyl group ofα-tocopherol generally remains beneath the interfacial region of the lipid bilayers and it shifts toward the bilayer mid-plane with an increase in temperature.At 350 K it flip-flops between the upper and lower leaflets in the four lipid bilayers.Second,α-tocopherol mainly forms hydrogen bonds with the carbonyl ester oxygen in the lipid head groups and hardly forms hydrogen bonds with the amino groups in the phosphatidylethanolamine(PE)bilayers.Hydrogen bonding with PEs is more stable than hydrogen bonding with phosphatidylcholines(PCs)at low temperatures.Third,α-tocopherol′s head group has fluctuating tilt angles relative to the normal of the lipid bilayers and the tail has many different conformations.Fourth,the lateral diffusion rate ofα-tocopherol is comparable to that of phospholipid molecules at low temperature and it diffuses much faster than lipids at 350 K.The diffusion rate in the direction perpendicular to the membrane surface is much slower than the lateral diffusion rate.
Key Words:Vitamin E;Phospholipid;Orientation;Flip-flop;Diffusion;Hydrogen-bond
Vitamin E was first separated from wheat-germ oil by chemist Evans et al.[1]in 1936 and was able to be synthesized artificially two years later.Natural vitamin E mainly exists in vegetable oil,malt,almond,peanut,and sunflower seed.It is the generic description for all tocol and tocotrienol derivatives.The biologically most active form is RRR-α-tocopherol(shown in Fig.1),which possesses a chromanol ring and a trimethyltridecyl phytol side chain and dominates in mammalian cells and tissues.
The principal role of α-tocopherol is to scavenge the lipid peroxyl radicals to prevent them from attacking the target lipid substrate[2-3].Another major function of α-tocopherol is to stabilize membranes by forming complexes with those membrane lipid components that tend to destabilize the bilayer structure[4].With very low solubility in water,the principal site of action is in cell membranes and lipoproteins.The putative role of tocopherols as membrane antioxidants and stabilizers depends on their localization and orientation in cell membranes.The manner in which they are arranged and distributed within membranes is presumed to govern the molecular mechanisms underlying these functions.There have been many studies of phospholipid model membranes which aim to explain how vitamin E interacts with biological membranes[5-6].Thermal studies of mixtures of α-tocopherol with saturated phosphatidylcholines showed that the presence of α-tocopherol reduced the pre-transition enthalpy or even eliminated the pre-transition.Wang et al.[7]examined the effect of α-tocopherol on the structures and phase behavior of dilauroyl-,dimyristoyl-,dipalmitoyl-,and distearoyl-phosphatidylcholines and observed that ripple phase formed in all of the mixtures at temperatures well below the pre-transition temperature of the corresponding pure phospholipid.The effect of α-tocopherol on the phase behavior of aqueous dispersions of phosphatidylethanolamines is different from that on dispersions of phosphatidylcholines[6].Similar to sterols[8],the structural role of α-tocopherol in membranes was proved to disorder the gel phase[9],and order the liquid crystal phase[10-12].Suzuki[11]and Wassall[13]et al.proved that when α-tocopherol was added into model membranes at gel phase the number of gauche rotamers in acyl chains of phospholipids increased in a manner consistent with ESR and NMR observations.2H NMR study of α-tocopherol in perdeuteropalmitate-containing PC showed that it increased the order of liquid crystal membranes[10-11,13].And this stabilization effect of α-tocopherol in liquid crystal membranes has also been proved by infrared[14],ESR[15],fluorescence[16],and NMR[17]studies of α-tocopherol/DPPC(dipalmitoylphosphatidylcholines)mixed bilayer.
The location and behavior of α-tocopherol in model membranes have been the subject of considerable interests.Srivastava et al.[18]suggested that α-tocopherol incorporated into phospholipid bilayer membranes with the chromanol ring situated in the aqueous interface based on paramagnetic resonance and13C magnetic resonance study of α-tocopherol and DPPC bilayers.Fragata and Bellemare[19]reported a location of the chromanol ring of α-tocopherol 1 nm from the aqueous interface.Several experiments using fluorescence quenching of probes have been performed to determine the location of the chromanol ring of α-tocopherol and found that it located close to the lipid/water interface of the phospholipid bilayer,but did not extend into the lipid/water interface[20].There has also been reports to suggest that the chromanol ring was deeply buried into the bilayer,closer to the poly unsaturated sites in lipid tails[21].Staying in a bilayer,α-tocopherol was found to rotate about an axis perpendicular to the plane of the phospholipid bilayer.The motion of the rigid chromanol ring was more constrained than the phytol chain,and the frequency of gauche rotamers increased with distance towards the center of the bilayer[17].
Hydrogen bonding between α-tocopherol and phospholipids has also been studied by various experimental methods.Gomez-Fernandez[22]and Salgado[23]et al.showed evidences of H-bonding of the hydroxyl group to either the carbonyl or phosphate oxygen of the phospholipid molecules by Fouriertransform infrared spectroscopy measurements.Urano et al.[24]concluded that the hydroxyl group of α-tocopherol was hydrogen bonded to the carbonyl group of the ester carbonyl bond of the phospholipids.

In addition to the location and orientation of α-tocopherol inside biomembranes,the mobility of α-tocopherol measured by diffusion coefficient has also been of interest within the community.The value of the coefficient,however,was reported differing by several orders of magnitude depending on the method used to determine it.Aranda et al.[25]assessed the lateral diffusion coefficient of α-tocopherol to be 4.8 × 10-6cm2·s-1,which is about 100 times faster than 1-palmitoyl-2-oleoyl-phosphatidylcholine(POPC)diffusing in membranes,at steady state by monitoring the fluorescence quenching of α-tocopherol by 5-NS(5-(N-oxy-4,4-dimethyioxazolidin-2-yl)stearic acid).Gramlich et al.[26]studied the quenching of α-tocopherol by a novel fluorescent lipid probe in the environment of POPC liposomes,and found that the mutual lateral diffusion coefficient for α-tocopherol in POPC liposomes was(1.8±0.1)×10-7cm2·s-1,about a factor of 2 greater than that of mutual diffusion of POPC.
The behavior of α-tocopherol in biomembranes is still under conjecture.In our previous work[27],we performed molecular dynamics(MD)simulations of α-tocopherol with DPPC,DPPE (dipalmitoylphosphatidylethanolamine),POPC,and POPE(1-palmitoyl-2-oleoyl-phosphatidyl-ethanolamine)model membranes.To see whether the tail length of lipid bilayers will affect α-tocopherol's behavior,we further conducted simulations in this work with model membranes either possessing shorter acyl chains(DMPC and DMPE)or longer acyl chains(DSPC and DSPE).We also calculated the vertical diffusion coefficients of both α-tocopherol and phospholipid molecules in the binary lipid systems,which will help us to deepen the understanding on the dynamic property of α-tocopherol/lipid mixtures.
1 Computational details
1.1 Simulation conditions
Molecular dynamics simulation has become a powerful method in understanding the molecular mechanisms of various physical processes including phase transition and diffusion process[27].The simulation in this work included one α-tocopherol molecule in the upper leaflet,63 phospholipid molecules(31 in the upper layer and 32 in the lower),and more than 43 water molecules per lipid molecule to attenuate the influence of the image bilayer.The molar concentration of α-tocopherol was in the same order as in Golgi membranes and lysosomes,where the molar ratio of α-tocopherol/lipids was found to be 1:65[28-31].Periodic boundary conditions in x,y,and z directions were used.Simulations of the binary α-tocopherol/phospholipid systems have been carried out at three different temperatures:280,310,and 350 K.Four phospholipid molecules were included in our study:DMPC,DMPE,DSPC,and DSPE,which were differentiated by structure of head group and length of lipid tails.Most of the simulations started with the configuration that α-tocopherol was vertically inserted into the middle of phospholipid bilayer with its head group being close to lipid-water interface.The simulation time for each system was at least 240 ns and only the trajectory for the last 40 ns was used for analyzing.
