一种四氢苯并吡喃及其衍生物的水相合成
2014-09-02周红英朱婉莉应安国
高 洁, 刘 硕, 周红英, 朱婉莉, 刘 璐, 应安国,4*
(1.台州学院 医药化工学院,浙江 台州 318000; 2.天津大学 化工学院,天津 300072;3.浙江建业化工股份有限公司,浙江 杭州 311604; 4.台州学院 应用化学研究所,浙江 台州 318000)
一种四氢苯并吡喃及其衍生物的水相合成
高 洁1, 刘 硕2, 周红英3, 朱婉莉1, 刘 璐1, 应安国1,4*
(1.台州学院 医药化工学院,浙江 台州 318000; 2.天津大学 化工学院,天津 300072;
3.浙江建业化工股份有限公司,浙江 杭州 311604; 4.台州学院 应用化学研究所,浙江 台州 318000)
制备了一种以三乙烯二胺(DABCO)为基础的离子液体;在水溶剂中,以该离子液体为催化剂催化三组分一锅法制备四氢苯并吡喃的反应,得到2-氨基-3-氰基-4-芳基-7,7-二甲基-5-氧代-4H-5,6,7,8-四氢苯并[b]吡喃;考察了反应时间、催化剂用量、催化剂加入时间、反应温度对反应收率的影响,确定了最优反应条件;由不同的芳香醛和活性亚甲基化合物制备了一系列四氢苯并吡喃衍生物,并讨论了可能的反应机理. 结果表明,所选用的合成反应条件温和、时间短、收率高、后处理简单,且催化剂重复使用4次催化效果变化不大;就反应机理而言,所用催化剂具有双重催化活性.
三乙烯二胺;离子液体;四氢苯并吡喃;衍生物;水相合成
四氢苯并[b]吡喃衍生物在医药领域是一类非常重要的有机杂环化合物. 这些化合物具有多种生物活性和药理活性,广泛用于抗凝剂,利尿剂,解痉药,抗癌药和抗过敏药[1]. 此外,它们可以用作认知增强剂,治疗神经退化性疾病,包括老年痴呆症,肌萎缩性脊髓侧索硬化症,亨廷顿氏病,帕金森病,艾滋相关的痴呆,唐氏综合症,以及精神分裂症和肌阵挛[2-3]. 一些2-氨基-四氢苯并[b]吡喃可以作为光敏材料[4]. 因此,这类化合物的合成引起了人们极大的兴趣.
四氢苯并[b]吡喃的合成通常需要强碱,强酸和较高的温度,并用到挥发性有机溶剂[5-9]. 因此这些反应都具有反应条件苛刻,时间长,收率低,需要有机溶剂等缺点.
随着人们对人类生存环境的日益重视,绿色合成逐渐成为科学家的研究重点,提倡在有机合成过程中采用无毒溶剂(如水)和催化剂,或无溶剂条件,实现绿色全合成. 靳通收等[10]报道了以十二烷基苯磺酸为催化剂,水中一锅法制备四氢苯并[b]吡喃的反应,收率较高. 史达清等[11]使用三乙基苄基氯化铵为催化剂,水中一锅法制备四氢苯并[b]吡喃. 张慧等[12]以碳酸钾为碱,无溶剂研磨条件下制备四氢苯并[b]吡喃. 以上催化体系,反应时间大部分偏长,适用范围窄. 离子液体因其熔点低、蒸汽压可忽略、液程宽、溶解性强、热稳定性高、对环境无污染等优点,逐渐被人们应用于有机合成中. BALALAIE等[13]合成了氢氧化四甲基铵盐离子液体,在水中催化一锅法合成四氢苯并[b]吡喃的反应;KOLEKAR等[14]利用4-氨基吡啶与3-氯-1,2-丙二醇合成了一系列离子液体,催化芳香醛、丙二腈、双甲酮合成四氢苯并[b]吡喃的反应. 相比之前的反应体系,这些反应体系有了较大提升. 但是,由于该反应体系研究较少,开发更高效、新颖的催化剂还是很有必要的.
DABCO(三乙烯二胺)是一种有机碱,WANG等[15]报道了以DABCO为基础的一系列离子液体用于催化Knoevenagel缩合反应. 而KOLEKAR等[14]利用4-氨基吡啶与3-氯-1,2-丙二醇合成了一系列离子液体. 受上述两方法的启发,我们以DABCO和3-氯-1,2-丙二醇为原料,制备含DABCO的离子液体. 本文报道了以离子液体[DABCO][CF3SO3]为催化剂在水相介质中一锅法催化芳醛,丙二腈和双甲酮三组分反应合成四氢苯并[b]吡喃的反应.
1 实验部分
1.1 仪器与试剂
Bruker 400核磁共振仪;Carlo Erba 1160元素分析仪;HK-2A型超级恒温水浴;Mettler Doledo 电子天平. 实验所用试剂全为阿拉丁公司所购买,纯度为分析纯.
