Cathodic and Thermal Stabilities of the P(VdF-HFP)-Based Ionic Liquid Composite Polymer Electrolyte
2014-10-14CUIWenYuANMaoZhongYANGPeiXiaZHANGJinQiu
CUI Wen-Yu AN Mao-Zhong YANG Pei-Xia ZHANG Jin-Qiu
(School of Chemical Engineering and Technology,Harbin Institute of Technology,Harbin 150001,P.R.China)
Cathodic and Thermal Stabilities of the P(VdF-HFP)-Based Ionic Liquid Composite Polymer Electrolyte
CUI Wen-Yu AN Mao-Zhong*YANG Pei-Xia ZHANG Jin-Qiu
(School of Chemical Engineering and Technology,Harbin Institute of Technology,Harbin 150001,P.R.China)
Abstract:We report on a composite polymer electrolyte containing the ionic liquid 1-ethyl-3-methylimidazolium hexafluorophosphate(EMIPF6).This composite polymer electrolyte is based on the poly(vinylidene fluoride-co-hexafluoropropylene)(P(VdF-HFP))polymer matrix and is a potential electrolyte for use in lithium ion batteries.The ionic conductivity of the composite polymer electrolyte was measured by electrochemical impedance spectroscopy(EIS).Linear sweep voltammetry(LSV)was performed to investigate the electrochemical stability window of the polymer electrolyte.The thermal properties for the composite polymer electrolyte were also characterized by thermogravimetry(TG)and by a flammability test.The results show that the presence of the EMIPF6ionic liquid increases the ion transport properties greatly but a better cathodic stability is only obtained by the addition of organic additives such as ethylene carbonate-propylene carbonate(EC-PC),which extends the cathodic stability to 0.3 V.This corresponds to an electrochemical stability window of 0.3-4.3 V.The selected Li4Ti5O12anode and LiCoO2cathode materials exhibit acceptable electrochemical performance in combination with the prepared P(VdF-HFP)/LiPF6/EMIPF6/EC-PC composite polymer electrolyte.At a charge-discharge rate of 0.1C,Li/LiCoO2and Li/Li4Ti5O12have reversible capacities of 130 and 144 mAh·g-1,respectively.However,the corresponding thermal performance is suppressed because of the presence of organic additives.
Key Words:Lithium ion battery;Ionic liquid;Composite polymer electrolyte;Cathodic stability;Thermal stability
Commercial lithium ion batteries using non-aqueous organic solvents as electrolyte has been utilized extensively in portable electronic devices and the promising applications in electric vehicles are also in near future by virtues of attractive high energy density and prolonged cycle life[1].However,the dangers of liquid leakage,flammability,or even explosion are inevitable in abuse conditions due to the flammable and volatile natures of organic solvents.Accordingly,exploiting new type of electrolyte with the enhanced safety is currently the urgent investigation.Polymer electrolyte[2-3]is one of the possible options for improved safety by conversing the liquid into polymer structure.But the technological problem of polymer electrolyte is the relative low ionic conductivity compared with conventional organic electrolyte.During previous years,many methods have been reported to enhance the ionic conductivity of polymer electrolyte,such as addition of nano-inorganic fillers[4-7],incorporation of organic solvent[8-10].However,their electrochemical properties are still unsatisfactory compared with organic electrolyte.
In recent years,ionic liquid,consisted of only cation and anion,is becoming the hot issue in energy storage fields by virtue of low vapor pressure,high conductivity,low toxicity,excellent thermal and electrochemical stabilities[11-12],which are attractive for overcoming the inherent ionic conductivity limitations of polymer electrolytes.In the previous reports,poly(ethylene oxide)(PEO)-based polymer electrolyte containing the ionic liquid of N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide(PYR13TFSI)[13],N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide(PYR14TFSI)[14],1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide(BMPyTFSI)[15]have been investigated extensively,but the ionic conductivity rarely reached 10-3S·cm-1at room temperature.On the other hand,poly(vinylidene fluoride)(PVdF)[16]or poly(vinylidene fluoride-co-hexafluropropylene)P(VdF-HFP)[17-18]polymer electrolyte containing ionic liquid has also aroused much attention due to their higher ionic conductivities.Though the ionic conductivity reaches 10-3S·cm-1at room temperature,the electrochemical performance of cathode or anode materials using such ionic liquid-PVdF(or P(VdF-HFP))composite polymer as electrolyte is not reported in details elsewhere.