All the simulations were carried out in the NPT ensemble,at constant pressure of 1.01325×105Pa and various temperatures.The time step was 2 fs for all simulations,and the pair list for computing non-bonded pair forces was updated every 10 steps with a list-cutoff of 0.9 nm.The coulomb and van der Waals interactions were computed using the cutoff algorithm,and the cutoff radii for both real-space coulomb and van der Waals interactions were 0.9 nm.The long range electrostatic interaction was corrected using the PME method[32-33]with the maximum spacing for the fast Fourier transform(FFT)grid of 0.12 nm and the interpolation order of 4.The weak coupling scheme of Berendsen and coworkers[34-35]was used for both temperature and pressure control,with the temperature coupling time constant of 0.1 ps.For pressure coupling,we used the semiisotropic coupling type,in which the x,y,and z directions of the simulation box could be varied independently,with the coupling time constant of 1.0 ps and the compressibility of 4.6×10-10Pa-1.The trajectory was collected every 2 ps,and all the simulations were performed with Gromacs 3.3.1 package[36-37]in parallel under a Windows Compute Cluster Server.
1.2 Molecular models
We adopted the α-tocopherol and lipid models from our recent work[27],where the α-tocopherol model was verified,and the lipid model had been validated in previous work[27].Briefly,for lipid models the bonded parameters were from the Gromos force field[38]and the Ryckaert-Bellemans potential[39]was used as the torsion potential of the lipid hydrocarbon chains.For nonbonded parameters we used values extracted form Berger et al.[40]for lipid tails and OPLS values[41-42]for head groups and the partial atomic charges were obtained from Chiu et al.[43].For water molecules,the SPC model[44]was used with settle algorithm[45]to constrain bonds and angles.All the CH3,CH2,and CH groups were treated as united atoms.And hydrogen atoms in the amino group of phosphatidyl enomines were considered explicitly due to their capability to form hydrogen bonds.
For α-tocopherol we proposed a united atom model and the hydrogen atom in the hydroxyl group was described explicitly.The Lennard-Jones parameters of the CH2and CH3groups were from the original OPLS Lennard-Jones parameter set[41].The harmonic potentials describing bonds,angles,and dihedral angles were taken from the Amber 2003 united atom force field[46].We have carefully evaluated the energetical profile by calculating the torsional barriers in three isopentyl fragments at HF/6-31G*level using Gaussian 98[47],and the MD model by vacuum simulations of the same isopentyl fragments.This comparison gave a good agreement between MD simulations and quantum chemical calculations.The charges in the molecule were determined by the 2-stage resp method[48]from a HF/6-31G*optimized α-tocopherol geometry.
2 Simulation results

The main purpose of this work is to find out the preferential location of α-tocopherol inside biomembranes.To get a general view of how α-tocopherol locates in various lipid bilayers,selected snapshots of α-tocopherol/DMPC system at 280,310,and 350 K are shown in Fig.2 as representative pictures.At280 K α-tocopherol keeps staying in the initial upper leaflet of the DMPC bilayer,while at 310 and 350 K α-tocopherol flips over between the upper and lower leaflets of the bilayer,indicating the“flip-flop”moving mode of the molecule at high temperatures in DMPC bilayer.The α-tocopherol molecule,or separately its head and tail can take many different conformations.The“flip-flop”phenomenon of tocopherol has also been observed when DMPC is replaced with DMPE at 310 and 350 K,but for DSPC and DSPE bilayers it only happens at 350 K.Detailed statistical analysis of the simulation results are discussed below.
2.1 Position of α-tocopherol in lipid bilayers
2.1.1 α-Tocopherol/DMPC system
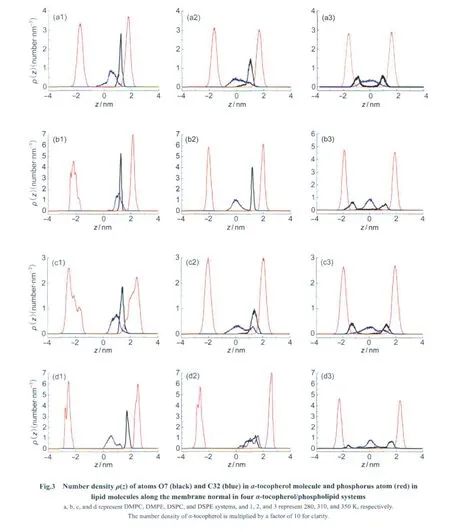
To get some knowledge of the situation when α-tocopherol lies in saturated phosphatidylcholine with short tails we performed the simulations of α-tocopherol/DMPC system.According to the experimental conclusions reported before[20,49]the head group of α-tocopherol was initially put near the glycerol groups of lipid.The simulations were set at 280,310,and 350 K.Fig.3 shows the number densities of atom O7 and C32 in α-tocopherol molecule and phosphorus atoms in lipid molecules along the membrane normal at 280,310,and 350 K,respectively.The data shows that,on average,the hydroxyl group locates always beneath the plane defined by the average positions of phosphorus atoms.At 280 K,the distance between the peak locations of the density profiles of atom O7 in α-tocopherol and that of the phosphorus atoms in DMPC is 0.52 nm(Fig.3(a1)),which means that atom O7 in α-tocopherol locates close to the membrane surface due to the hydrophilicity of hydroxyl group.It keeps staying in one side of DMPC bilayer with a narrow and sharp distribution,while the distribution of atom C32 is much wider,indicating a larger moving range.
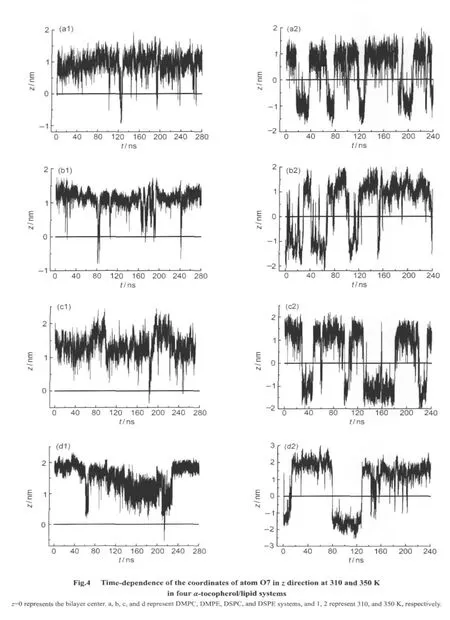
At 310 K,the distance between the peak locations of the density profiles of atom O7 in α-tocopherol and that of the phosphorus atoms in DMPC is 0.64 nm(Fig.3(a2)),which means that atom O7 moves a little bit away from DMPC head groups in comparison with the situation at 280 K.Occasionally,we noticed that O7 crossed the bilayer center and contacted with the head groups of the other side of the bilayer,a phenomenon of flip-flop.This is shown in Fig.4(a1).Over the time range of 280 ns,we only noticed that theZcoordinate of atom O7 is below-0.5 nm from 121.1 to 127.6 ns in the DMPC bilayer.In most of the time,α-tocopherol stays in the upper leaflet of the bilayer,and the density profiles of atom O7 is calculated according to the last 40 ns trajectory in which α-tocopherol stays in the upper leaflet of the bilayer.
At 350 K,the α-tocopherol's head group appears in both upper and lower leaflets of the DMPC bilayer,representing a“flip-flop”moving mode.We can see from Fig.4(a2)that,α-tocopherol flips much faster than situation at 310 K.This indicates that temperature is the key factor that triggers the flipflop behavior:high temperature increases the kinetic energy of α-tocopherol molecule,which makes it possible to overcome the energy barrier to come through the hydrophobic interior of a lipid bilayer.High temperature also increases the fluidity of the bilayer matrix,which makes α-tocopherol molecule easier to flip over.The atom C32 stays in the middle of DMPC bilayer,which is due to the hydrophobic character of α-tocopherol tail.Snapshots in Fig.2 also show the conformations of α-tocopherol during the flip-flop process at 310 and 350 K.
