紫外光下Ag掺杂TiO2薄膜基底对VO2相转变温度的影响*
2014-10-12郑金玉吴梁鹏王学伟周凤玲李新军
郑金玉,吴梁鹏,王学伟,周凤玲,徐 刚,李新军†
(1. 中国科学院广州能源研究所,中国科学院可再生能源重点实验室,广州 510640;2. 深圳市环境工程科学技术中心有限公司,深圳 518057;3. 莫纳什大学克雷顿校区化学系,维多利亚,澳大利亚 3800)
紫外光下Ag掺杂TiO2薄膜基底对VO2相转变温度的影响*
郑金玉1,2,吴梁鹏1,王学伟1,周凤玲3,徐 刚1,李新军1†
(1. 中国科学院广州能源研究所,中国科学院可再生能源重点实验室,广州 510640;2. 深圳市环境工程科学技术中心有限公司,深圳 518057;3. 莫纳什大学克雷顿校区化学系,维多利亚,澳大利亚 3800)
利用溶胶-凝胶法和浸渍提拉技术制备了不同结构银掺杂二氧化钛薄膜为基底材料的 VO2薄膜,考察了Ag分级配置的二氧化钛薄膜基底材料对VO2薄膜相变温度的影响。在紫外灯照射下测试面内电阻随温度,电压随时间的变化,结果表明基底材料为Ag分级配置的VO2/TiO2薄膜相变温度点明显降低。这可能是由于光照条件下空穴载流子从基底材料注入到 VO2薄膜导致相变温度点偏移。因此,不同结构银掺杂二氧化钛薄膜为基底材料的VO2薄膜能够根据环境温度和太阳光线变化而应用于光热致变色智能窗。
银掺杂二氧化钛;VO2;相转变温度
0 Introduction
Vanadium dioxide (VO2) has been extensively studied since long time for its ultra-fast (sub-ps) thermally-driven metal-insulator transition (MIT) occurring at a critical temperature of 68°C[1]. It is one of the most promising materials for temperature sensing devices, electrical and infrared light switching device, storage medium and especially thermochromic windows which enable automatic solar/heat control in response to environmental temperature[2-6].
MIT of VO2is characterized by a thermal hysteresis that reflects in hysteretic optical, electrical and structural properties. In pure single crystals, this MIT is sharp and hysteresis width is limited in a range of few degrees Celsius[7]. In thin VO2films grown on different substrates such as Al2O3[8], TiO2(001)[9]and amorphous SiO2[10], the MIT temperature, its amplitude and sharpness as well as the width of the hysteresis depend on many parameters such as strain, film quality, sample micro-structure (grain size, punctual defects, grain orientation), chemical doping and lattice internal stress[11,12]. Gao et al. fabricatedVO2@TiO2core/shell nanoparticles with clear heteroepitaxial coherent interfaces using a sol-gel based coating method. The TiO2overcoating not only increased the luminous transmittance of VO2based on an antireflection effect, but also modified the intrinsic colour of VO2films from yellow to light blue. The TiO2also enhanced the chemical stability of VO2against oxidation[13]. Jin et al. prepared and characterized the VO2/TiO2core-shell particles, and found that an ultrathin TiO2shell coating on VO2can lower the hysteresis from 23.5°C to 12.0°C due to interfacial stress and confinement[14].
In recent years, there have been efforts in the case of oxide heterostructures to achieve carrier control, which open a possibility to use them as promising materials for electronic devices. The phase transitions can be tuned by appropriate dopant element for dielectric constant modulation such as titanium with dopant element, or by external fields such as electric field[15,16]. In a La0.8Sr0.2MnO3film grown on an insulating SrTiO3substrate, Katsu et al. observed a decrease in the Curie temperature by 10 K under ultraviolet (UV) light irradiation[17]. Hiroi et al. studied the efficiency of photocarrier injection in a VO2/TiO2:Nb heterostructure by measuring I-V characteristics at room temperature under ultraviolet light irradiation. It is revealed that photogenerated hole carriers in the TiO2:Nb substrate are injected and accumulated in the VO2film by the photovoltaic effect[18]. Muraoka et al. presented a highly efficient and tunable photocarrier injection (PCI) method by using TMO heterostructures. A dramatic decrease in resistance in VO2/TiO2: Nb heterostructures was observed by UV light irradiation, but the shift of phase transition temperature was little mentioned[19]. Since the phase transition is a function of carrier concentration, it could be induced by carrier injection from the substrate. In our previous work, we presented silver-modified nano-structured TiO2thin films with various Ag/TiO2molar ratios, which use a layer-by-layer dip-coating (LLDC) technique to cause a highly efficient photogenerated carrier separation[20]. Herein, we investigate the effect of an Ag doping TiO2film as a substrate on the phase transition temperature shift of VO2.