Among various ionic liquids,imidazolium cation(EMI+)-based ionic liquid owns the low viscosity and high ion conductivity,which is favorable features to promote the ion transport properties of polymer electrolyte.However,EMI+ionic liquid encounters the problem of cathodic stability[19-22]and trends to discompose around 1.5 V(vs Li/Li+),which makes it unusable in combination with lithium anode or carbon anode.Some literature[18,23]demonstrates that addition of organic solvents(such as ethylene carbonate(EC),propylene carbonate(PC))can improve the cathodic stability effectively by formation of passivation film,which suppresses the decomposition of ionic liquid and improves the interfacial compatibility.However,although the electrochemical performance is improved,it is most likely that the organic additives may reduce the thermal stability of ionic liquid-polymer composite electrolyte again.In this point,related investigations are not reported according to our knowledge and it is important to verify this concern as soon as possible for gaining both better electrochemical performance and higher safety.
In this study,ionic liquid-P(VdF-HFP)composite polymer electrolyte comprised of EMIPF6ionic liquid,P(VdF-HFP)polymer,LiPF6salt,and EC-PC organic additives were synthesized and investigated as possible electrolyte for lithium ion batteries.The performance of Li/LiCoO2and Li/Li4Ti5O12coin cells using prepared composite polymer electrolyte were investigated.Furthermore,thermal stability of the prepared composite polymer electrolyte was evaluated.
1 Experimental
P(VdF-HFP)(MW=500000,Aldrich,USA)and LiPF6(Merck,USA)were received and dried under vacuum for 48 h at 50°C.EMIPF6(Acros Organics,Belgium)was used as received.LiCoO2(Shanshan,Hunan,China)and Li4Ti5O12(Altairnano,USA)were dried under vacuum at 150°C.
Composite polymer electrolyte was prepared as follows:P(VdF-HFP)was dissolved in NMP(N-methyl pyrrolidone)and stirred for 4 h to get transparent solution.EMIPF6and LiPF6were added into the solution and stirred homogeneously for another 6 h.Then the solution was cast onto polytetrafluoroethylene(PTFE)plate and dried at 80°C for 24 h under vacuum to form a thin polymer film.After cooling down naturally to room temperature,the composite polymer P(VdF-HFP)/LiPF6/xEMIPF6(x=m(MIPF6)/m(LiPF6)=m(EMIPF6)/m(P(VdF-HFP)),was obtained.The mixed solvents of EC-PC were further added onto the surface of composite polymer(m(EC):m(PC)=1:1,m(EC-PC):m(P(VdF-HFP))=9:17)for modification.
The scanning electron microscopy(SEM,KYKY-EM3200)was tested to observe morphology of the prepared composite polymer films.The thermal stability test was performed by thermogravimeter(TG,ZRY-2P).The flammability test was conducted by igniting the composite polymer to check the burning degree and time.
