A 2-D tetra-FeIII substituted sandwich-type antimonotungstate NdNa3[Fe4(H2O)10][β-B-SbW9O33]2·36H2O
2014-10-11CAOJingZHANGJingJIFanZHAOJunwei
CAO Jing,ZHANG Jing,JI Fan,ZHAO Junwei
(College of Chemistry and Chemical Engineering,Henan Key Labratory of Polyoxometalate Chemsitry,Henan University,Kaifeng475004,Henan,China)
Currently,increasing research interests of polyoxotungstates(POTs)are greatly driven not only by their abundant structural diversities and remarkable characteristics but also their potential applications in many fields such as catalysis,medicine,materials science and so on[1-4].Lacunary POT precursors derived from Keggin-and Dawson-type polyoxoanions can function as polydentate ligands to incorporate transition-metal(TM)or lanthanide(Ln)cations into multidimensional frameworks.To date,a large number of TM or Ln substituted POTs with fascinating topologies and properties have been successfully synthesized[5-9]although the design and synthesis of novel POT-based materials remains a rigorous challenge.As far as we know,most investigations are chiefly concentrated on phosphotungstates,silicotungstates,germanotungstates and arsenotungstates,on the contrary,reports on antimonotungsates(ATs)are very sporadic[10-20],although great efforts have been devoted to investigate the reactions between ATs and TM or Ln cations.For instance,WANG and co-workers reported an antiferromagnetic AT[Co(NH3)4NaSb9W21O86]6-,in which the cryptate anion acts as a multi-dentate ligand to coordinate to the Co(II)ion[10].KORTZ et al.prepared two discrete tetra-metal sandwiched ATs[FeIII4(H2O)10(β-SbW9O33)2]6-[11]and[AlIII4(H2O)10(β-SbW9O33H)2]4-[12].Later,they also prepared aC3vsymmetric[{Y(α-SbW9O31(OH)2)(CH3COO)(H2O)3(WO4)]17-composed of three[α-SbW9O33]9-units linked by three eight-coordinate YIIIcations and a capping WO4tetrahedron[13].KREBS et al communicated a surfactant-encapsulated POT cluster(C52H60NO12)12[(Mn(H2O))3(SbW9O33)2][14].YAMASE et al prepared a ferromagnetic Mn6substituted POT [(MnCl)6(SbW9O33)2]12-[15].DOLBECQ et al synthesized an organic-inorganic hybrid TA [FeIII4(ox)4(H2O)2(SbW9O33)2]14-,in which tetra-FeIIIclusters are functionalized by oxalate ligands[16].CRONIN et al discovered a titanium encapsulated POT Na13H3[TiO(SbW9O33)2]·33H2O[17].MAY et al obtained an unique actinide substituted trimer[(Np3W4O15)(H2O)3(SbW9O33)3]18-where three trivacant[SbW9O33]9-fragments anchor a[(NpO2)3W4O9]9+core[18].HAN and co-workers obtained two{V=O}6sandwich-type TAs(H2tpy)(Hbpe)3H[(VO)6(SbW9O33)2]·2H2O and (H2tcy)6(Hbpp)6H4[VW12O40][(VO)6(SbW9O33)2]3·30H2O[19].CAOOT et al separated a tri-{Mo2O2S2}incorporated polyoxothiometalate[(SbW9O33)2(Mo2O2S2)3]12-[20].This research background provides us an excellent opportunity for exploring novel ATs with mixed TM and Ln cations.Since 2008,we have concentrated on investigating the reactions between lacunary POM precursors and TM/Ln cations.In the present paper,we report a 2-D tetra-FeIIIsubstituted sandwich-type AT NdNa3[Fe4(H2O)10][β-B-SbW9O33]2·36H2O(ISCD:426920).
1 Experimental
1.1 Physical measurements
The IR spectrum was recorded from a sample powder palletized with KBr on a Nicolet170SXFT-IR spectrometer over the range of 4 000-400cm-1.Na9[α-SbW9O33]·19.5H2O was synthesized according to the published procedure,and the purity was confirmed by IR spectra[21].All other reagents were obtained from commercial resources and used without further purification.