2.1.2 α-Tocopherol/DMPE system
Fig.3(b1-b3)shows the simulation results of α-tocopherol/DMPE system.The graphs are not very different from those of α-tocopherol/DMPC system.The distances between the peak locations of the density profiles of atom O7 in α-tocopherol and that of the phosphorus atoms in DMPE are 0.90 and 0.70 nm at 280 and 310 K,respectively.Atom O7 stays closer to DMPE head groups at 310 K than at 280 K,which is different from that of the α-tocopherol/DMPC system.Fig.4(b1)shows thezcoordinates of atom O7 during the entire simulation of 280 ns at 310 K.It can be seen that the“flip-flop”occurs occasionally,for example from 81.3 to 86.2 ns in the figure.At 350 K,α-tocopherol flips much more frequently as can be seen in Fig.4(b2),which demonstrates again that high temperature is the key factor to trigger the flip-flop process.
2.1.3 α-Tocopherol/DSPC system
Results of α-tocopherol/DSPC system are shown in Fig.3(c1-c3).The peak to peak distances of atom O7 in α-tocopherol head group and the phosphorus atoms in DSPC head groups are 1.10 and 0.66 nm at 280 and 310 K,respectively.The head of α-tocopherol moves much closer to the DSPC head group when temperature increases,which is obviously different from the α-tocopherol/DMPC system discussed above.According to our previous studies on the phase behavior of DSPC bilayer[50],it should be in the“mixed”ripple phase at 280 K basing on the lipid model we adopted in the simulation,which has the coexistence of ordered and disordered domains.And α-tocopherol tends to locate in the disordered region,which has smaller coordinates in Z direction,thus α-tocopherol stays further away from the lipid head at 280 K.And at 310 K the simulated DSPC bilayer is already in liquid crystal phase[50],thus α-tocopherol has comparable location to α-tocopherol/DMPC system.
Fig.4(c1-c2)shows that in DSPC bilayer the“flip-flop”occurs only at 350 K,which indicates that the length of lipid tail is another factor that impacts the flip-flop behavior:the longer the lipid tail is,the harder for α-tocopherol to flip over.
2.1.4 α-Tocopherol/DSPE system
Fig.3(d1-d3)shows the results of α-tocopherol/DSPE system.The peak to peak distances of the density profile of atom O7 in α-tocopherol and that of phosphorus atoms in DSPE are 0.78 and 0.85 nm at 280 and 310 K,respectively,indicating that the head group of α-tocopherol moves away from the head group of DSPE bilayer when temperature increases from 280 to 310 K.Fig.4(d1)shows that during the simulated time of 280 ns at 310 K,atom O7 stays very close to the bilayer centerfrom 130 to 230 ns but does not flip over to the lower leaflet of the DSPE bilayer.Combined with our previous reports on the DSPE phase behavior[50],DSPE bilayer should be in gel phase at 310 K,thus the DSPE molecules in the lower leaflet should be tightly packed,which prevents α-tocopherol molecule from flipping over.
In summary,α-tocopherol molecule tends to move towards the bilayer center from 280 to 310 K in DMPC and DSPE bilayers but to move away from the bilayer center in DMPE and DSPC bilayers,until it flips over at 350 K.The apparent inconsistence will be explained in the discussion section.There are three factors that impact the happening of flip-flop process:temperature,the length of lipid tail,and the fluidity of the lipid.The third one can be seen in Fig.4.For DMPC and DMPE bilayers,since both bilayers have higher fluidity at 350 K,α-tocopherol has higher“flip-flop”frequency at 350 K than 310 K.For DSPC and DSPE bilayers,since DSPC bilayer has higher fluidity,α-tocopherol flips much more frequently in it than in DSPE bilayer.
2.1.5 Average atom positions
In order to get more accurate information about the relative position of the hydroxyl group ofα-tocopherol in the four lipid bilayers,we calculated the averagez-axis value of atom O7 inα-tocopherol and the C1'―C10'carbon atoms in the lipidsn-1 andsn-2 acyl chains numbered from C=O to the tail end.The results are listed in Table 1 and Table 2.Three main conclusions can be drawn.First,the hydroxyl group locates preferably between C2'and C5'in the lipidsn-1 tail and between C3'and C6'in the lipidsn-2 tail at 280 and 310 K,respectively,which indicates that thesn-2 tail has higher position relative to thesn-1 tail.This shows clearly that the hydrophilic group of α-tocopherol prefers to stay at a position below the polar region of a leaflet of the lipid bilayer.
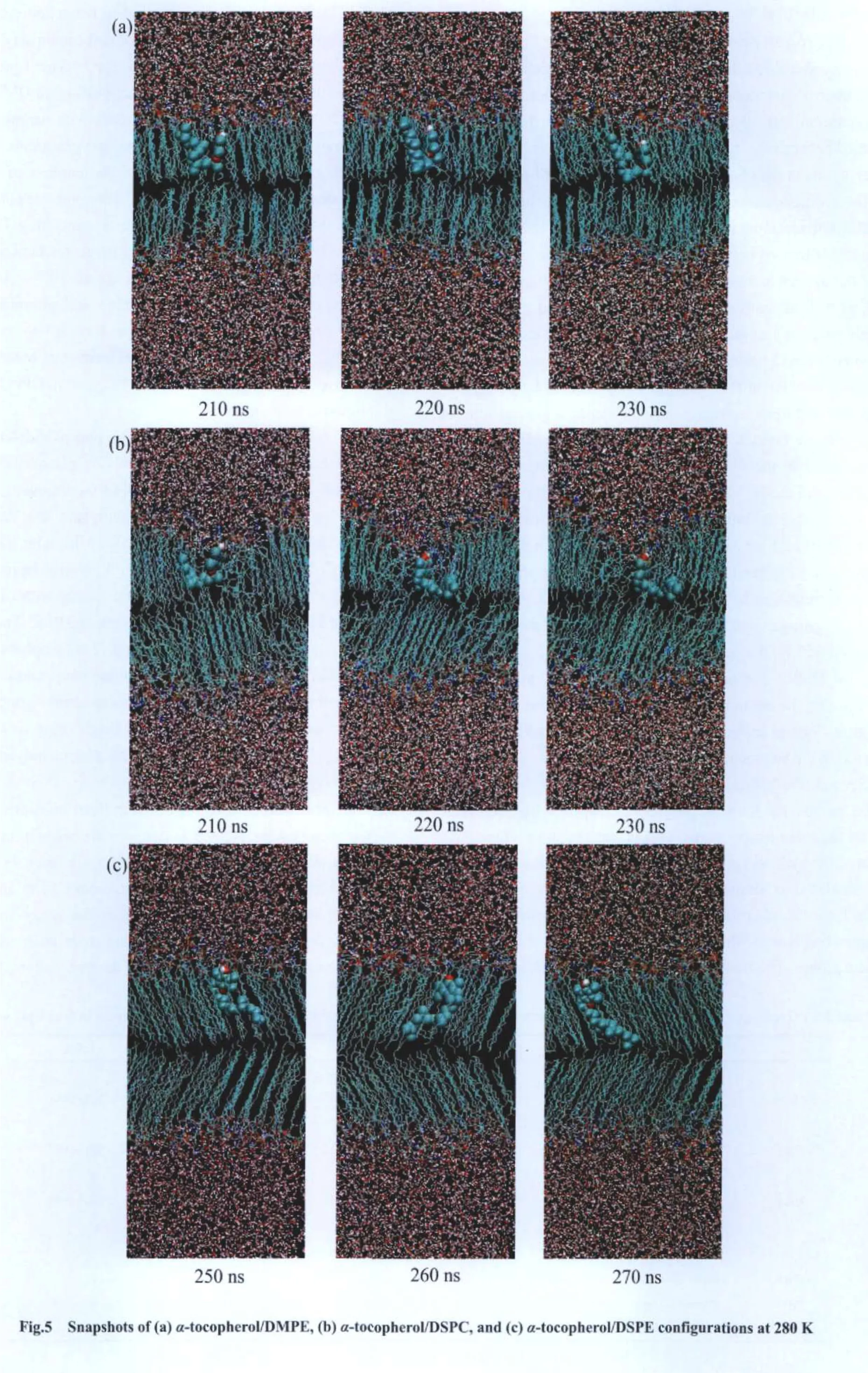
Second,the hydroxyl group moves towards the bilayer center with increasing temperature from 280 to 310 K forα-tocopherol/DMPC andα-tocopherol/DSPE systems.Exceptions were seen forα-tocopherol/DMPE andα-tocopherol/DSPC systems.In the former,as shown in the snapshots in Fig.5(a),theα-tocopherol tail has to be folded to stay in the upper leaflet of DMPE bilayer at 280 K,which leads to the lower location of atom O7.In the latter,as we have pointed out in Section 2.1.3,α-tocopherol tends to locate in the disordered region of DSPC bilayer,leading to smaller coordinates ofα-tocopherol inzdirection as shown in Fig.5(b).And we can see from Fig.4(b1-b2)and Fig.4(d1-d2)thatα-tocopherol can locate very close to the center of the DMPE bilayer from 160 to 200 ns and from 130 to 230 ns in the DSPE bilayer,respectively.