The electrochemical stability window was performed by linear sweep voltammetry(LSV)technique with three-electrode cell at the rate of 0.5 mV s-1,where stainless steel(SS)was used as working electrode and metal lithium was used as both counter and reference electrodes.The ionic conductivities dependence of temperature(30-80°C)was measured by electrochemical impedance spectroscopy(EIS)with blocking cell(SS/composite polymer film/SS)in frequency range of 105-10-2Hz by electrochemical analyzer(CHI604b).The blocking cell was thermally equilibrated at each temperature for at least 2 h prior to measurements.The value of ion conductivity was calculated by following equation:σ=L/(Rb×S),where L is the thickness of the composite polymer film,S is the area of the electrode,and Rbis the bulk resistance accessed from Nyquist plots.Ion trans-ference numbers(t+)were determined by EIS and chronoamperometry(CA)with symmetric cell(Li/IL-gel film/Li)according to opening literature[16,24].The value was calculated by following equation:
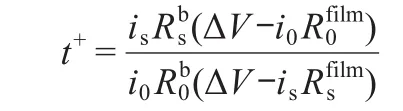
where,the subscripts“0”and“s”indicate initial values and steady-state values,Rbis the bulk resistance,Rfilmis the passive film resistance,ΔV is the applied bias potential(0.01 V),and i is the current.The values of all resistances were obtained from the EIS of symmetric cell before and after application of bias potential.
The performance of selected LiCoO2or Li4Ti5O12was estimated with coin cell,where metal lithium was used as counter electrode and the prepared composite polymer film was used as both electrolyte and separator.The electrodes were prepared by casting slurry(80%(w)active powder,10%(w)acetylene black,10%(w)PVdF)on current collector(copper foil for Li4Ti5O12and aluminum foil for LiCoO2)and dried at 120°C for 10 h under vacuum.The electrodes were pressed and cut into disks as the research electrodes.Coin cells were assembled in argon-filled glove box and cycled within the voltage of 1-3 V(Li/Li4Ti5O12)or 2.7-4.2 V(Li/LiCoO2).
2 Results and discussion
Fig.1 shows the typical optical images of the composite polymer film P(VdF-HFP)/LiPF6/EMIPF6,which presents the flexible,freestanding and transparent features.The thickness of the films is tested to be 140-160 μm.
Fig.2 is the SEM images of the composite polymer film P(VdF-HFP)/LiPF6/xEMIPF6.For x=0 in Fig.2(a),the surface of polymer film is found to be heterogeneous with heaves on surface,which is probably due to the incomplete miscibility between polymer and LiPF6.Small pores less than 5 μm are formed on most surfaces but partial surface is still imperforate,which is disadvantageous for ion transportation during electrochemical reaction.In Fig.2(b),the surface of polymer film becomes homogeneous with smooth surface,indicating that the addition of EMIPF6can enhance the solubility of LiPF6in polymer and facilitate the homogenization of polymer film.Micropores in size of 4-10µm are evidenced over the surface with the pore-wall thickness of 5-15µm.However,as the EIMPF6concentration increases to x=2,it is found in Fig.2(c)that the smooth surface of the polymer film is disappeared and becomes much rough with the decreased pores amounts.Partial pores are jammed by collapsed pore walls,which is tend to obstruct the movement of ions or reduce the mechanical strength of the polymer film.This morphology is adverse for satisfactory performance during long cycle life of batteries.Based on these analyses,further discussion was emphasized on P(VdFHFP)/LiPF6/EMIPF6(x=1).

Fig.3 shows the electrochemical stability windows of the composite polymer film P(VdF-HFP)/LiPF6/EMIPF6(x=1)with or without EC-PC additives.Both stabilities on anodic and cathodic sides are present.On anodic side,the current onset is less sensitive to the EC-PC additives and the anodic stability is only enhanced a little from 4.1 to 4.3 V,which suggests that the obtained composite polymer electrolyte can besafely used in combination with most common cathode materials(such as LiCoO2,LiFePO4).On the cathodic side,however,the current onset is sensitive to EC-PC additives and for no EC-PC additives,the cathodic current starts to increase quickly from 1.5 V,indicating the decomposition of EMIPF6ionic liquid.This cathodic instability of EMIPF6ionic liquid has been reported elsewhere[25]and is attributed to the drawback of poor cathodic electrochemical stability caused by attacking to hydro-gen at C(2)carbo)site[26].After the presence of EC-PC,the cathodic stability is improved obviously.The current onset is retarded to 1.3 V and the subsequent cathodic current is much weaker than that of P(VdF-HFP)/LiPF6/EMIPF6.It is known that EC can also act as the solid electrolyte interface film(SEI film)formation solvent and thus it is reasonable to deduce that the addition of EC-PC forms a SEI film,which suppresses the reduction of EMIPF6ionic liquid and gives rise to a weak current.However,it seems that although the SEI film is formed,the decomposition of ionic liquid is not suppressed completely,because the cathodic current does not return to zero but still keeps increasing very slowly up to 0.3 V.Below 0.3 V,the cathodic current starts to increase relative quickly again,indicating an extended cathodic stability of 0.5 V is gained by addition of EC-PC.Considering rigorously,anode material with working voltage extended to 0 V should be excluded and relative high voltage anode material can be used(such as Li4Ti5O12).