1.2 离子液体[DABCO][CF3SO3]的制备
向100 mL 圆底烧瓶中加入10.15 g (90.5 mmol) 三乙烯二胺 (DABCO)和10 g(7.57 mL,90.5 mmol)3-氯-1,2-丙二醇, 加入50 mL乙醇. 在磁力搅拌下,温度设置为80 ℃ , 加热回流反应24 h. 旋蒸得到[DABCO][Cl]. 取3 g(13.4 mmol) [DABCO][Cl]与2.53 g (13.4 mmol)CF3SO3K于15 mL甲醇中,65 ℃下,加热回流反应8 h,真空旋蒸得到淡黄色液体,即为离子液体[DABCO][CF3SO3]. 具体合成路线见图1.
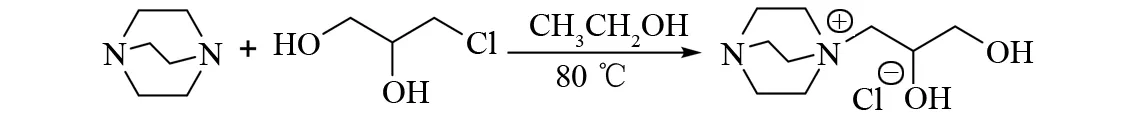
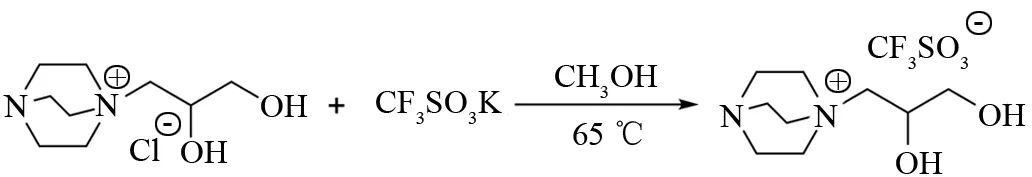
图1 离子液体[DABCO][CF3SO3]的合成路线Fig.1 Synthesis route of [DABCO][CF3SO3]
1.3 三组分一锅法制备四氢苯并吡喃及其衍生物
三组分一锅法制备四氢苯并吡喃及其衍生物的反应方程式如图2.
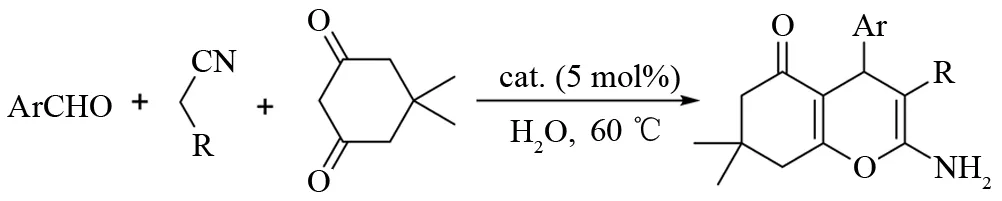
图2 三组分一锅法制备四氢苯并吡喃及其衍生物Fig.2 One-pot three-component synthesis of tetrahydrobenzo[b]pyran
以2-氨基-3-氰基-4-芳基-7,7-二甲基-5-氧代-4H-5,6,7,8-四氢苯并[b]吡喃的合成为例,取双甲酮0.5 g,苯甲醛0.36 mL,丙二腈0.23 mL加入 50 mL 圆底烧瓶中,加入离子液体0.06 g,60 ℃搅拌. 用TLC监测反应进行程度(展开剂,石油醚与乙酸乙酯体积比1∶1). 反应结束后用乙酸乙酯萃取,收集有机相,旋干乙酸乙酯,用乙醇重结晶,抽滤、旋干得到黄色固体0.97 g,收率93%.1H NMR (400 MHz, CDCl3)δ: 7.24~7.31(m, 5H, Ph), 4.56(s, 2H, NH2), 4.43(s, 1H, CH), 2.48(s, 2H, CH2), 1.61(s, 2H, CH2), 1.13(s, 3H, CH3), 1.06(s, 3H, CH3);MSm/z=294.1; Anal. Calcd for C18H18N2O2(%): C 73.45,H 6.16,N 9.52,O 10.87;found: C 73.41,H 6.23,N 9.44,O 10.92.
1.4 产物的结构表征数据
2-Amino-7,7-dimethyl-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体1.14 g,产率94%.1H NMR (400 MHz, CDCl3)δ: 8.19(d,J= 8.8 Hz, 2H, Ph), 7.44(d,J=8.4 Hz, 2H, Ph), 4.69(s, 2H, NH2), 4.54(s, 1H, CH), 2.51(s, 2H, CH2), 2.24~2.27(m, 2H, CH2), 1.15(s, 3H, CH3), 1.06(s, 3H, CH3);MSm/z=339.1; Anal Calcd for C18H17N3O4(%): C 63.71,H 5.23,N 12.38,O 18.86;found: C 63.51,H 5.23,N 12.28,O 18.98.