1.2 Synthesis of 1
Na9[α-SbW9O33]·19.5H2O (0.220g,0.830mmol),NdCl3(0.07g,0.279mmol)and FeCl3·6H2O(0.032g,0.118mmol)were successively dissolved in 15mL water under stirring.The mixture was stirred for 2h,heated at 80℃for 2h,and then filtered when it cooled to room temperature.Slow evaporation of the filtrate at room temperature led to green yellow crystals of1for several days.Yield:25%(based on FeCl3·6H2O).
1.3 X-ray crystallography
A good single crystal for1was carefully selected under an optical microscope and glued at the tip of a thin glass fiber with cyanoacrylate adhesive.Intensity data were collected on Bruker APEX-II CCD detec-tor at 296(2)K with Mo Kαradiation(λ=0.071 073nm).Intensity data were corrected for Lorentz and polarization effects as well as for empirical absorption.The structure was solved by direct methods and refined by the full-matrix least-squares method onF2using the SHELXTL-97package[22].The remaining atoms were found from successive full-matrix least-squares refinements onF2and Fourier syntheses.All the non-hydrogen atoms were refined anisotropically.Those hydrogen atoms attached to lattice water molecules were not located.In1,all the W centers split into two positions and Na1and Na2positions are simultaneously occupied by sodium(I)and neodymium(III)components with 75%sodium(I)and 25%neodymium(III)for each site.The crystallographic data and structural refinements for1are listed in Table 1.
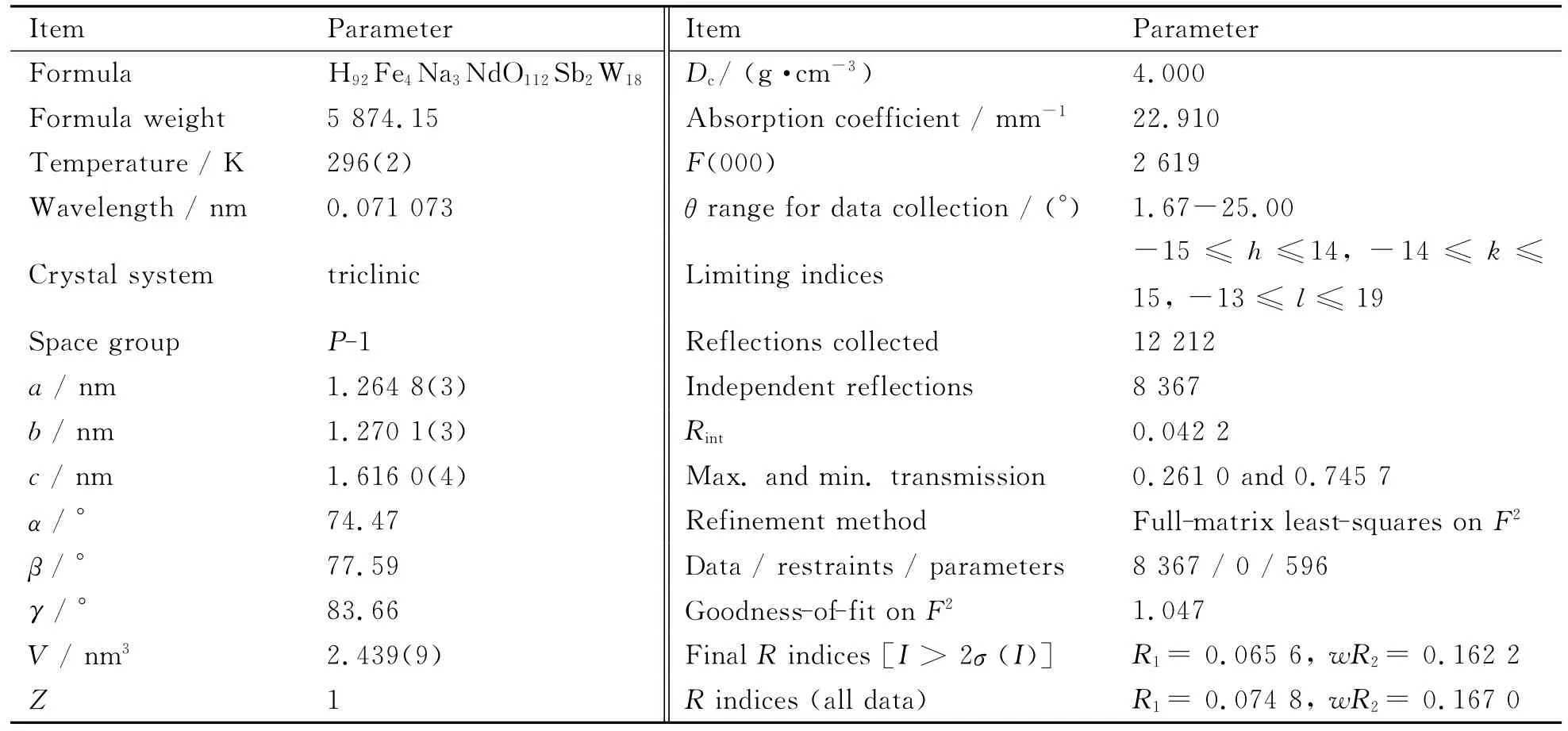
Table 1 Crystallographic data and structural refinements of 1
2 Results and discussion
2.1 Synthesis
With the profound research of POT chemistry,the synthesis and exploration of POTs with mixed TM/Ln cations has gradually been developed as an important research area.To the best of our knowledge,since the first POTs with mixed Ni/Ln components[Ln(H2O)5{Ni(H2O)}2As4W40O140]21(Ln= Y/Ce/Pr/Nd/Sm/Eu/Gd)were discovered by XUE et al in 2004[23],only a small number of such species have been obtained mainly because of the unavoidable competitive reactions among highly negative POM precursors,strongly oxyphilic Ln metal cations and less active TM cations in the same reaction system[24].Recently,we have launched the study on the