Fig.3 Electrochemical windows of the P(VdF-HFP)/LiPF6/EMIPF6(x=1)with or without EC-PC additives
Table 1 shows the ionic conductivity(σ)dependence of P(VdF-HFP)/LiPF6/xEMIPF6(x=0,1)on temperature(30-80°C).Sample without ionic liquid(x=0)is present for comparison.It is seen that the ion conductivity is enhanced gradually as the temperature increases from 30 to 80 °C.For instance of 30 °C,theσof P(VdF-HFP)/LiPF6(x=0)is calculated to be 0.129mS·cm-1and improved pronouncedly to 1.295 mS·cm-1(x=1)by addition of EMIPF6ionic liquid,which is much higher than previously reportedσvalues for other gel polymer electrolytes[12-13,27].Effects of EC-PC onσare also investigated here and it is seen that the EC-PC additives can further improve the value ofσto 1.650mS·cm-1(x=1)due to the high dielectric constant of EC and PC.

Table 1 Ion conductivity of P(VdF-HFP)/LiPF6/xEMIPF6with or without EC-PC additives
The relationship between ionic conductivity(σ)and temperature is shown in Fig.4.For all samples,the logarithmic plots of ionic conductivityversus1/Tare approximated to Vogel-Tammann-Fulcher(VTF)equation(σ(T)=AT--0.5eB/T-T0)over the examined temperature range,whereAis the pre-exponential factor,T0=Tg-const(const=20-50 K),andBis a pseudo-activation energy for the charge-carrier motion.No abrupt changes are exhibited in the conductivityversustemperature plots,suggesting that these composite polymer films likely retain the same ionic conduction mechanism in the examined temperature range.
Table 2 shows the cationic transference numbers(t+)of the composite polymer films.It is seen that P(VdF-HFP)/LiPF6possesses thet+of 0.529,which is reduced to 0.479 by addition of EMIPF6due to the relative high viscosity of ionic liquid.However,the value oft+is enhanced again to 0.547 by EC-PC additives.As is known,Li+cations move between the anode and cathode electrodes.However,the other ions in the electrolyte(i.e.,EMI+and P)are also mobile and may polarize a cell when current is passed.Ideally,all charges are carried by Li+which gives a Li transference number of unity(t+=1).In practice,most electrolytes have much lower values than unity due to other counter anions.By further application of equation:σLi+=t+×σ,the Li+conductivity can be obtained,which reflects the actual transport properties of Li+involved in electrochemical reactions.As is seen,a tiny Li+conductivity of 0.068 mS·cm-1is obtained in P(VdF-HFP)/LiPF6,indicating that most Li+ionsare blocked in polymer matrix and few can participate in the electrochemical reaction.However,the presence of EMIPF6ionic liquid(x=1)and EC-PC additives(x=1,with EC-PC)are both effective in improving the Li+conductivity to 0.620 and 0.902 mS·cm-1,which is suggested by other reports18],i.e.,addition of EC or PC additive can improve the properties of ion transport by virtue of high dielectric constant.Based on above discussion,P(VdF-HFP)/LiPF6/EMIPF6/EC-PC with the best ion transport properties is used as the electrolyte to assess the cell performance.