2-Amino-7,7-dimethyl-4-(3-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体1.15 g,产率95%.1H NMR (400 MHz, CDCl3)δ: 8.10(d,J=8.4 Hz, 1H, Ph), 8.06(s, 1H, Ph), 7.70(d,J=8.4 Hz, 1H, Ph), 7.49~7.53(m, 1H, Ph), 4.74(s, 2H, NH2), 4.55(s, 1H, CH), 2.47~2.57(m, 2H, CH2), 2.20~2.30(m, 2H, CH2), 1.15(s, 3H, CH3), 1.07(s, 3H, CH3);MSm/z=339.1;Anal Calcd for C18H17N3O4(%): C 63.71,H 5.23,N 12.38,O 18.86;found: C 63.58,H 5.21,N 12.25,O 18.96.
2-Amino-4-(2-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体1.09 g,产率93%.1H NMR (400 MHz, CDCl3)δ: 7.34(d,J=7.6 Hz, 1H, Ph), 7.21~7.23(m, 2H, Ph), 7.14~7.18(m, 1H, Ph), 4.87(s, 1H, CH), 4.64(s, 2H, NH2), 2.47(s, 2H, CH2), 2.18~2.28(m, 2H, CH2), 1.14(s, 3H, CH3), 1.09(s, 3H, CH3);MSm/z=328.1;Anal Calcd for C18H17ClN2O2(%): C 65.75,H 5.21,Cl 10.78,N 8.52,O 9.73;found: C 65.71,H 5.29,Cl 10.73,N 8.48,O 9.81.
2-Amino-4-(3-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:白色固体1.03 g,产率90%.1H NMR (400 MHz, CDCl3)δ: 7.21~7.25(m, 1H, Ph), 6.84(d,J=7.6 Hz, 1H, Ph), 6.75~6.79(m, 2H, Ph), 4.61(s, 2H, NH2), 4.39(s, 1H, CH), 3.81(s, 3H, OCH3), 2.47(s, 2H, CH2), 2.25(d,J=2.4Hz, 2H, CH2), 1.13(s, 3H, CH3), 1.07(s, 3H, CH3),MSm/z=324.1;Anal Calcd for C19H20N2O3(%): C 70.35,H 6.21,N 8.64,O 14.80;found: C 70.25,H 6.29,N 8.57,O 14.89.
2-Amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:白色固体1.02 g,产率89%.1H NMR (400 MHz, CDCl3)δ: 7.17(d,J=8.4 Hz, 2H, Ph), 6.84(d,J=8.4 Hz, 2H, Ph), 4.53(s, 2H, NH2), 4.38(s, 1H, CH), 3.79(s, 3H, OCH3), 2.46(s, 2H, CH2), 2.23(d,J=5.2Hz, 2H, CH2), 1.13(s, 3H, CH3), 1.06(s, 3H, CH3),MSm/z=324.1;Anal Calcd for C19H20N2O3(%): C 70.35,H 6.21,N 8.64,O 14.80;found: C 70.21,H 6.30,N 8.53,O 14.96.
2-Amino-4-(2-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:白色固体0.99 g,产率86%.1H NMR (400 MHz, CDCl3)δ: 7.18~7.22(m, 1H, Ph), 7.11~7.13(m, 1H, Ph), 6.87~6.91(m, 2H, Ph), 4.73(s, 1H, CH), 4.46(s, 2H, NH2), 3.86(s, 3H, OCH3), 2.46(s, 2H, CH2), 2.23(d,J=8.4Hz, 2H, CH2), 1.14(s, 3H, CH3), 1.07(s, 3H, CH3),MSm/z=324.1;Anal Calcd for C19H20N2O3(%): C 70.35,H 6.21,N 8.64,O 14.80;found: C 70.26,H 6.29,N 8.59,O 14.86.
2-Amino-4-(4-hydroxy-3,5-dimethoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体1.06 g,产率80%.1H NMR (400 MHz, CDCl3)δ: 6.46(s, 2H, Ph), 5.47(s, 1H, CH), 4.62(s, 2H, NH2), 4.34(s, 1H, OH), 3.88(s, 6H, OCH3), 2.43~2.53(m, 2H, CH2), 2.22~2.31(m, 2H, CH2), 1.14(s, 3H, CH3), 1.09(s, 3H, CH3). MSm/z=370.1;;Anal Calcd for C20H22N2O5(%): C 64.85,H 5.99,N 7.56,O 21.60;found: C 64.76,H 6.11,N 7.46,O 21.67.
2-Amino-7,7-dimethyl-5-oxo-4-(thiophen-2-yl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体0.88 g,产率82%.1H NMR (400 MHz, CDCl3)δ: 7.15(d,J=4.4 Hz, 1H, Ph), 7.01(d,J=3.2 Hz, 1H, Ph), 6.91~6.93(m, 1H, Ph), 4.80(s, 1H, CH), 4.63(s, 2H, NH2), 2.45(s, 2H, CH2), 2.30(s, 2H, CH2), 1.14(s, 3H, CH3), 1.09(s, 3H, CH3),MSm/z=300.1;Anal Calcd for C16H16N2O2S(%): C 63.98,H 5.37,N 9.33,O 10.65,S 10.67;found: C 63.87,H 5.49,N 9.29,O 10.62,S 10.72.