Third,when temperature further increases to 350 K,α-tocopherol molecules are able to move much closer to the bilayer center,allowing the“flip-flop”to happen.This is reflected in the absolute values of the average positions of atom O7 of the overall trajectory in the last 40 ns.It can be seen from the tables that atom O7 lies much closer to the bilayer center at high temperature than at lower temperatures.It can also be found that atom O7 in PC bilayers has smaller absoluteZcoornidates than in PE bilayers at 350 K,which can be ascribed to thegreater fluidity of PC bilayers thus higher flip-flop frequency ofα-tocopherol at the same temperature.
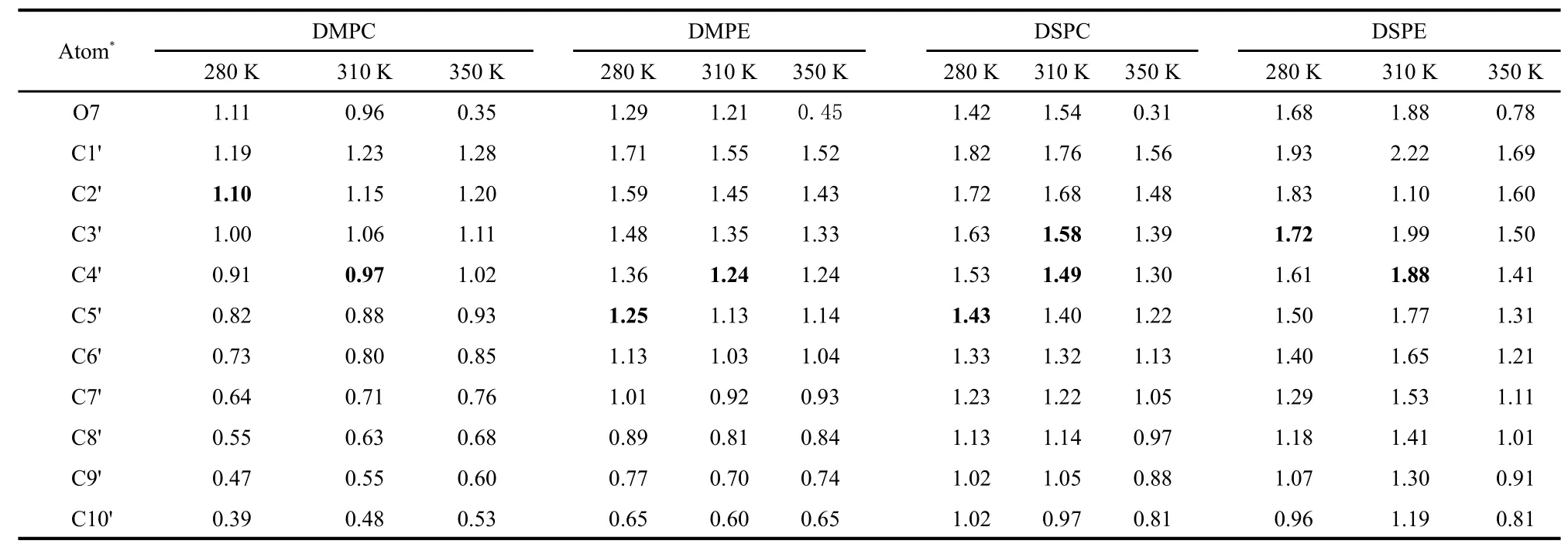
Table 1 Averagedz-axis values(nm)of O7 inα-tocopherol and C1'―C10'in the lipidsn-1 tail at 280 and 310 K

Table 2 Averagedz-axis values(nm)of O7 inα-tocopherol and C1'―C10'in the lipidsn-2 tail at 280 and 310 K
2.2 Hydrogen bond(HB)
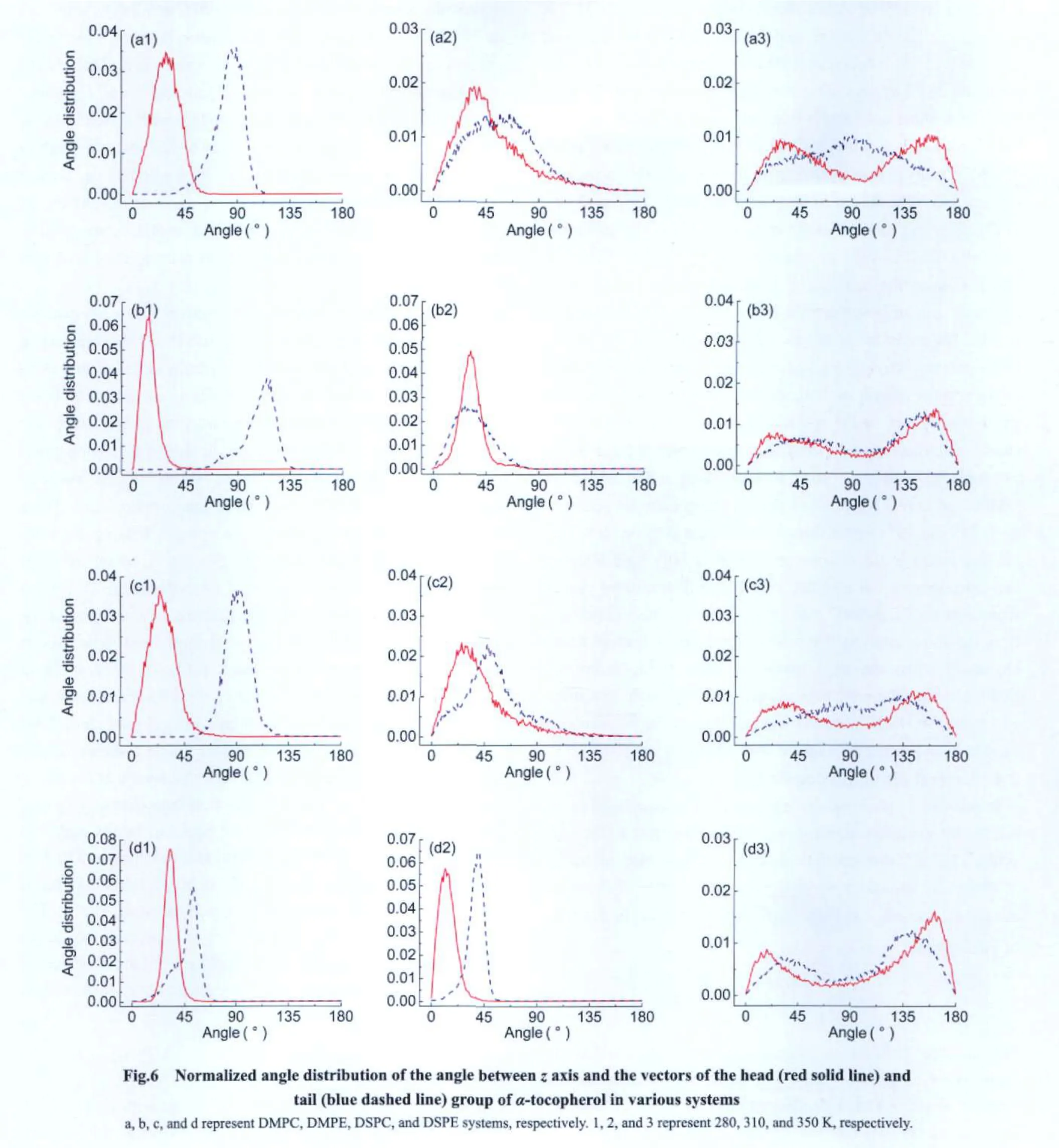
α-Tocopherol has the potential to form hydrogen bonds with a number of functional groups of lipid molecules.To address the question,we calculated the percentage of hydrogen bonds formed between the hydroxyl group inα-tocopherol and the oxygen atoms in the phosphate and ester groups of lipids,as well as the hydrogen atoms in the amino groups in the case of phosphatidylethanolamines.First we counted the number of hydrogen bonds formed between the hydroxyl group inα-tocopherol and the oxygen atoms in the phosphate and ester groups of lipids,as well as the hydrogen atoms in the amino groups separately over the last 40 ns of simulation,then we calculated the ratio between the number of each kind of hydrogen bond and the total number of the hydrogen bonds formed betweenα-tocopherol and lipid molecules.By considering a general form of the hydrogen-bond X―H…Y,the criteria of the HB formation we adapted in the work are:the X―Y distance is less than 0.35 nm and the H―X―Y angle is less than 30°[51-52].The results are listed in Table 3.Several conclusions can be drawn from the table.First,the hydrogen bonds between the hydroxyl group ofα-tocopherol and the amino group in the phosphatidylethanolamines can be ignored.At 280 and 310 K,the percentages of hydrogen bonds in the two lipid systems are counted as zero.At 350 K,the percentages are merely 1%for DMPE and 3%for DSPE.The results are understandable,as the amino group is on the out most surface of a bilayer,whilst the hydroxyl group locates preferably below the glycerol backbone as discussed in the last section.