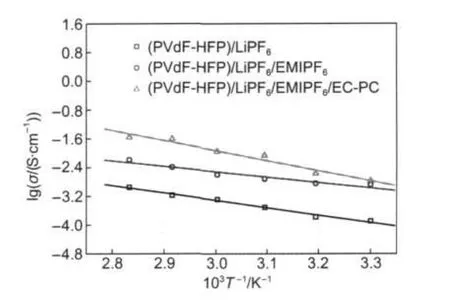
Fig.4 Relationships between ionic conductivity and temperature

Table 2 Transference numbers of P(VdF-HFP)/LiPF6/xEMIPF6with or without EC-PC additives
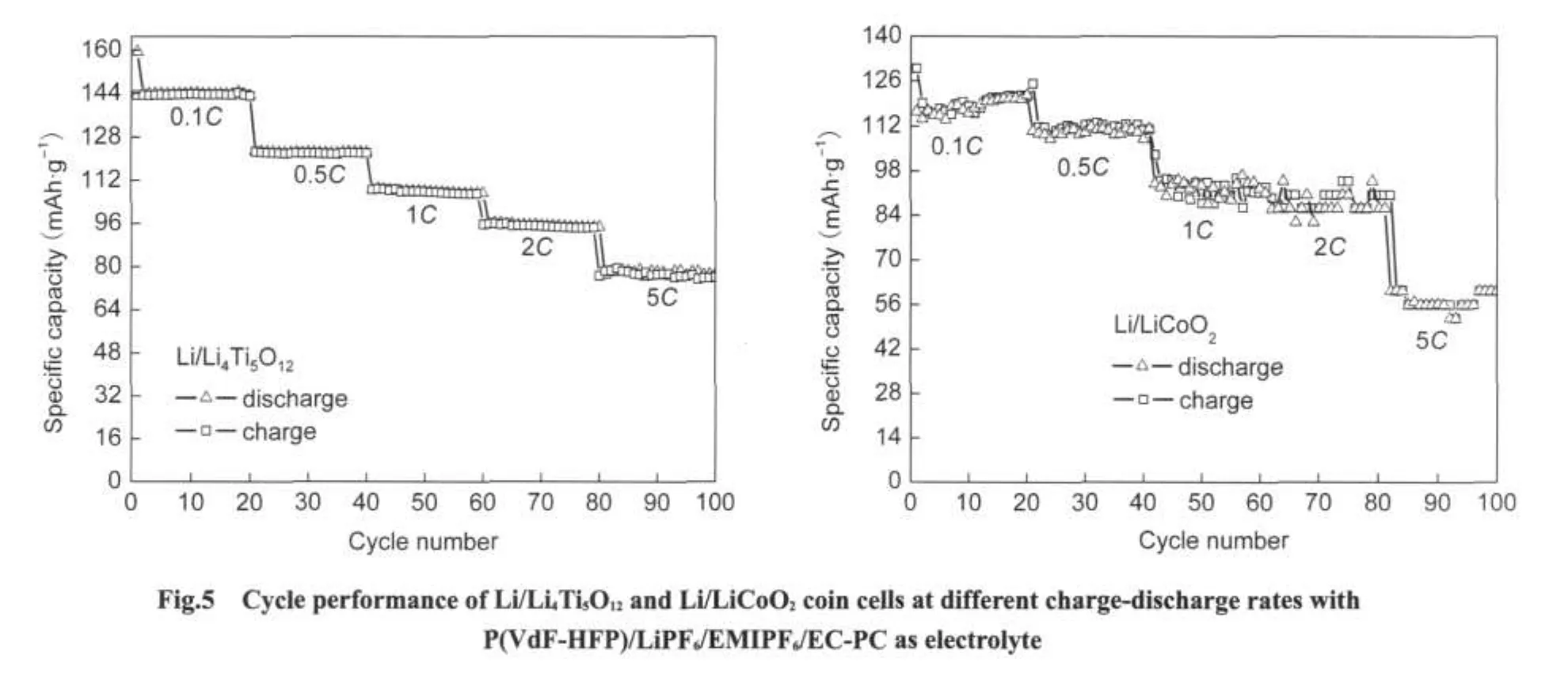
Fig.5 shows the cycle performance of Li/Li4Ti5O12and Li/LiCoO2cell with P(VdF-HFP)/LiPF6/EMIPF6/EC-PC as electrolyte at different charge-discharge rates(0.1C,0.5C,1C,2C,5C).As is interpreted,Li/Li4Ti5O12exhibits the initial coulombic efficiency of 90%and stable reversible capacities at each rate.For instance,a stable reversible capacity 144 mAh·g-1is exhibited at 0.1Cand 107 mAh·g-1at 1C.As the charg-discharge rate is advanced to 5C,53%capacity is remained relative to initial capacity.In case of Li/LiCoO2,the initial coulombic efficiency is 89.6%and the reversible capacity of 130 mAh·g-1is given out at 0.1C.At 5C,the reversible capacity decreases to 56 mAh·g-1,i.e.,43%capacity relative to initial capacity.Seen from the battery performance,it is objective to say that the prepared P(VdF-HFP)/LiPF6/EMIPF6/EC-PC composite polymer electrolyte can be used in combination with high voltage cathode material or relative high voltage anode material and an acceptable electrochemical performance can be obtained compared with conventional organic liquid electrolyte.
Fig.6 displays the TG analysis of P(VdF-HFP)/LiPF6/EMIPF6and P(VdF-HFP)/LiPF6/EMIPF6/EC-PC.LiPF6and EMIPF6are also present for comparison.As is shown,LiPF6salt starts to decompose at 173.1°C and a total mass loss of 78%is estimated up to 400.0°C.EMIPF6ionic liquid shows a high thermal stability and the mass loss occurs as high as 352.7 °C with