2-Amino-7,7-dimethyl-4-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体1.02 g,产率83%.1H NMR (400 MHz, CDCl3)δ: 8.40(d,J=8.4 Hz, 1H, Ph), 7.85(d,J=8.0 Hz, 1H, Ph), 7.74(d,J=8.0 Hz, 1H, Ph), 7.59~7.61(m, 1H, Ph), 7.57(s, 1H, Ph), 7.48~7.52(m, 1H, Ph), 7.25~7.43(m, 1H, Ph), 5.23(s, 1H, CH), 4.53(s, 2H, NH2), 2.53~2.60(m, 2H, CH2), 2.17~2.28(m, 2H, CH2), 1.15(s, 3H, CH3), 1.09(s, 3H, CH3),MSm/z=344.1,Anal Calcd for C22H20N2O2(%): C 76.73,H 5.85,N 8.13,O 9.29;found: C 76.67,H 5.93,N 8.06,O 9.34.
2-Amino-4-(1H-indol-3-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile:黄色固体0.93 g,产率78%.1H NMR (400 MHz, CDCl3)δ: 8.10(s, 1H, NH), 7.40(d,J=8.0 Hz, 1H, Ph), 7.35(d,J=8.0 Hz, 1H, Ph), 7.21(s, 1H, Ph), 7.14~7.18(m, 1H, Ph), 7.05~7.09(m, 1H, Ph), 4.75(s, 1H, CH), 4.54(s, 2H, NH2), 2.43~2.55(m, 2H, CH2), 2,15~2,25(m, 2H, CH2), 1.12(s, 3H, CH3), 0.97(s, 3H, CH3),MSm/z=333.1; Anal Calcd for C20H19N3O2(%): C 72.06,H 5.74,N 12.60,O 9.60;found: C 72.00,H 5.83,N 12.53,O 9.64.
Ethyl 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate:黄色固体1.08 g,产率89%.1H NMR (400 MHz, CDCl3)δ: 7.27~7.29(m, 2H, Ph), 7.20~7.24(m, 2H, Ph), 7.10~7.14(m, 1H, Ph), 6.18(m, 2H, NH2), 4.72(s, 1H, CH), 3.99~4.09(m, 2H, CH2), 2.45(s, 2H, CH2), 2.16~2.27(m, 2H, CH3), 1.16~1.20(m, 3H, CH3), 1.12(s, 3H, CH3), 0.99(s, 3H, CH3);MSm/z=341.1; Anal Calcd for C20H23NO4(%): C 70.36,H 6.79,N 4.10,O 18.75;found: C 70.27,H 6.87,N 4.05,O 18.81.
Ethyl 2-amino-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate:黄色固体1.17 g,产率91%.1H NMR (400 MHz, CDCl3)δ: 7.22~7.26(m, 2H, Ph), 6.88~6.93(m, 2H, Ph), 6.19(s, 2H, NH2), 4.70(s, 1H, CH), 4.03~4.08(m, 2H, CH2), 2.44(s, 2H, CH2), 2.16~2.27(m, 2H, CH2), 1.15~1.19(m, 3H, CH3), 1.12(s, 3H, CH3), 0.99(s, 3H, CH3);MSm/z=359.1; Anal Calcd for C20H22FNO4(%): C 66.83,H 6.17,F 5.29,N 3.90,O 17.81;found: C 66,76,H 6.25,F 5.31,N 3.82,O 17.86.
2 结果与讨论
2.1 反应条件优化
2.1.1 催化剂用量对反应的影响
以苯甲醛为底物,离子液体[DABCO][CF3SO3]为催化剂,反应在60℃下进行,只改变催化剂的用量,由此考察了催化剂的用量对反应时间以及产率的影响,结果见表1.