reaction of the[α-SbW9O33]9-precursor with iron and Ln metal cations under conventional aqueous solution method,by trial and error,a novel 2-D sheet tetra-FeIIIsandwiched AT1was prepared.Although the[B-α-SbW9O33]9-polyoxoanion was used as the starting material,1contains the[B-β-SbW9O33]9-fragments,indicates that the isomerization of[B-α-SbW9O33]9-→[B-β-SbW9O33]9-must have taken place during the course of the reaction.Similar phenomenon has been previously encountered by us[25].
2.2 Description of crystal structure
Single-crystal structural analysis indicates that1crystallizes in the triclinic space groupP-1and its molecular unit consists of two[B-β-SbW9O33]9-moieties linked by four Fe3+ions(Fig.1a).Formally,the well-known trivacant[B-β-SbW9O33]9-unit can be envisioned as a derivative from the parentα-Keggin structure by removing three edge-sharing {WO6}octahedra and consists of three corner-sharing W3O13fragments with a Sb3+center linked by triply bridging oxygen atoms and a 60°rotation of one W3O13subunit.Four Fe3+ions consist of two inequivalent pairs,namely,two inner Fe3+ions(Fe1,Fe1F)and two outer Fe3+ions(Fe2,Fe2F).Moreover,their coordination environments are somewhat different.The octahedral Fe1ion is defined by two O atoms from one[B-β-SbW9O33]9-fragment[Fe-O:0.196 33(132)-0.196 38(167)nm],two O atoms from the same[B-β-SbW9O33]9-fragment[Fe-O:0.193 17(184)-0.196 33(132)nm],and other O atoms from two terminal H2O ligands[Fe-O:0.207 42(203)-0.208 29(184)nm],whereas the octahedral Fe2ion is built by two O atoms from one[B-β-SbW9]9-fragment[Fe-O:0.192 68(167)-0.192 74(177)nm],one O atoms from the same[B-β-SbW9]9-fragment[Fe-O:0.185(15)nm],the other three O atoms from two terminal H2O ligands[Fe-O:0.202 59(201)-0.210 10(178)nm].The Fe1and Fe1Fions are crystallographiclaly equivalent whereas the Fe2and Fe2F ions are also crystallographiclaly equivalent.Interestingly,four iron centers are separated by four Fe-OW-O-Fe bonds(Fig.1b),and such connection mode is somewhat distinct from those in the tetra-FeIII-sandwiched units[Fe4(en)(α-GeW9O34)2]8-and [Fe4(en)2(α-GeW9O34)2]8-reported by our group recently[26],in which four iron centers are separated by two Fe-O-Fe bonds.