a mass loss of 79%up to 400.0 °C,which is above the flash point of most common organic electrolyte(~200.0 °C)[28-29].Generally,P(VdF-HFP)polymer decomposes around 140.0°C[30],so in case of P(VdF-HFP)/LiPF6/EMIPF6,the first mass loss stage ranged in 133.3-198.6°C is mainly attributed to the decomposition of LiPF6salt and P(VdF-HFP)polymer(a mass loss of 33.5%).The second mass loss stage happens in range of 372.7-400.0°C,which is related to the decomposition of EMIPF6ionic liquid.After addition of EC-PC,the mass loss of 73%is mainly ranged in 145.0-194.8°C due to the decomposition of LiPF6salt,P(VdF-HFP)polymer,andEC-PC.Comparing the thermal properties between P(VdF-HFP)/LiPF6/EMIPF6and P(VdF-HFP)/LiPF6/EMIPF6/EC-PC,it is seen that the presence of EC-PC additives debases the thermal stability of the composite polymer film due to the low evaporation or decomposition temperature of EC and PC.

Fig.7 presents the flammability tests of 1 mol·L-1LiPF6+EC/DEC/EMC(1:1:1,volume ratio),P(VdF-HFP)/LiPF6/EMIPF6and P(VdF-HFP)/LiPF6/EMIPF6/EC-PC.The results are summarized in Table 3.It is seen that 1 mol·L-1LiPF6+EC/DEC/EMC(1:1:1)electrolyte burns strongly for 10 s.In case of P(VdF-HFP)/LiPF6/EMIPF6,the non-inflammable feature is exhibited without burning phenomenon.However,incorporating the additives of EC-PC makes the composite polymer film flammable again but the weak burning and reduced burning time of 4 s are observed,indicating that the non-flammable EMIPF6ionic liquid may have a certain positive effect on suppressing the burning degree of P(VdF-HFP)/LiPF6/EMIPF6/EC-PC compared with 1 mol·L-1LiPF6+EC/DEC/EMC(1:1:1)electrolyte.