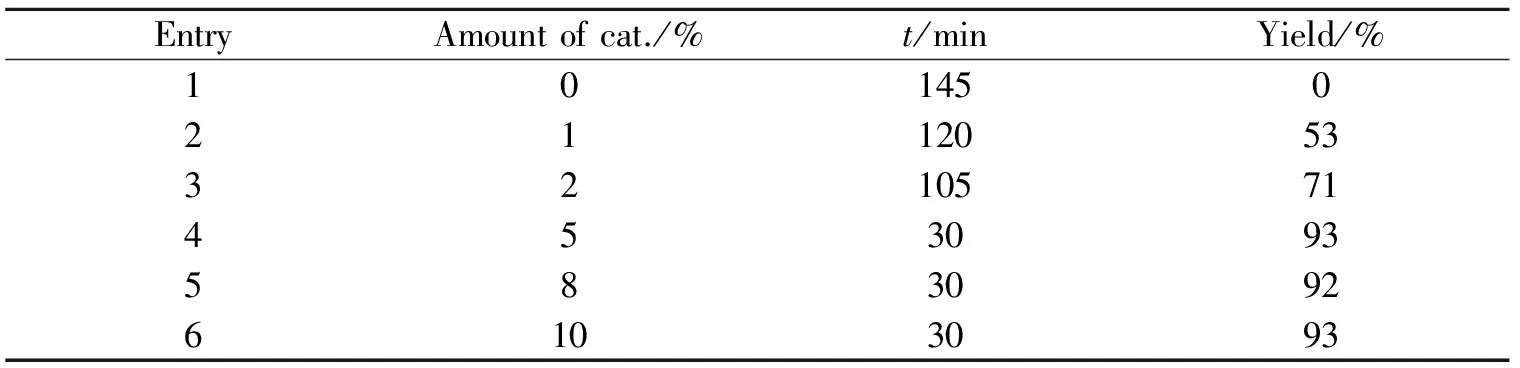
表1 催化剂用量对反应时间和产率的影响Table 1 Effects of the amount of catalyst on reaction yields
上表的实验结果表明,催化剂的用量对反应是有影响的. 在没有催化剂存在时,反应可以进行,但是没有得到多组分缩合产物. 当催化剂用量为1.0%(物质的量分数,下同)时,产率为53%,反应时间为120 min;当催化剂用量为2.0%时,产率为71%,反应时间为105 min;当催化剂用量为5%时,产率达到93%,且反应时间缩短为30 min;当催化剂用量为8%时,产率为92%,反应时间为30 min;当催化剂用量为10%时,产率为93%,反应时间为30 min. 由上述实验结果我们可以知道,催化剂对反应的产率和时间都有影响,但催化剂的用量也有一个最适值,由实验可得,催化剂用量为5%较合适,此时产率最高,反应时间也最短.
2.1.2 温度对反应的影响
以苯甲醛作为底物,离子液体[DABCO][CF3SO3]为催化剂,催化剂用量为5 mol%,只改变反应的温度,由此考察了反应温度对反应时间和产率的影响,结果见表2.
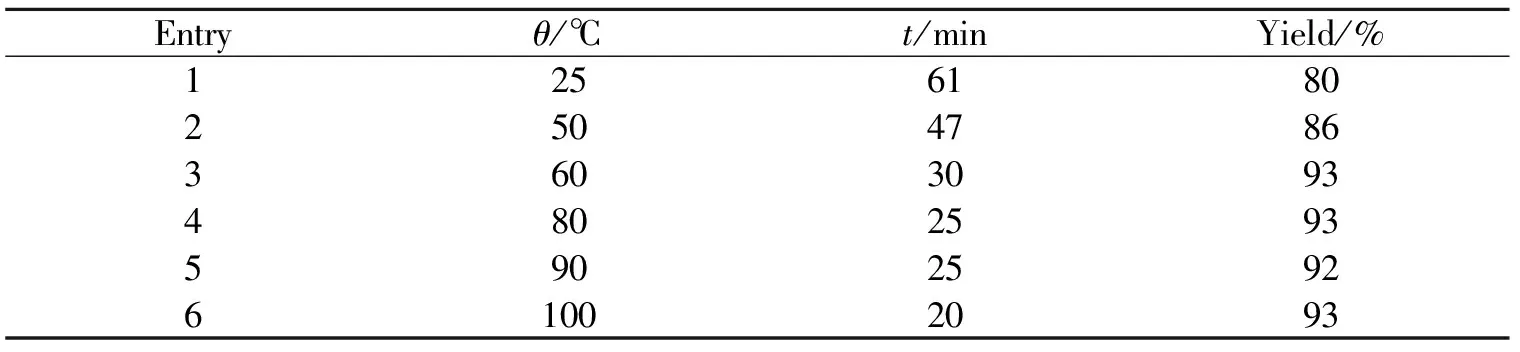
表2 反应温度对反应时间和产率的影响Table 2 Effects of reaction temperature on reaction yields
实验结果表明,温度对反应有影响. 当温度为60 ℃时,产率最高为93%,且反应时间也相对较短;而当温度继续升高或降低时,反应产率并没有提高,反应时间也没有相应缩短. 由此,本实验的最佳温度为60 ℃.
3.1.3 反应时间对反应的影响
以苯甲醛为底物,离子液体[DABCO][CF3SO3]为催化剂,反应在60 ℃下进行,催化剂用量为5%,控制反应时间,由此考察反应时间对产率的影响. 结果见图3.
反应时间过短,反应不完全;在一定范围内,反应时间越长,反应越完全,收率越高. 由图3可知,当反应时间在30 min之前时,随着时间的增加,反应产率逐渐的提高,但继续延长反应时间,产率基本不变.
最终我们得到的以苯甲醛为底物进行反应的最佳反应条件为:催化剂用量5%(物质的量分数),反应温度为60 ℃,反应时间为30 min.