1 Experimental
The TiO2and Ag doping TiO2film as a substrate were prepared according to the reference[21]. First, TiO2sol was prepared through hydrolyzation of tetrabutyl titanate. The preparation of Ag-TiO2sol was similar to that of TiO2sol. The only difference was the addition of 0.5% mole of AgNO3and ammonia during the hydrolyzation process. The clean soda lime glass slices were dipped into TiO2sol or Ag-TiO2sol and withdrawn at a speed of 2 mm·s-1. They were baked at 100°C for 10 minutes. The above process was repeated according to table 1 in order to obtain a hierarchical TiO2structure. Finally, the glass slices with various TiO2hierarchical structures were annealed at 500°C for 2 hours. According to the coating order and the nature of the TiO2sol gel, three types of the TiO2thin films were constructed and marked as AT (the bottom layer was Ag-modified, and the surface layer was pure TiO2), TT (pure TiO2thin film) and AA (TiO2thin film was uniformly Ag-modified). The VO2film was fabricated on titanium oxide substrates by sol-gel method. 5g V2O5powders were heated to 900°C, kept for 0.5 hours, then the melting V2O5was rapidly poured into 200 mL water, stirred violently, to obtain a fulvous V2O5sol. The titanium oxide substrates were dipped into fulvous V2O5sol and withdrawn at a speed of 2 mm·s-1. They were baked at 100°C for 10 minutes. The second process was repeated. Finally, the above substrates were annealed under vacuum at 500°C for 2 hours.
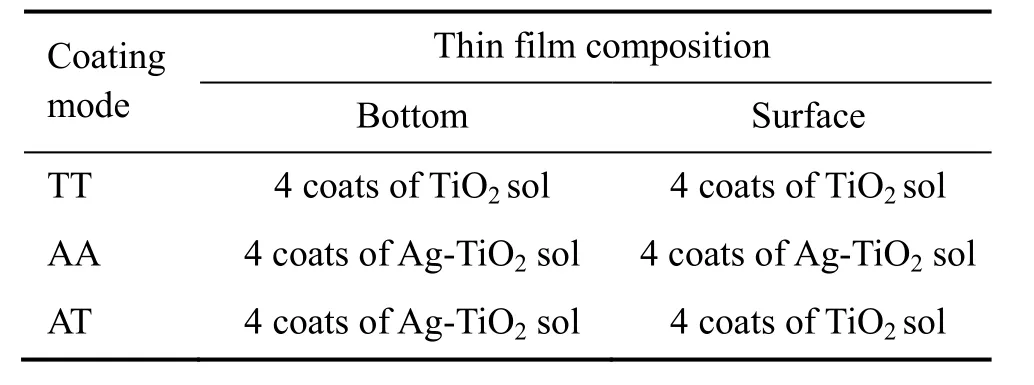
Table 1 Preparation of TiO2 thin films using the lay-by-layer dip coating technique
The temperature dependence of in-plane resistance was measured with the standard four-probe method in the dark or under UV light irradiation. Electrochemical characterization was performed in a three-electrode system made of quartz cells linked with an electrochemical station (CHI660, Chenhua). A TiO2/VO2thin film (on ITO glass) electrode, a platinum sheet and an Ag/AgCl electrode served as the working electrode, counter electrode and reference electrode, respectively. A 0.5 mol·L-1Na2SO4aqueous solution was employed as a supporting electrolyte. The photoelectrochemical tests were carried out under the illumination of a mercury lamp (5 W, λp= 365 nm) at room temperature.
2 Results and discussion
The large width of the MIT of VO2grown on the cantilever compared to that of single crystals[7]or films grown on TiO2(001)[9]is linked to the progressive formation of metallic clusters that, in turn, depends on the presence of grains, defects and lattice strain. Figure 1 shows the temperature dependences of in-plane resistance of VO2/Ag-TiO2composite films measured in the dark or under light irradiation by four-probe method. Temperature is changed over the whole sample by heating the sample holder. The solid lines show little changes for the in-plane resistance curves of the composite films without UV irradiation. There exhibits an abrupt phase transition around a critical temperature of 68°C, at which the film resistance varies with a magnitude of four. This implies that the VO2films had been successfully synthesized by the sol-gel process. The red lines show the variation of the in-plane resistances of the composite films under UV irradiation. For the VO2/AA film, the phase transition point almost keeps unchanged, whereas the shifts of about 3.5°C and 1.5°C for the VO2/AT and VO2/TT films can be obviously observed, respectively.