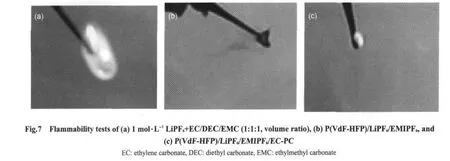
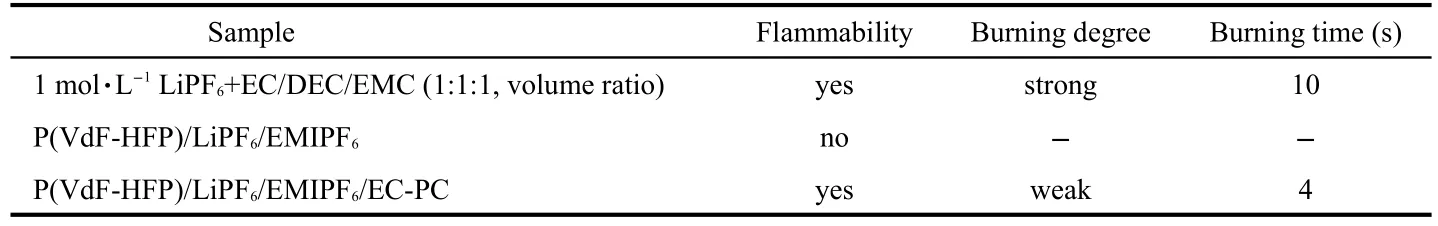
Table 3 Results of flammability tests for different samples
3 Conclusions
In this study,P(VdF-HFP)composite polymer containing EMIPF6ionic liquid is reported and used as electrolyte for lithium ion batteries.The ion transport properties are improved greatly by addition of EMIPF6ionic liquid and EC-PC additives.On the other hand,EC-PC additives can extend the cathodic stability to 0.3 V by forming SEI film and an electrochemical stability window of 0.3-4.3 V can gain for P(VdF-HFP)/LiPF6/EMIPF6/EC-PC electrolyte.The selected Li4Ti5O12anode and LiCoO2cathode show acceptable battery performance,indicating the potential application of such composite polymer electrolyte in lithium ion batteries.However,the thermal stability is debased due to the relative low vapor or decomposition temperatures of EC-PC.
1 Sung,M.G.;Hattori,K.;Asai,S.Materials and Design,2009,30:387
2 Song,J.Y.;Wang,Y.Y.;Wan,C.C.J.Power Sources,1999,7:183
3 Alper,J.Science,2002,296:1224
4 Scrosati,B.;Croce,F.;Persi,L.J.Electrochem.Soc.,2000,147:1718
5 Croce,F.;Appetecchi,G.B.;Persi,L.;Scrosati,B.Nature,1998,394:456
6 Croce,F.;Curini,R.;Martinelli,A.;Persi,L.;Ronci,F.;Scrosati,B.;Caminiti,R.J.Phys.Chem.B,1999,103:10632
7 Wieczorek,W.;Lipka,P.;Zukowska,G.;Wycislik,H.J.Phys.Chem.B,1998,102:6968
8 Saito,Y.;Stephan,M.;Kataoka,H.Solid State Ionics,2003,16:149
9 Forsyth,M.;Meakin,P.M.;MacFarlane,D.R.Electrochim.Acta,1995,40:2339
10 Adebahr,J.;Forsyth,M.;MacFarlane,D.R.;Gavelin,P.;Jacobsson,P.Solid State Ionics,2002,14:303
11 Noda,A.;Hayamizu,K.;Watanabe,M.J.Phys.Chem.B,2001,105:4603
12 Tokuda,H.;Hayamizu,K.;Ishii,K.;Susan,M.A.B.H.;Watanabe,M.J.Phys.Chem.B,2004,108:16593
13 Shin,J.H.;Henderson,W.A.;Passerini,S.Electrochem.Commun.,2003,5:1016
14 Shin,J.H.;Henderson,W.A.;Appetecchi,G.B.;Alessandrini,F.;Passerini,S.Electrochim.Acta,2005,5:3859
15 Cheng,H.;Zhu,C.;Huang,B.;Lu,M.;Yang,Y.Electrochim.Acta,2007,52:5789
16 Fortunato,R.;Branco,L.C.C.;Afonso,A.M.;Benavente,J.;Crespo,J.G.J.Membrane Science,2006,270:42