3.2 [DABCO][CF3SO3]一锅法制备四氢苯并吡喃及其衍生物
各种芳香醛与丙二腈、双甲酮的三组分一锅法反应结果见表3. 由表可知,带有不同取代基的芳香醛都能在离子液体[DABCO][CF3SO3]的催化下,在60℃下与丙二腈,双甲酮等活性亚甲基化合物发生反应,反应时间短,产率高. 芳香醛的取代基不同会影响反应时间和收率. 带有吸电子基的芳香醛如硝基,氯(表3,序号2-4)比带给电子基的芳香醛如甲氧基(表3,序号5-7)反应效果更好. 这是由于芳香醛上取代基团对羰基碳正电性的影响所致,具有更强羰基碳正电性的醛所需的时间更短,产率更高. 当苯环上有多基团取代(表3,序号8)时,由于存在空间位阻,所需的反应时间更长,产率相对较低. 作者也尝试了杂原子取代的芳香醛,如2-噻吩醛,1-萘醛,吲哚-3-甲醛(表3,序号9-11),反应仍很迅速,产率也可以达到80%左右. 将丙二腈替换为活性相对较差的氰乙酸乙酯,反应产率可达到85%以上(表3,序号12,13).
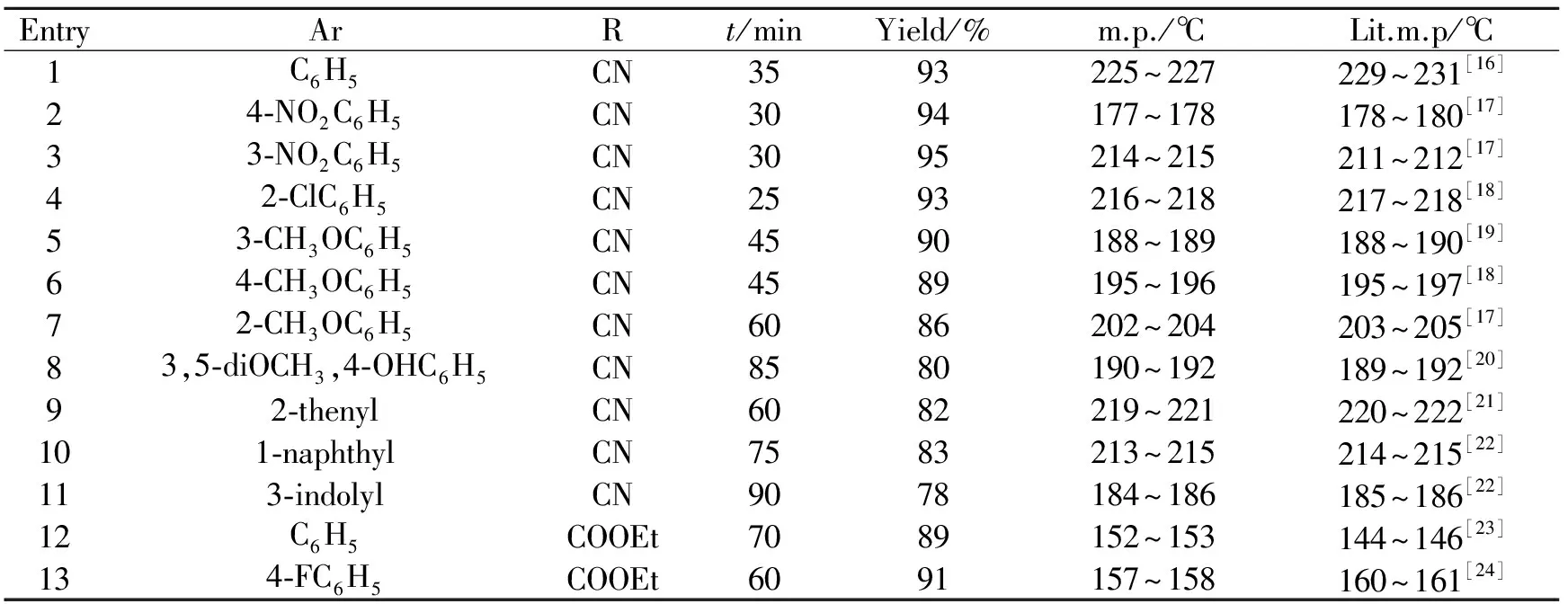
表3 一锅法制备四氢苯并吡喃及其衍生物Table 3 One-pot synthesis of various 4H-benzo[b]pyran derivatives via a three-component condensation in aqueous media
3.3 催化剂循环使用性能
以苯甲醛为底物,离子液体[DABCO][CF3SO3]为催化剂,反应在60℃下进行,初始催化剂用量为5%,由此考察催化剂的重复使用性能. 反应结束后,过滤析出固体产物,产物用适量乙醇重结晶. 由于该离子液体不溶于乙酸乙酯,只需要将滤液通过简单的乙酸乙酯萃取分离,然后通过真空干燥除水即可直接用于催化下次反应. 催化剂重复使用结果见图4. 结果表明,催化剂重复使用4次以后仍保持较好的活性.