Second,the hydrogen bonds are mainly with the fatty acid ester groups,not with the phosphate group in a lipid molecule at all the three temperatures.This is because the average position of the hydroxyl group is a few angstroms below the glycerol backbone as shown in Table 1 and Table 2.Exception is seen forα-tocopherol/DMPC system at 280 K,where 38%hydrogen bonds are with the ester groups and 62%are with phosphate groups.The reason might be that DMPC molecule has acyl tails shorter than the length ofα-tocopherol molecule.At 280 K,DMPC molecules are arranged in better order than at higher temperatures.The tail ofα-tocopherol cannot stay favorably in the bilayer center.As a result,the hydroxyl group inα-tocopherol is forced to stay at a higher position in DMPC bilayer at 280 K and forms hydrogen bonds with nearby phosphate group.Since there are totally four oxygen atoms in one phosphate group and two ester groups,the numbers of HB in the table concerning these two types of acceptors are comparable.For the other three binary systems containing DMPE,DSPC,and DSPE at 280 K,the HB percentages between hydroxyl group and the fatty acid esters are all 100%.And the HB percentages between hydroxyl group and phosphate are 0%.The general conclusion is the same at other two temperatures,which is in agreement with the experimental observation that the hydrogen bond is with carbonyl oxygen in DPPC bilayer at liquid-crystal phase[53].
Third,the hydrogen bonds involving phosphatidylethanolamines are more stable than that involving phosphatidylcholines at low temperatures.This is based on the following observations:the HB percentages with phosphate are zero for DMPE and DSPE at 280 and 310 K;the HBs with the ester groups of DMPE are predominantly with glycerol oxygen;the HBs with the ester groups of DSPE are mainly with carbonyl oxygen at 280 K and with glycerol oxygen at 310 K.These are the situations when the hydroxyl group ofα-tocopherol stays in a position stably.This is because at these temperatures,there are cross-linking hydrogen bonds between amino groups and the phosphate and ester groups in the neighboring molecules,stabilizing the head group regions of phosphatidylethanolamine bilayers[54].
2.3 Orientation ofα-tocopherol in lipid bilayers
Two vectors were defined to describe the orientation of the head and tail ofα-tocopherol molecule in a bilayer.For head group it was defined as the vector from atom C15 to atom O7 ofα-tocopherol;for tail it was defined as the vector from the geometry center of carbon atoms in the main chain of α-tocopherol tail to atom C15.For each of the two vectors,there isa tilting angle between the vector and the Z axis at any moment,with possible values ranging from 0°to 180°.By dividing the angle range into 180 sectors,angle distribution could be evaluated as the total number of angles that fall into each sector normalized by the overall number of angles we sampled over a time span of 40 ns.
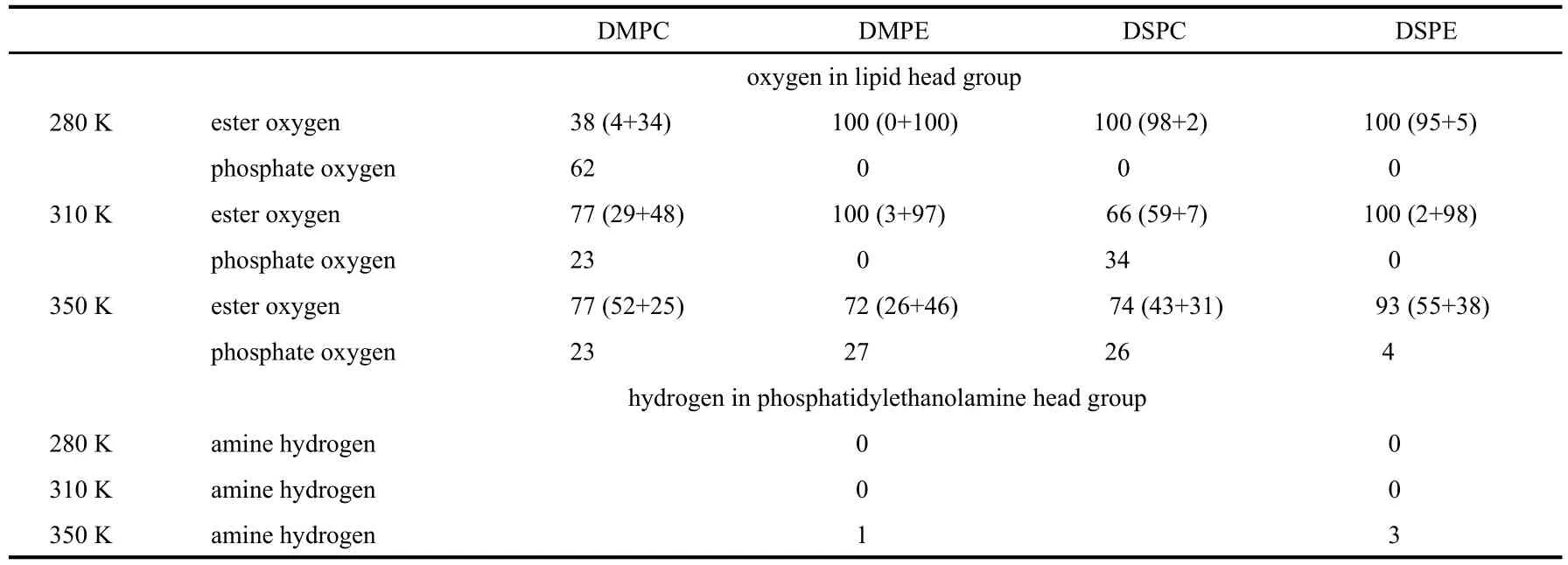
Table 3 Percentage(%)of hydrogen bonds between hydroxyl group inα-tocopherol and oxygen and hydrogen atoms in four lipid species
Shown in Fig.6 are the tilting angle distributions of both head and tail vectors of the four lipid mixtures at three temperatures.Generally,for the head vector in each of the four systems,one peak is seen at 280 and 310 K,while two peaks appear at 350 K.The double-peak feature at the high temperature represents the“flip-flop”moving mode of the tocopherol molecule.Specifically for phosphatidylcholines systems,at 280 K the distribution curves of α-tocopherol head group take a maximum value at 28°in α-tocopherol/DMPC system and 23°in α-tocopherol/DSPC system;for tail group at the temperature,it has the peak value at 85°in α-tocopherol/DMPC system and 90°in α-tocopherol/DSPC system.This may be due to that at 280 K DSPC bilayer is in the“mixed”ripple phase consisting of both ordered and disordered domains,and α-tocopherol tends to lie horizontally in the disordered domain,leading to larger tilt angles for tail group,as shown in Fig.5(b).At 310 K,the distribution curves of α-tocopherol head and tail take a maximum value at 37°and 60°in lipid DMPC,respectively,and in DSPC bilayer α-tocopherol head and tail has its maximum value at 32°and 68°,respectively.Compared to DSPC,α-tocopherol head has a larger tilt angle in DMPC bilayer,which may be due to the higher fluidity of DMPC bilayer.At 310 and 350 K,the distribution curves of α-tocopherol tail group for both systems show broader peaks than that at 280 K,indicating more random moving modes.