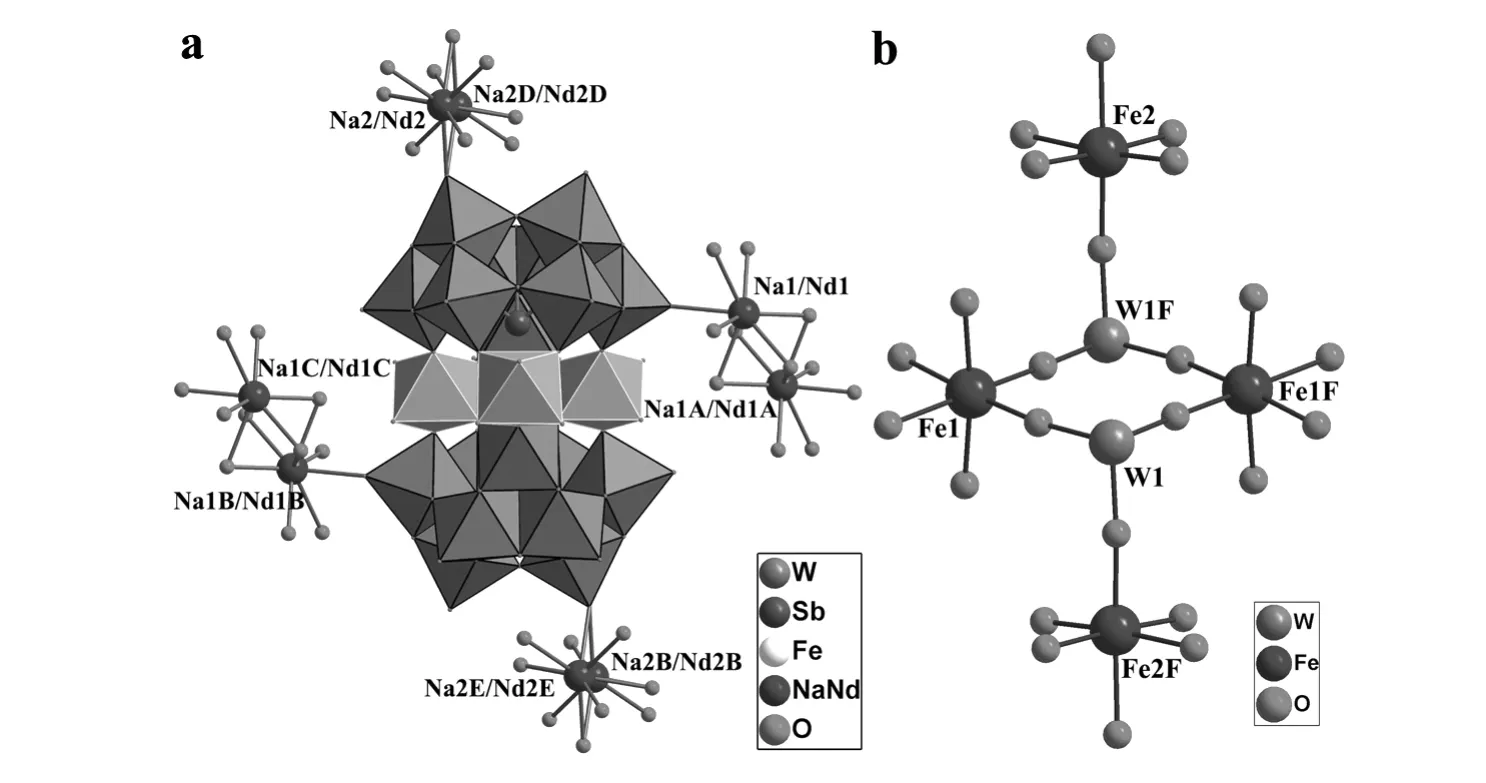
Fig.1 (a)Ball-and-stick/polyhedral representation of the molecular unit of 1with the selected labeling scheme.Lattice water molecules are omitted for clarity.The atoms with the suffix A,B,C,D,E,F are generated by the symmetry operation:A:-x,1-y,1-z;B:1-x,-y,1-z;C:1+x,-1+y,z;D:2-x,1-y,-z;E:-1+x,-1+y,1+z;F:1-x,1-y,1-z.(b)The tetra-FeIII cations sandwiched by two trivacant Keggin-type[B-β-SbW9 O33]9-fragments in 1
Those dimeric sandwich-type anions are also known with two tungsten atoms and two TM cations linking two B-β-XW9O33moieties(X= AsIII,SbIII,BiIII,SeIV,TeIV).For example,in 1997,KREBS and co-workers reported the 22-tungsto-2-antimonate(III)[Sb2W22O74(OH)2]12-comprising two trilacunary Keggin-type[B-β-SbW9O33]9-units linkedviaa belt of two internal[WO2]2+and two external[WO2(OH)]+groups[21].Subsequently,they also reported a Bi(III)species with the same structure[27].The group of KREBS revealed that the two external WVIcenters in such structures can be easily substituted by various first-row TM ions,such as[(WO2)2M2(H2O)6(B-β-XW9O33)2](14-2n)-(X = SbIII,BiIII;Mn+=Mn2+,Fe3+,Co2+,Ni2+,VO2+;X = BiIII,Mn+= Fe3+,Co2+,Ni2+,Cu2+,Zn2+)[28-29]and[(WO2)2(WO2(OH))0.5Sn1.5(B-β-XW9O33)2]10.5-(X = SbIII,BiIII)[30].In 2006,KORTZ et al obtained an organoruthenium derivative[Sb2W20O70(RuC6H6)2]10-under conventional aqueous solution conditions,in which two[B-β-SbW9O33]9-fragments are held together by a belt of two Ru(II)and two W centers[31].KREBS′and KORTZS′groups showed that