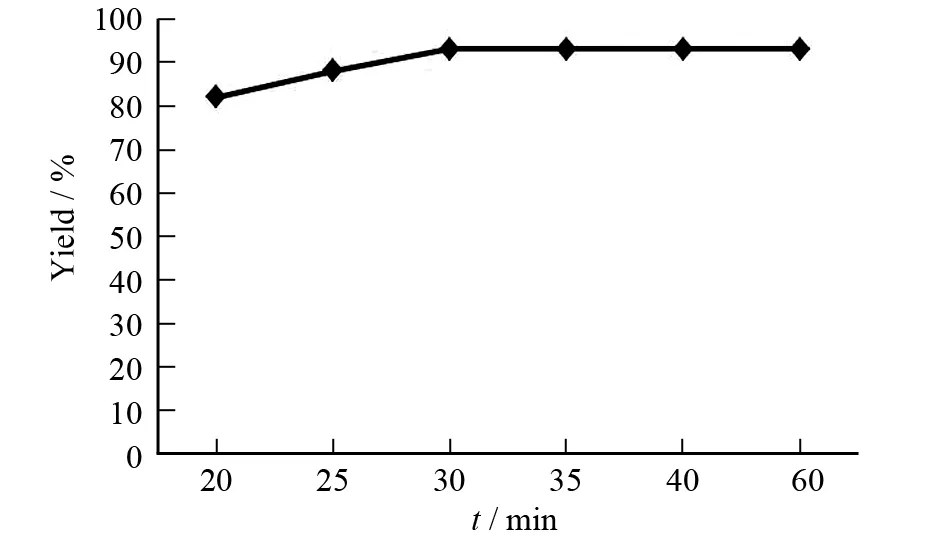
图3 反应时间对反应产率的影响Fig.3 Effects of the reaction time on reaction yields
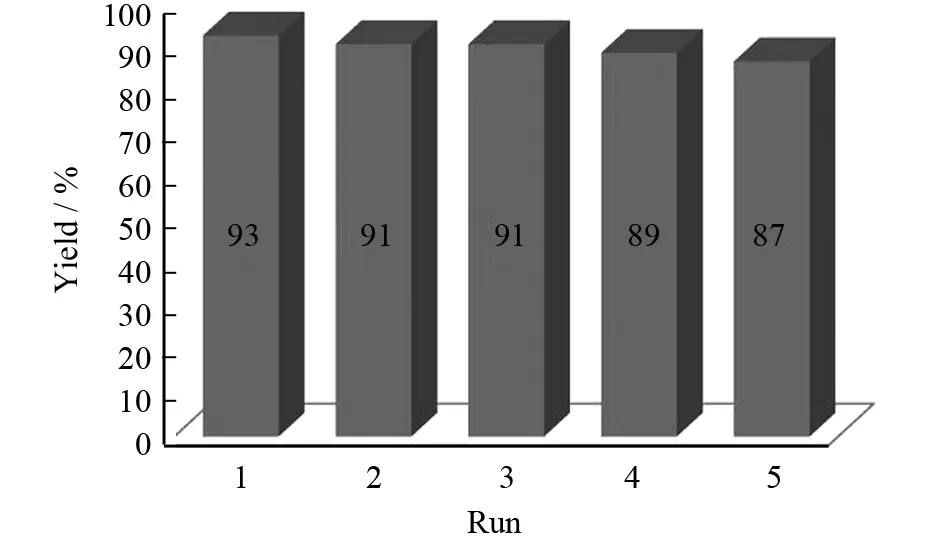
图4 催化剂循环使用性能Fig.4 The resuabilities of the catalyst
3.4 催化剂的催化机理
可能的反应机理(见图5):[DABCO][CF3SO3]中未与3-氯-1,2-丙二醇连接的氮原子显碱性,可以夺去丙二腈活性亚甲基的氢原子;该分子中的羟基可与芳香醛的羰基形成氢键,使羰基碳的电正性更强,容易受到碳负离子进攻. 通过Knoevenagel缩合反应,得到中间体A. 同时,[DABCO][CF3SO3]中未与3-氯-1,2-丙二醇连接的氮原子也可以夺去双甲酮活性亚甲基的氢原子,形成的碳负离子与A进行加成反应,得到中间体B,B经过烯醇化、环合、互变异构等过程得到目标产物.