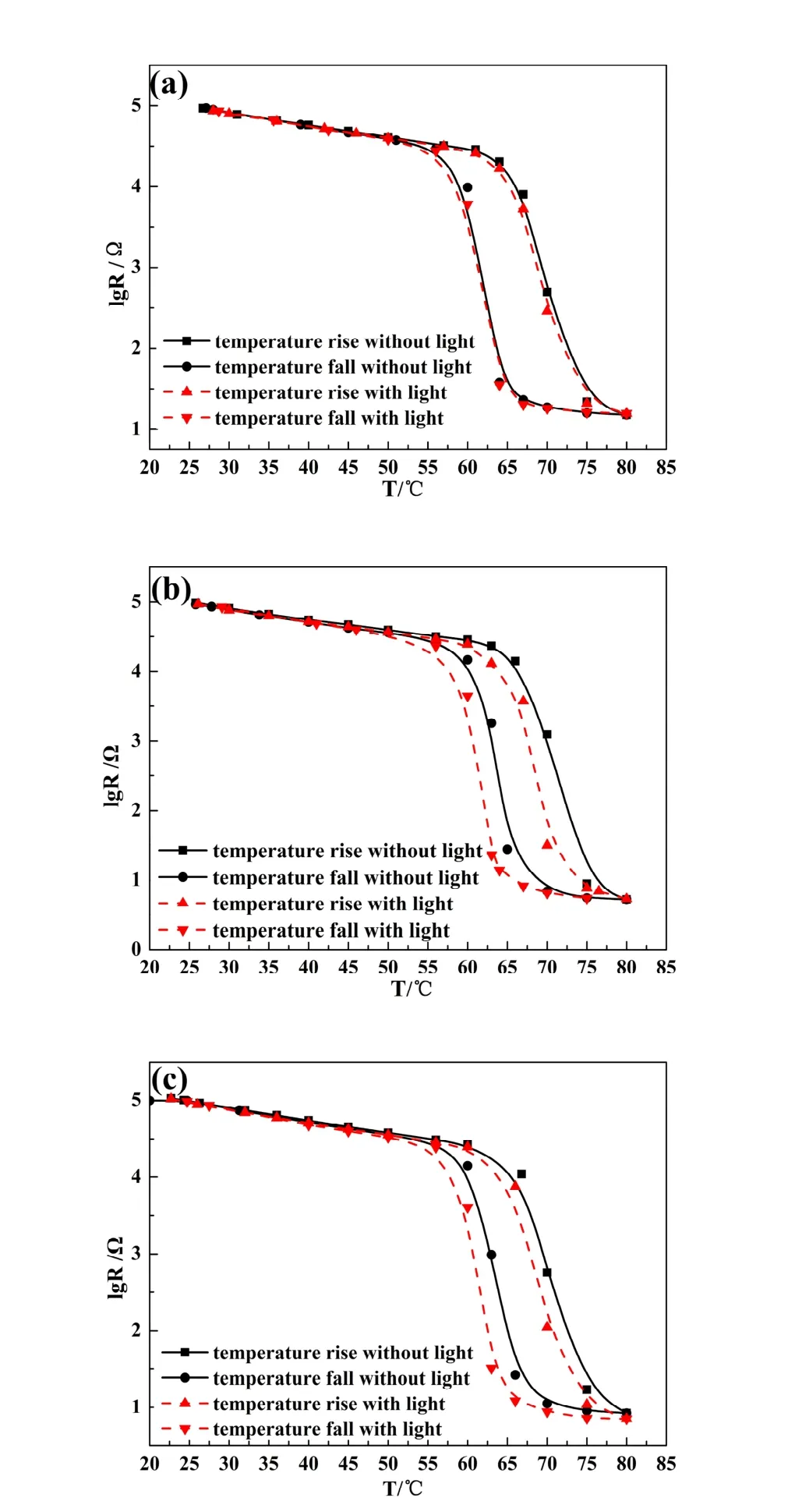
Fig. 1 The comparison of the resistance switching property of three different composite films under and without UV irradiation, (a) VO2/AA, (b) VO2/AT, (c) VO2/TT
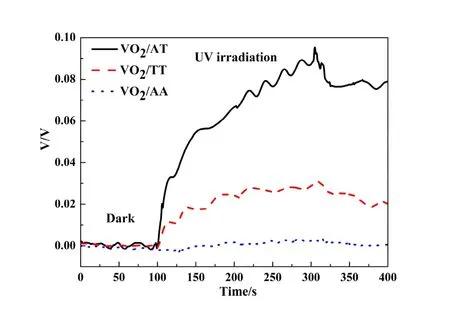
Fig. 2 The open circuit potential varies with time for the composite films
It is important to note that the efficiency of photo carrier injection is governed by the light absorption process at low irradiance, while is determined by the capacitance at high irradiance. Figure 2 shows the variation of open-circuit voltage, VOC, with VO2/TiO2thin film electrodes in a three-electrode system. Interestingly, the values of VOCfor the VO2/AA, VO2/TT and VO2/AT are near zero, 0.027 V and 0.073 V, respectively, which are consistent to their phase transition temperature shifts. The observation of positive photovoltage for VO2/TT and VO2/AT films implies that the hole carriers of TiO2are injected to the VO2film during irradiation[19]. The Ag doping mode has an effect on the injected hole carriers of TiO2under UV light irradiation. For the AA film, Ag was uniformly distributed in the TiO2film, the photogenerated holes would be consumed because the Ag acts as recombination centers. So AA film could contribute little hole carriers to VO2film. For the AT thin films, the Ag in the bottom would attract the electrons from the interface layer between TiO2and VO2to the bottom layer of the TiO2film, and the holes will be left on the interface layer of the thin film[7]. The enriched holes in the interface layer can be prone to injecting into the VO2film and cause the phase transition point of VO2shifting to lower temperature effectively. The results suggest that distribution of non-volatile clusters determines the progressive enlargement or formation of new metallic regions. These clusters may act as seeds for the temperature induced phase transition mediated by electrical current that flows through the device in percolative manner[22].
3 Conclusion
An obvious decrease in the phase transition temperature point was observed for VO2/TiO2with a silver hierarchical configuration under ultraviolet light irradiation. The TMO film could be applied as a photo-thermochromic smart window to enable automatic solar/heat control in response to environmental temperature and solar light.