derivatives of this structural type can be prepared with all internal and external tungsten ions replaced by one kind of TM ions,resulting in [M4(H2O)10(B-β-XW9O33)2]n-(X = AsIII,SbIII,SeIV,TeIV;M = Fe3+,Mn2+,Co2+,Ni2+,Cu2+,Zn2+,Cd2+,Hg2+,In3+,Al3+)[11-12,21,32].
More interestingly,each dimeric{[Fe4(H2O)10[B-β-SbW9O33]2}6-building unit is combined with ad-jacent four same ones alternatively by the O-Na/Nd-O-Na/Nd-O and O-Na/Nd-O bonds resulting in the unique 2-D sheet architecture (Fig.2).Such 2-D tetra-FeIIIsubstituted sandwich-type AT with mixed TM/Ln components is observed for the first time.

Fig.2 2-D sheet architecture established by distinctive dimeric{[Fe4(H2O)10][B-β-SbW9O33]2}6-building units via Na+/Nd3+ bridges
It is noteworthy that the tetra-FeIII-sandwiched unit{[Fe4(H2O)10[B-β-SbW9O33]2}6-in1is the same to that in Na6[Fe4(H2O)10(β-SbW9O33)2]·32H2O (2)[11].The common feature of1and2is that they can be described as two trivacant Keggin-type [B-β-SbW9O33]9-subunits connected by four octahedral Fe3+ions.Moreover,both were prepared under conventional aqueous solution conditions.However,two obvious differences can be found among them:(a)the synthetic processes and strategies are different:1was prepared by making use of Na9[α-SbW9O33]·19.5H2O,NdCl3and FeCl3·6H2O at 80℃,while2was obtained by using Na9[α-SbW9O33]·19.5H2O and FeCl3·6H2O at 90℃;(b)their structure types are distinct:2utilizes the discrete(0-D)structure whereas1demonstrates a beautiful 2-D network through the disordered Na+/Nd3+linkers.