In α-tocopherol/phosphatidylethanolamine systems,at 280 K the distribution curves of α-tocopherol head group take its maximum at 12°in α-tocopherol/DMPE system and 30°in α-tocopherol/DSPE system,and for tail group it has the peak value at 115°in α-tocopherol/DMPE system and 49°in α-tocopherol/DSPE system.This may be due to that DMPE molecule has a short tail(only 14 carbon sites),at 280 K they are tightly packed and α-tocopherol can not enter the lower leaflet of DMPE bilayer,in order to stay in the upper leaflet,α-tocopherol tail has to be folded to have a tilt angle of 113°,as shown in Fig.5(a);and its head group chooses to have a small tilt angle since DMPE head groups are tightly packed.While for DSPE bilayer,the lipid tail is longer(18 carbon sites),α-tocopherol tail can locate upright in the bilayer,which leads to a smaller tilt angle in its tail as shown in Fig.5(c).At 280 K,DSPE bilayer is tightly packed in gel phase with a tilting angle relative to the bilayer normal,thus α-tocopherol head has a tilt angle of 30°due to the restriction from nearby DSPE molecules,which can also be illustrated by Fig.5(c).At 310 K the α-tocopherol head group takes its peak value at 32°and 12°in DMPE and DSPE bilayers and the tail group takes its peak value at 31°and 40°respectively.At 350 K,the α-tocopherol head has two peaks in the distribution curves in both lipid bilayers,and α-tocopherol tail also has very broad distributions,which represents the“flip-flop”moving mode.It can be summarized from the above analysis that α-tocopherol head has more tilting alignment when the lipid matrix is more fluid,except for DSPE bilayer,where the α-tocopherol tail movement is restricted by nearby DSPE molecules,which forces the α-tocopherol head to have a larger tilt angle at 280 K than that at 310 K.
2.4 Lateral diffusion coefficient
In addition to the structural properties of α-tocopherol in the bilayer,we are also interested in the lateral mobility of the molecule.The diffusion coefficients can be determined by means of molecular dynamics simulations from the ratio of the mean square displacement(MSD)to time in the limit of infinite time:

The brackets indicate an average over different time of the mass center of the molecules.The parameterNfdescribes the number of translational degrees of freedom.For an isotropic liquid it is three,whereas for the case of the lateral diffusion of a lipid within a quasi-two-dimensional bilayer,it is two.In order to describe the dynamic properties of α-tocopherol and lipid bilayers more specifically,we calculated the lateral diffusion coefficients in z direction and xy plane separately.
The lateral diffusion coefficient for each system is obtained by calculating the slopes of the MSD plots at various intervals with a minimum of 10 ns and then calculating the average value of all the slopes we have gotten.The error is estimated by calculating the standard deviation of all the slopes.The results are listed in Table 4 and Table 5.There have been few experimental reports in this aspect for comparison.For pure lipid bilayers,the reported diffusion rate of DMPC is 1.2×10−7cm2·s−1at 308 K[55].Since quantitative comparison between experimental and calculated diffusion coefficient values is difficult due to some complications such as finite size effects[56],and our simulation with pure lipids are of the same size with mixed lipid bilayers(32 lipids in both upper and lower leaflets),the simulation results could be considered as in qualitative agreement with these experimental values.Our simulation results are of the same order as the experimental values,thus they qualitatively reflect the dynamic properties of α-tocopherol/lipid systems.
As can be seen in Table 4,α-tocopherol has a comparable diffusion rate with phospholipid molecules in the bilayers at gel phase,like the situation of four systems at 280 K and α-tocopherol/DSPE system at 310 K;while it moves much faster than phospholipid molecules at liquid-crystal phase,except for DMPE bilayer at 310 K,which may be due to that α-tocopherol disturbs the alignment of the tail of DMPE bilayers drastically that DMPE molecules move faster than α-tocopherol.There is also experimental report on α-tocopherol helping form reversed hexagonal phase in DMPE bilayers[57],which indicates the remarkable disturbance effect of α-tocopherol on DMPE bilayers.Finally,we can see that the addition of α-tocopherol enhances the lateral diffusion rate of phospholipid molecules at gel state and decreases their lateral diffusion rates at liquid crystal state,which is in accordance with the conclusion that α-tocopherol could disturb lipid bilayers in gel phase and stabilize lipid bilayers in liquid crystal phase.Table 5 shows the diffusion coefficients in z direcrtion.It can be seen that α-tocopherol diffuses much slower in z direction than that in xy plane,only a few percent of the latter and thus can be ignored.The diffusion coefficients of α-tocopherol in z direction at 350 K is much larger than those at 280 and 310 K,and this may result from the“flip-flop”behavior of α-tocopherol at 350 K.The diffusion coefficients of lipid bilayers in z direction are larger than those of pure lipids at all the three temperature,especially at 350 K,which indicates that α-tocopherol may disturb lipid bilayers in z direction.
3 Discussion
We simulated the systems of α-tocopherol with several model membranes at different temperatures to study the position,conformation,and dynamic properties ofα-tocopherol in saturated lipid bilayers with various lengths of acyl tails.When the acyl chains are short,namely in the cases of DMPC and DMPE bilayers,α-tocopherol molecule stays in one side of lipid bilayers at 280 K.At 310 K,it starts to flip-flop between the two monolayers.When the temperature increases to 350 K,the flip-flop can take place in a much fast manner.When the acyl chains are longer,i.e.,in DSPC and DSPE bilayers,the vitamin molecule is seen to flip over at 350 K,but not at 310 K and lower temperatures.This is in line with the results of our last work[27]which reported thatα-tocopherol only flips over at 350 K in lipid bilayers with 16 or more carbon sites in their acyl tails(DPPC,DPPE,POPC,and POPE).

Table 4 Lateral diffusion coefficients(×10-7cm2·s-1)ofα-tocopherol and lipids inxyplane at various temperatures
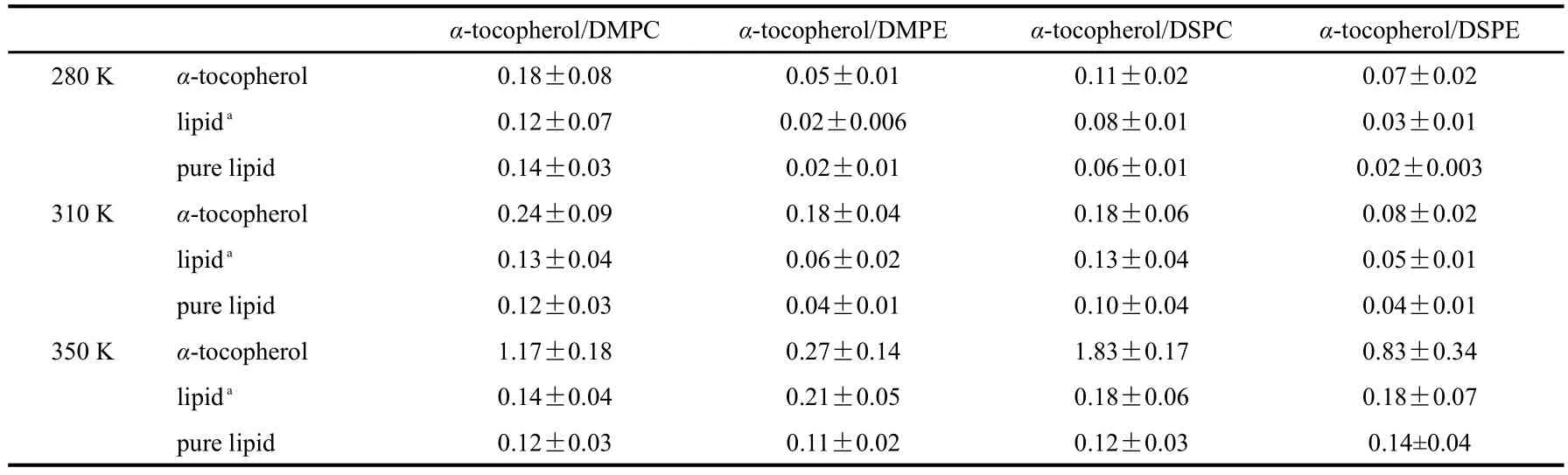
Table 5Lateral diffusion coefficients(×10-7cm2·s-1)ofα-tocopherol and lipids inzdirection at various temperatures
To illustrate the flip-flop properties ofα-tocopherol,a shorttime movie of the vitamin molecule in a DMPC bilayer at 350 K and 1.01325×105Pa is attached to this manuscript as supplementary material.It displays the process ofα-tocopherol firstly leaving the polar region of the upper leaflet of DMPC bilayer,then coming through the non-polar bilayer center and finally contacting with the head groups of the lower leaflet of the bilayer.The movie also illustrates representatively the conformational properties ofα-tocopherol in phospholipid bilayers.