Acknowledgment
This work is supported by the Science&Technology Plan Project of Guangzhou City, China (No.2013J4300035), the National Natural Science Foundation of China (No.51172233) and the Foundation of the Key Laboratory of Water and Air Pollution Control of Guangdong Province (GD2012A05).
[1] Morin F J. Oxide which show a metal to insulator transition at the neel temperature[J]. Physical Review Letters, 1953, 9: 34-36.
[2] Chen S H, Ma H, Dai J, et al. Nanostructured vanadium dioxide thin films with low phase transition temperature[J]. Applied Physics Letters, 2007, 90(10): 101117.
[3] Ganqvist C G, Avendano E, Azens A. Electrochromic coatings and devices: Survey of some recent advances[J]. Thin Solid Films, 2003, 442: 201-211.
[4] Yan J Z, Zhang Y, Huang W X, et al. Effect of Mo-W Co-doping on semiconductor-metal phase transition temperature of vanadium dioxide film[J]. Thin Solid Films, 2008, 516: 8554-8558.
[5] Xu G, Jin P, Tazawa M, et al. Tailoring of Luminous Transmittance upon Switching for Thermochromic VO2Films by Thickness Control[J]. Japanese Journal of Applied Physics, 2004, 1: 186-187.
[6] Kivaisi R T, Samiji M. Optical and electrical properties of vanadium dioxide films prepared under optimized RF sputtering conditions[J]. Solar Energy Materials and Solar Cells, 1999, 57: 141-152.
[7] Mun B S, Chen K, Yoon J, et al. Nonpercolative metal-insulator transition in VO2single crystals[J]. Physical Review B, 2011, 84: 113109.
[8] Lysenko S, Vikhnin V, Fernandez F, et al. Photoinduced insulator-to-metal phase transition in VO2crystalline films and model of dielectric susceptibility[J]. Physical Review B, 2007, 75: 075109.
[9] Nagashima K, Yanagida T, Tanaka H, et al. Interface effect on metal-insulator transition of strained vanadium dioxide ultrathin films[J]. Journal of Applied Physical, 2007, 101: 026103.
[10] Rúa A, Fernández F E, Sepúlveda N. Bending in VO2-coated microcantilevers suitable for thermally activated actuators[J]. Journal of Applied Physical, 2010, 107: 074506.