17 Fuller,J.;Breda,A.C.;Carlin,R.T.J.Electrochem.Soc.,1997,144:L67
18 Fuller,J.;Breda,A.C.;Carlin,R.T.J.Electroanal.Chem.,1998,459:29
19 Nishida,T.;Tashiro,Y.;Yamamoto,M.J.Fluorine Chem.,2003,120:135
20 Hagiwara,R.;Hirashige,T.;Tsuda,T.;Ito,Y.J.Fluorine Chem.,1999,99:1
21 Matsumoto,H.;Miyazakj,Y.Chem.Lett.,2000:922
22 Bonhôte,P.;Dias,A.P.;Papageorgiou,N.;Kalyanasundaram,K.;Grätzel,M.Inorg.Chem.,1996,35:1168
23 Ye,H.;Huang,J.;Xu,J.J.;Khalfan,A.;Greenbaum,S.G.J.Electrochem.Soc.,2007,154:A1048
24 Evans,J.;Vincent,C.A.;Bruce,P.G.Polymer,1987,28:2324
25 Zhang,S.M.;Hou,Y.W.;Huang,W.G.;Shan,Y.K.Electrochim.Acta,2005,50:4097
26 Kim,K.S.;Park,S.Y.;Choi,S.;Lee,H.J.Power Sources,2006,155:385
27 Tokuda,H.;Hayamizu,K.;Ishii,K.;Susan,M.A.B.H.;Watanabe,M.J.Phys.Chem.B,2005,109:6103
28 Botte,G.G.;White,R.E.;Zhang,Z.M.J.Power Sources,2001,97-98:570
29 Wang,Q.S.;Sun,J.H.;Yao,X.L.;Chen,C.H.Journal of Loss Prevention in the Process Industries,2006,19:561
30 Saikia,D.;Kumar,A.Electrochim.Acta,2004,49:2581
P(VdF-HFP)-基离子液体复合聚合物电解质的阴极稳定性及热稳定性
崔闻宇 安茂忠*杨培霞 张锦秋
(哈尔滨工业大学化工学院,哈尔滨150001)
以聚偏氟乙烯-六氟丙烯P(VdF-HFP)聚合物为基体,制备了含离子液体1-甲基-3-乙基咪唑六氟磷酸盐(EMIPF6)、用于锂离子电池的离子液体复合聚合物电解质[P(VdF-HFP)/LiPF6/EMIPF6/EC(碳酸乙烯酯)-PC(碳酸丙烯酯)].采用热重分析法以及燃烧实验测试了复合聚合物电解质的热稳定性.离子电导率测试表明,离子液体的存在显著改善了复合聚合物电解质的离子传输;循环伏安测试表明,添加剂EC和PC的加入提高了复合电解质的阴极稳定性,制得的离子液体复合聚合物电解质在0.3-4.3 V电压范围内稳定存在.Li4Ti5O12和LiCoO2为电极材料、P(VdF-HFP)/LiPF6/EMIPF6/EC-PC为电解质的半电池表现出优良的循环性能,0.1C充放电倍率下,Li/LiCoO2和Li/Li4Ti5O12半电池的可逆容量分别为130和144 mAh·g-1.但EC、PC在一定程度上降低了离子液体复合聚合物电解质的热稳定性.
锂离子电池;离子液体;复合聚合物电解质;阴极稳定性;热稳定性
O646
Received:September 20,2010;Revised:October 28,2010;Published on Web:November 25,2010.
∗Corresponding author.Email:mzan@hit.edu.cn;Tel/Fax:+86-451-86413721.
The project was supported by the Natural Science Foundation of Heilongjiang Province,China(B2007-05).
黑龙江省自然科学基金(B2007-05)资助项目
猜你喜欢
杂志排行
物理化学学报的其它文章
- Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Electronic Structures and Optical Properties of Ilmenite-Type Hexagonal ZnTiO3
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
- Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol