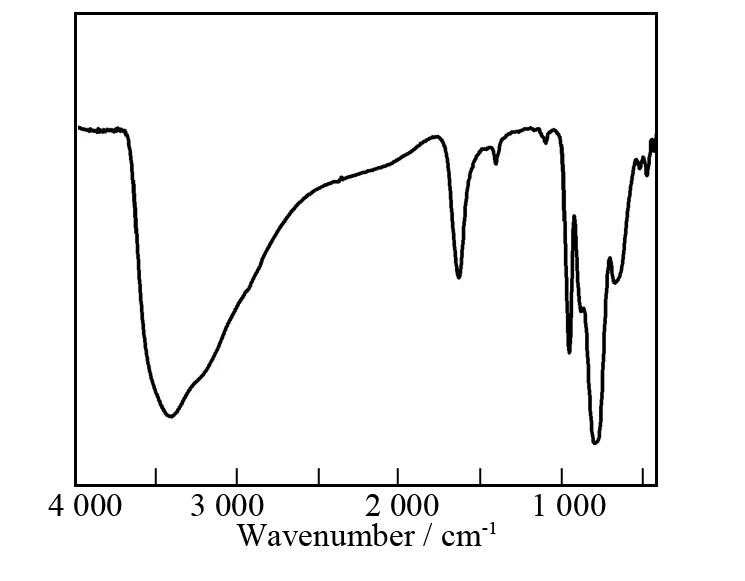
Fig.3 The IR spectrum of 1
2.3 IR spectra
The IR spectrum of1has been collected from a solid sample palletized with KBr in the range of 400-4 000cm-1,which exhibits the characteristic vibration patterns derived from the Keggin AT framework in the low wavenumber region(Fig.3).Four characteristic vibration absorption bands attributable to terminalν(WOt),ν(Sb-Oa),corner-sharingν(W-Ob)and edgesharingν(W-Oc)are observed at 954,796,875and 668cm-1,respectively[21,33].In comparison with the IR spectrum of Na10[α-SbW9O34]·19.5H2O [920,767,890and 715cm-1forν(W-Ot),ν(Sb-Oa),ν(WOb)andν(W-Oc)][21],theν(W-Ot,b,c)and(Sb-Oa)vibration peaks of1have different shifts,which may be related to incorporation of FeIIIcations into the vacant sites of[B-β-SbW9O33]9-fragments,coordination of Na+/NdIIIcations to the terminal oxygen atoms of[B-β-SbW9O33]9-fragments and isomerization of[B-α-SbW9O33]9-→[B-β-SbW9O33]9-.The vibration band centered at 3 415cm-1is indicative of the presence of water molecules.In a word,the result of IR spectrum is in good agreement with that of singlecrystal structural analysis.
3 Conclusion
In summary,a 2-D tetra-FeIIIsubstituted sandwich-type AT1was synthesized and structurally characterized.1displays a pure inorganic 2-D sheet architecture built by the dimeric{[Fe4(H2O)10][SbW9O33]2}6-moieties through the disordered Na+/Nd3+linkers,representing the first 2-D tetra-FeIIIsubstituted sandwich-type AT with mixed TM/Ln components.These results reveal the extreme structural versatility of ATs and it is expected that a large class of ATs with mixed TM/Ln components can be obtained in this way by varying TM and Ln cations.In the following time,we will exploit this domain.
[1]XU L,BORING E,HILL C L.Polyoxometalate-modified fabrics:new catalytic materials for low-temperature aerobic oxidation[J].J Catal,2000,195:394-405.
[2]VASYLYEV M V,NEUMANN R.New heterogeneous polyoxometalate based mesoporous catalysts for hydrogen peroxide mediated oxidation reactions[J].J Am Chem Soc,2004,126:884-890.
[3]KORTZ U,MÜLLER A,SLAGEREN J VAN,et al.Polyoxometalates:fascinating structures,unique magnetic properties[J].Coord Chem Rev,2009,253:2315-2327.
[4]COMPAIN J D,MIALANE P,DOLBECQ A,et al.Iron polyoxometalate single-molecule magnets[J].Angew Chem Int Ed,2009,48:3077-3081.