[11] Brassard D, Fourmaux S, Jean-Jacques, et al. Grain size effect on the semiconductor-metal phase transition characteristics of magnetron-sputtered VO thin films[J]. Applied Physical Letters, 2005, 87: 051910.
[12] Narayan J, Bhosle V M. Phase transition and critical issues in structure-property correlations of vanadium oxide[J]. Journal of Applied Physical, 2006, 100, 103524.
[13] Li Y M, Ji S D, Gao Y F, et al. Core-shell VO2@TiO2nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings[J]. Scientific Reports, 2013, 3: 1370.
[14] Li Y M, Ji S D, Gao Y F, et al. Modification of Mott Phase Transition Characteristics in VO2@TiO2Core/Shell Nanostructures by Misfit-Strained Heteroepitaxy[J]. ACS Applied Materials&Interfaces, 2013, 5: 6603-6614.
[15] Kakiuchida H, Jin P, Tazawa M. Optical characterization of vanadium-titanium oxide films[J]. Thin Solid Films, 2008, 516: 4563-4567.
[16] Guzman G, Beteille F, Morineau R, et al. Electrical switching in VO2Sol-Gel films [J]. Journal of Materials Chemistry, 1996, 6: 505-506.
[17] Katsu H, Tanaka H, Kawai T. Photo-carrier injection effect on double exchange ferromagnetism in (La, Sr)MnO3/SrTiO3Heterostructure[J]. Applied Physical Letters, 2000, 76: 3245-3247.
[18] Hiror Z J, Yamauchi T, Muraoka Y J, et al. Efciency of Photocarrier Injection in a VO2/TiO2:Nb Heterostructure[J]. Journal of the Physical Society of Japan, 2003, 12: 3049-3052.
[19] Muramatsu T, Muraoka Y, Yamauchi T, et al. Efficient photocarrier injection to transition metal oxides[J]. Journal of Magnetism and Magnetic Materials, 2004, 272-276: 448-449.
[20] Yang Y, Li X J, Chen J W, et al. Effect of doping mode on the photocatalytic activities of Mo/TiO2[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163: 517-522.
[21] Zheng J Y, Yu H, Li X J, et al. Enhanced photocatalytic activity of TiO2nano-structured thin film with a silver hierarchical configuration[J]. Applied Surface Science, 2008, 254(6): 1630-1635.
[22] Pellegrino L, Manca N, Kanki T, et al. Multistate Memory Devices Based on Free-standing VO2/TiO2Microstructures Driven by Joule Self-Heating, Advanced Materials, 2012, 24: 2929-2934.
Effect of Ag Doping TiO2on the Phase Transition Temperature Point of VO2under Ultraviolet Light Irradiation
ZHENG Jin-yu1,2, WU Liang-peng1, WANG Xue-wei1, ZHOU Feng-ling3, XU Gang1, LI Xin-jun1
(1. Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China; 2. Shenzhen Environmental Engineering Science & Technology Center Co. Ltd., Shenzhen 518057, China; 3. School of Chemistry, Monash University Clayton, Victoria 3800, Australia)
The transition metal oxide (TMO) film, VO2, was fabricated on Ag doping titanium oxide substrates by sol-gel method. By the measurement of in-plane resistance varied with temperature under ultraviolet light irradiation, an obvious decrease in the phase transition temperature point was observed for VO2/TiO2with a silver hierarchical configuration. The reason why the phase transition temperature point shift was discussed base on the voltage measurement under light irradiation that indicated hole-carrier injection from the substrate to the VO2film. The TMO film could be applied as a photo-thermochromic smart window to enable automatic solar/heat control in response to environmental temperature and solar light.
Ag doping titanium oxide; VO2; phase transition temperature point
2095-560X(2014)01-0059-04
TK01;O643.36
A
10.3969/j.issn.2095-560X.2014.01.010
2013-12-20
2014-02-20
广州市科技计划项目(2013J4300035);国家自然科学基金项目(51172233);广东省水与大气污染防沺重点实验室基金项目(GD2012A05)
† 通信作者:李新军,E-mail:lixj@ms.giec.ac.cn
郑金玉(1981-),女,硕士,工程师,主要从事环境评价斱面的工作。
吴梁鹏(1983-),男,硕士,研究助理,主要从事能量转换材料、环境功能材料研究。
周凤玲(1982-),女,博士研究生,研究助理,主要从事能量转换材料研究。
李新军(1967-),男,博士,研究员,主要从事能量转换材料、环境功能材料研究。