Analysis of the average position of atom O7 inα-tocopherol head reveals that the hydroxyl group stays below the polar region of the simulated lipid bilayers.Combined with our previous results on the average positions of atom O7 ofα-tocopherol in lipid bilayers[32],we conclude that the head group ofα-tocopherol generally moves towards the bilayer center with increasing temperature.Exceptions observed in DSPC and DMPE bilayers can be explained as follows.The O7 ofα-tocopherol are around the 4th carbon of the acyl chains of either DSPE or DMPE at 310 K.This is in agreement with other lipid bilayers.The problem is that the positions at 280 K are too low in these two bilayers.At this particular temperature,DSPC is in“mixed”ripple phase consisting of ordered and disordered domains,andα-tocopherol tends to stay in the disordered one leading to a lower-than-normal position of the O7 atom.For DMPE system at 280 K,DMPE bilayer is in gel phase,and the tocopherol tail has to be folded to stay in the upper leaflet of DMPE bilayer,which leads to the lower location of atom O7 than in other phospholipids.
Hydrogen bond analysis shows thatα-tocopherol mainly forms hydrogen bonds with carbonyl ester groups of phospholipid molecules.This is in consistent with our previous results[27],except that forα-tocopherol/DMPC system at 280 K whereα-tocopherol mainly forms hydrogen bonds with phosphate groups rather than ester groups.This is due to the high position of the hydroxyl group ofα-tocopherol relative to DMPC molecules and the short tails that DMPC molecules possess.
Calculations on tilt angle distributions ofα-tocopherol head and tail show that only one peak exists in the distribution curves ofα-tocopherol head when“flip-flop”behavior does not occur,while two peaks could be observed whenα-tocopher-ol head flips over.And α-tocopherol head usually has larger tilt angles when the lipid matrix is more fluid.Compared with head group,distribution curves for α-tocopherol acyl tails always have broader distributions corresponding to more freely moving modes.
We also calculated the lateral diffusion coefficients of α-tocopherol and lipid bilayers at all three temperatures,the results are basically consistent with our last study[27]on α-tocopherol/lipid systems.In both works,α-tocopherol has a comparable diffusion rate with the phospholipid molecules at low temperature while it moves much faster than the lipids at high temperatures.And α-tocopherol disturbs DMPE and DPPE bilayers drastically at 310 K,which is consistent with the fact that α-tocopherol helps form reversed hexagonal phase in PE bilayers.Additionally,in this work we calculated the diffusion coefficients in the direction perpendicular to the bilayer surface.The results show that it is only a few percent of the lateral diffusion coefficients.
4 Conclusions
(1)Our study reveals that the relative positions of atom O7 in α-tocopherol head in the simulated lipid bilayers depend on both temperature and the structure of lipid molecules.Generally,the head group of α-tocopherol moves towards the bilayer center with increasing temperature,and it flips over in the lipid bilayers at 350 K.However,the length of the lipid tails and the phase of the lipid bilayers also influence the position of atom O7.For example,α-tocopherol can flip-flop at 310 K in DMPC and DMPE bilayers,due to their much shorter acyl tails than DSPC and DSPE bilayers;and α-tocopherol stays close to the DSPC bilayer center at 280 K due to the ripple phase of the DSPC bilayer at this temperature.Thus the α-tocopherol head position is determined compositely by temperature,lipid structure and lipid phase.
(2)The hydrogen bond formation largely depends on the position of the α-tocopherol head.Since α-tocopherol head stays below the polar region of the simulated lipid bilayers,it mainly forms hydrogen bonds with carbonyl ester groups,rather than phosphate and amine groups of the phospholipid molecules.The hydrogen bonds with PEs are more stable than those with phosphatidylcholines(PCs)at low temperatures,which demonstrates that the molecular structure of lipids and the phase of lipid bilayers could also affect the hydrogen bond formation.
(3)The head of α-tocopherol has fluctuating tilt angles relative to the normal of lipid bilayers and the tail is able to occupy many different conformations.The tilt angle distribution is determined by the phase of lipid bilayers and the lipid structure.
(4)The analysis of the dynamic property of α-tocopherol reveals that it has a comparable lateral diffusion rate to phospholipid molecules at low temperature while it moves much faster than the lipids at 350 K.The diffusion rate in the direction perpendicular to the membrane surface is much slower than lateral diffusion rate and can be ignored.
Supporting Information Available:A short-time movie of the vitamin molecule in a DMPC bilayer at 350 K and 1.01325×105Pa is attached to this manuscript as supplementary material.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
Acknowledgments:We are very grateful to Prof.SHUAI Zhi-Gang from the Department of Chemistry at Tsinghua University for computational resources support.