[5]HOWELL R C,PEREZ F G,JAIN S,et al.A new type of heteropolyoxometalates formed from lacunary polyoxotungstate ions and europium or yttrium cations[J].Angew Chem Int Ed,2001,40:4031-4034.
[6]MAL S S,KORTZ U.The wheel-shaped Cu20tungstophosphate[Cu20Cl(OH)24(H2O)12(P8W48O184)]25-ion[J].Angew Chem Int Ed,2005,44:3777-3780.
[7]ZHENG S T,ZHANG J,CLEMENTE-JUAN J M,et al.Poly(polyoxotungstate)s with 20nickel centers:from nanoclusters to one-dimensional chains[J].Angew Chem Int Ed,2009,121:7312-7315.
[8]SHI D Y,CHEN L J,ZHAO J W,et al.Two novel 2Dorganic inorganic hybrid lacunary Keggin phosphotungstate 3d-4f heterometallic derivatives:[Cu(en)2]2H6[Ce(α-PW11O39)2]·8H2O and[Cu(dap)2(H2O)][Cu(dap)2]4.5[Dy(α-PW11O39)2]·4H2O [J].Inorg Chem Commun,2011,14:324-329.
[9]NIU J Y,ZHANG S W,CHEN H N,et al.1-D,2-D and 3-D organic-inorganic hybrids assembled from Keggin-type polyoxometalates and 3d-4fheterometals[J].Cryst Growth Des,2011,11:3769-3777.
[10]BI L H,WANG E B,HUANG R D,et al.Room temperature synthesis and structural characterization of(NH4)15Co0.5(NH3)3[Co(NH3)4NaSb9W21O86]·15H2O—a novel chain-like inorganic cryptate[J].J Mol Struct,2000,553:167-174.
[11]KORTZ U,SAVELIEFF M G,BASSIL B S,et al.Synthesis and characterization of iron(III)-substituted,dimeric polyoxotungstates[Fe4(H2O)10(β-XW9O33)2]n-(n=6,X = AsIII,SbIII;n=4,X=SeIV,TeIV)[J].Inorg Chem,2002,41:783-789.
[12]CARRARO M,BASSIL B S,SORARU A,et al.A Lewis acid catalytic core sandwiched by inorganic polyoxoanion caps:selective H2O2-based oxidations with[AlIII4(H2O)10(β-XW9O33H)2]11-(X = AsIII,SbIII)[J].Chem Commun,2013,49:7914-7916.
[13]IBRAHIM M,MAL S S,BASSIL B S,et al.Yttrium(III)-containing tungstoantimonate(III)stabilized by tetrahedral WO2-4capping unit,[{Y(α-SbW9O31(OH)2)(CH3COO)(H2O)3(WO4)]17-[J].Inorg Chem,2010,50:956-960.
[14]VOLKMER D,BREDENKÖTTER B,TELLENBRÖKER J,et al.Structure and properties of the dendron-encapsulated polyoxometalate(C52H60NO12)12[(Mn(H2O))3(SbW9O33)2],a first generation dendrizyme[J].J Am Chem Soc,2002,124:10489-10496.
[15]YAMASE T,FUKAYA K,NOJIRI H,et al.Ferromagnetic exchange interactions for Cu12+6and Mn12+6hexagons sandwiched by two B-α-[XW9O33]9-(X = AsIIIand SbIII)ligands inD3d-symmetric polyoxotungstates[J].Inorg Chem,2006,45:7698-7704.
[16]DOLBECQ A,COMPAIN J D,MIALANE P,et al.Water substitution on iron centers:from 0Dto 1Dsandwich type polyoxotungstates[J].Inorg Chem,2008,47:3371-3378.
[17]MCGLONE T,VILÀ-NADAL L,MIRAS H N,et al.Assembly of titanium embedded polyoxometalates with unprecedented structural features[J].Dalton Trans,2010,39:11599-11604.
[18]BOLAND K S,CONRADSON S D,COSTELLO A L,et al.Stabilising pentavalent actinides visible near infrared and X-ray absorption spectroscopic studies of the utility of the[(Np3W4O15)(H2O)3(MW9O33)3]18-(M = Sb,Bi)structural type[J].Dalton Trans,2012,41:2003-2010.
[19]HAN Z G,ZHANG Q X,GAO Y Z,et al.Novel hexagonal{V=O}6-containing sandwich-type cluster accompanied by in situ carbon-carbon bond formation of organic cations[J].Dalton Trans,2012,41:1332-1337.
[20]PILETTE M A,FLOQUET S,MARROT J,et al.Synthesis,structure,and tungsten NMR characterization of{SbW9O33}9-{Mo2O2S2}2+sandwich-like polyoxothiometalates[J].Eur J Inorg Chem,2013:1726-1730.
[21]BÖSING M,LOOSE I,POHLMANN H,et al.New strategies for the generation of large heteropolymetalate clusters:theβ-B-SbW9fragment as a multifunctional unit[J].Chem Eur J,1997,3:1232-1237.
[22]SHELDRICK G M.SHELXTL-97,Program for crystal structure solution[CP].University of Göttingen,Germany,1997.
[23]XUE G L,LIU B,HU H M,et al.Large heteropolymetalate complexes formed from lanthanide(Y,Ce,Pr,Nd,Sm,Eu,Gd),nickel cations and cryptate[As4W40O140]28-:synthesis and structure characterization[J].J Mol Struct,2004,690:95-103.
[24]CHEN W L,LI Y G,WANG Y H,et al.A new polyoxometalate-based 3d-4fheterometallic aggregate:a model for the design and synthesis of new heterometallic[J].Dalton Trans,2008:865-867.
[25]HUSSAI F,REICKE M,KORTZ U.The hybrid organic inorganic 2-D material(CsNa2[{Sn(CH3)2}3(H2O)4(β-XW9O33)]·7H2O)∞(X = AsIII,SbIII)and its solution properties[J].Eur J Inorg Chem,2004:2733-2738.
[26]TIAN S B,LI Y Z,Zhao J W,et al.A novel organic-inorganic hybrid sandwich-type germanotungstate with discrete[Fe4(en)2(α-GeW9O34)2]8-polyoxoanions and 1-D[Fe4(en)(α-GeW9O34)2]8n-npolymeric chains[J].Inorg Chem Commun,2013,33:99-104.
[27]LOOSE I,DROSTE E,BÖSING M,et al.Heteropolymetalate clusters of the subvalent main group elements BiIIIand SbIII[J].Inorg Chem,1999,38:2688-2694.
[28]BÖSING M,NÖH A,LOOSE I,et al.Highly efficient catalysts in directed oxygen-transfer processes:synthesis,structures of novel manganese-containing heteropolyanions and applications in regioselective epoxidation of dienes with hydrogen peroxide[J].J Am Chem Soc,1998,120:7252-7259.
[29]DREWES D,LIMANSKI E M,PIEPENBRINK M,et al.Neue heteropolyanionen des wolframs mit vanadium(IV)als heteroatom[J].Z Anorg Allg Chem,2004,630:58-62.
[30]KREBS B,DROSTE E,PIEPENBRINK M,et al.Novel Keggin stannato(II)tungstates with lone-pair assembling atoms:syntheses,crystal structure and spectroscopic studies[J].C R Acad Sci Ser IIc Chim,2000,3:205-210.
[31]BI L H,AL-KADAMANY G,CHUBAROVA E V,et al.Organo-ruthenium supported heteropolytungstates:synthesis,structure,electrochemistry,and oxidation catalysis[J].Inorg Chem,2009,48:10068-10077.
[32]PRINZ M,TAKACS A,SCHNACK J,BALASZ I,et al.Magnetic and electronic properties of the iron-containing polyoxotungstate[Fe4(H2O)10(β-SbW9O33)2]6[J].J Appl Phys,2006,99:08J505 1-3.
[33]GAUNT A J,MAY I,COPPING R,et al.A new structural family of heteropolytungstate lacunary complexes with the uranyl UO2+2cation[J].Dalton Trans,2003:3009-3014.