1 Evans,H.M.;Emerson,O.H.;Emerson,G.A.J.Biol.Chem.,1936,113:319
2 Atkinson,J.;Epand,R.F.;Epand,R.M.Free Radical Biol.Med.,2008,44:739
3 Fukuzawa,K.;Matsuura,K.;Tokumura,A.;Suzuki,A.;Terao,J.Free Radical Biol.Med.,1997,22:923
4 Wang,X.Y.;Quinn,P.J.Progress in Lipid Research,1999,38:309
5 Quinn,P.J.Eur.J.Biochem.,1995,233:916
6 Ortiz,A.;Aranda,F.J.;Gomez-Fernandez,J.C.Biochim.Biophys.Acta,1987,898:214
7 Wang,X.Y.;Semmler,K.;Richter,W.;Quinn,P.J.Arch.Biochem.Biophys.,2000,377:304
8 Gao,W.Y.;Chen,L.;Wu,F.G.;Yu,Z.W.Acta Phys.-Chim.Sin.,2008,24:1149 [高文颖,陈 琳,吴富根,尉志武.物理化学学报,2008,24:1149]
9 Wang,X.Y.;Quinn,P.J.Chem.Phys.Lipids,2002,114:1
10 Stillwell,W.;Ehringer,W.;Wang,L.J.;Wassall,S.R.Biophys.J.,1990,57:A271
11 Suzuki,Y.J.;Tsuchiya,M.;Wassall,S.R.;Choo,Y.M.;Govil,G.;Kagan,V.E.;Packer,L.Biochemistry,1993,32:10692
12 Wassall,S.R.;McCabe,M.A.;Ehringer,W.;Stillwell,W.Biophys.J.,1990,57:A473
13 Wassall,S.R.;Thewalt,J.L.;Wong,L.;Gorrisen,H.;Cushley,R.J.Biochemistry,1986,25:319
14 Villalain,J.;Aranda,F.J.;Gomez-Fernandez,J.C.Eur.J.Biochem.,1986,158:141
15 Wassall,S.R.;Phelps,T.M.;Wang,L.J.;Langsford,C.A.;Stillwell,W.Prog.Clin.Biol.Res.,1989,292:435
16 Massey,J.B.Chem.Phys.Lipids,2001,109:157
17 Srivastava,M.L.;Shukla,N.K.;Singh,S.K.;Jaiswal,M.R.J.Membr.Sci.,1996,117:39
18 Srivastava,S.;Phadke,R.S.;Govil,G.;Rao,C.N.R.Biochim.Biophys.Acta,1983,734:353
19 Fragata,Y.M.;Bellemare,Y.F.Chem.Phys.Lipids,1980,27:93
20 Kagan,V.E.;Quinn,P.J.Eur.J.Biochemistry,1988,171:661
21 Sanchez-Migallon,M.P.;Aranda,F.J.;Gomez-Fernandez,J.C.Biochim.Biophys.Acta,1996,1279:251
22 Gomez-Fernandez,J.C.;Aranda,F.C.;Villaloan,J.Location and dynamics of α-tocopherol in membranes//Membrane biotechnology.Gomez-Fernandez,J.C.;Chapman,D.;Packer,L.Eds.Basel:Birkhauser Verlag,1991:98-115
23 Salgado,J.;Villalõan,J.;Gomez-Fernandez,J.C.Arch.Biochem.Biophys.,1993,306:368
24 Urano,S.;Kitahara,M.;Kato,Y.;Hasegawa,Y.;Matsuo,M.J.Nutr.Sci.Vitam.,1990,36:513
25 Aranda,F.J.;Coutinho,A.;Berberan-Santos,M.N.;Prieto,M.J.E.;Gómez-Fernández,J.C.Biochim.Biophys.Acta,1989,985:26
26 Gramlich,G.;Zhang,J.;Nau,W.M.J.Am.Chem.Soc.,2004,126:5482
27 Qin,S.S.;Yu,Z.W.;Yu,Y.X.J.Phys.Chem.B,2009,113:16537
28 Liu,Y.C.;Wang,Q.;Lü,L.H.;Zhang,L.Z.Acta Phys.-Chim.Sin.,2005,21:63 [刘迎春,王 琦,吕玲红,章连众.物理化学学报,2005,21:63]
29 Zhuo,S.C.;Huang,Y.M.;Peng,C.J.;Liu,H.L.;Hu,Y.;Jiang,J.W.J.Phys.Chem.B,2009,114:6344
30 Buttriss,J.L.;Diplock,A.T.Biochim.Biophys.Acta,1988,963:61
31 Zhang,Y.;Turunen,M.;Appelkvist,E.L.J.Nutr.,1996,126:2089
32 Darden,T.;York,D.;Pedersen,L.J.Chem.Phys.,1993,98:10089
33 Essman,U.;Perera,L.;Berkowitz,M.L.;Darden,T.;Lee,H.;Pedersen,L.G.J.Chem.Phys.,1995,103:8577
34 Berendsen,H.J.C.Transport properties computed by linear response through weak coupling to a bath//Computer simulations in material science.Meyer,M.;Pontikis,V.Eds.Dordrecht:Kluwer,1991:139-155
35 Berendsen,H.J.C.;Postma,J.P.M.;DiNola,A.;Haak,J.R.J.Chem.Phys.,1984,81:3684
36 Berendsen,H.J.C.;van der Spoel,D.;van Drunen,R.Comput.Phys.Commun.,1995,91:43
37 Lindahl,E.;Hess,B.;van der Spoel,D.J.Mol.Model,2001,7:306
38 http://moose.bio.ucalgary.ca/index.php?page=Structures_and_Topologies
39 Ryckaert,J.P.;Bellemans,A.Chem.Phys.Lett.,1975,30:123
40 Berger,O.;Edholm,O.;Jähnig,F.Biophys.J.,1997,72:2002
41 Jorgensen,W.L.;Tirado-Rives,J.J.Am.Chem.Soc.,1988,110:1657
42 Essex,J.W.;Hann,M.M.;Richards,W.G.Philos.Trans.R.Soc.B,1994,344:239
43 Chiu,S.W.;Clark,M.;Balaji,V.;Subramaniam,S.;Scott,H.L.;Jakobsson,E.Biophys.J.,1995,69:1230
44 Berendsen,H.J.C.;Postma,J.P.M.;van Gunsteren,W.F.;Hermans,J.Interaction models for water in relation to protein hydration//Intermolecular forces.Pullman,B.Ed.Dordrecht:Reidel,1981:331-342
45 Miyamoto,S.;Kollman,P.A.J.Comput.Chem.,1992,13:952
46 Pearlman,D.A.;Case,D.A.;Cadwell,J.W.;Ross,W.S.;Cheatham III,T.E.;DeBolt,S.;Ferguson,D.;Siebel,G.;Kollman,P.Comput.Phys.Commun.,1995,91:1
47 Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 98.RevisionA.03.Pittsburgh,PA:Gaussian Inc.,1998
48 Bayly,C.I.;Cieplak,P.;Cornell,W.D.;Kollman,P.A.J.Phys.Chem.B,1993,97:10269
49 Stillwell,W.;Ehringer,W.;Wassall,S.R.Biochim.Biophys.Acta,1992,1105:237
50 Qin,S.S.;Yu,Z.W.;Yu,Y.X.J.Phys.Chem.B,2009,113:8114
51 Luzar,A.;Chandler,D.J.Chem.Phys.,1993,98:8160
52 Luzar,A.;Chandler,D.Nature,1996,397:55
53 Lefevre,T.;Picquart,M.Biospectroscopy,1996,2:391
54 Pink,D.A.;McNeil,S.;Quinn,B.;Zuckermann,M.J.Biochim.Biophys.Acta,1998,1368:289
55 Filippov,A.;Oradd,G.;Lindblom,G.Biophys.J.,2003,84:3079
56 Klauda,J.B.;Venable,R.M.;MacKerell Jr.,A.D.;Pastor,R.W.Considerations for lipid force field development//Computational modeling of membrane bilayers.Feller,S.E.Ed.San Diego:ElsevierAcademic Press,2008,60:1-48
57 Micol,V.;Aranda,F.J.;Villalain,J.;Gomez-Fernandez,J.C.Biochim.Biophys.Acta,1990,1022:194
α-生育酚在模型生物膜中的分子动力学模拟
秦姗姗 尉志武*
(清华大学化学系,生命有机磷与化学生物系教育部重点实验室,北京100084)
用分子动力学方法模拟了280,310和350 K下α-生育酚在二豆蔻酰磷脂酰胆碱、二豆蔻酰磷脂酰乙醇胺、二硬脂酰磷脂酰胆碱和二硬脂酰磷脂酰乙醇胺双层膜中的性质,包括了空间位置、氢键、取向和动力学性质,取得了如下的结论.第一,生育酚头部的羟基一般位于脂双层亲疏水界面的下方,升高温度将促进羟基向膜双层的中心移动,在350 K时观察到了在上下两个单层间的翻转.第二,生育酚主要与磷脂的酯基形成氢键,几乎不与磷脂酰乙醇胺的氨基形成氢键;比较生育酚与磷脂酰胆碱和乙醇胺形成的氢键后发现,后者更稳定.第三,生育酚的头部在膜中取向多变,与膜的法线夹角不固定,尾部的构象也很复杂.第四,在温度较低时,生育酚的侧向扩散系数与磷脂的相当,但在350 K时其扩散速度明显加快;在垂直方向生育酚的扩散速度很慢.
维生素E; 磷脂;取向;翻转;扩散;氢键
O641
Received:September 13,2010;Revised:October 22,2010;Published on Web:November 23,2010.
∗Corresponding author.Email:yuzhw@tsinghua.edu.cn;Tel:+86-10-62792492;Fax:+86-10-6277114.
The project was supported by the National Natural Science Foundation of China(20633080,20973100).
国家自然科学基金(20633080,20973100)资助项目
猜你喜欢
杂志排行
物理化学学报的其它文章
- Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Cathodic and Thermal Stabilities of the P(VdF-HFP)-Based Ionic Liquid Composite Polymer Electrolyte
- 表面活性剂对驱油聚合物界面剪切流变性质的影响
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol