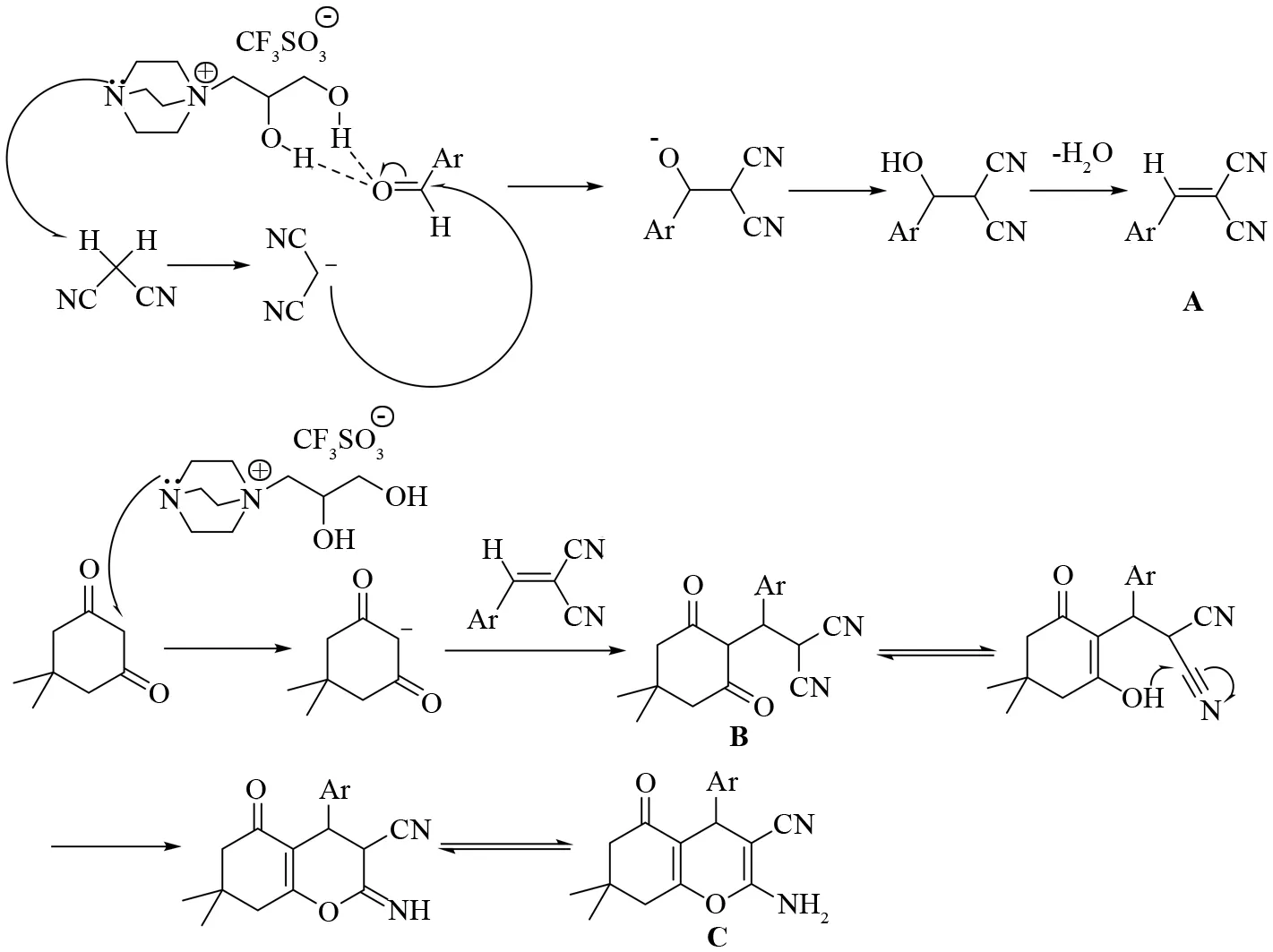
图5 [DABCO][CF3SO3]的催化机理Fig.5 Catalytic mechanism of [DABCO][CF3SO3]
结论:[DABCO][CF3SO3]是一种环保,简单易得,催化效率高,使用安全的离子液体催化剂. 能够催化以水为溶剂的三组分一锅法制备四氢苯并吡喃及其衍生物的反应. 与文献相比,该方法具有反应条件温和,反应时间短,反应覆盖率大,产率高(80%~95%),同时催化剂制作简便,且重复使用率高,反应后处理方便等优点,为四氢苯并吡喃的制备提供了一种有效,环保的合成方法,在精细化学品合成方面有一定的工业化应用潜力.
[1]BONSIGNORE L, LOY G, SECCI D, et al. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives [J]. Eur J Med Chem, 1993, 28(6): 517-520.
[2]CHINIGO G M, PAIGE M, GRINDROD S, et al. Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity [J]. J Med Chem, 2008, 51: 4620-4631.
[3]COHEN E, KLARBERG B, VAUGHAN J R. Quinazolinone sulfonamides. A new class of diuretic agents [J]. J Am Chem Soc, 1960, 82: 2731-2735.
[4]ARMETSO D, HORSPOOL W M, MARTIN N, et al. Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted, 2-amino-3, 5-dicyano-6-phenyl-4H-pyrans [J]. J Org Chem, 1989, 54(13): 3069-3072.
[5]WANG Xiangshan, SHI Daqing, TU Shujiang, et al. A convenient synthesis of 5-oxo-5,6,7,8-tetrahydro-4H-benzo-[b]pyran derivatives catalyzed by KF-alumina [J]. Synth Commun, 2003, 33: 119-126.
[6]BALALAIE S, BARARJANIAN M, AMANI A M, et al. (S)-Proline as a neutral and efficient catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media [J]. Synlett, 2006, 2: 263-266.
[7]SINGH K, SINGH J, SINGH H. A synthetic entry into fused pyran derivatives through carbon transfer reactions of 1,3-oxazinanes and oxazolidines with carbon nucleophiles [J]. Tetrahedron, 1996, 52(45): 14273-14280.
[8]PORE D M, UNDALE K A, DONGARE B B, et al. Potassium phosphate catalyzed a rapid three-component synthesis of tetrahydrobenzo[b]pyran at ambient temperature [J]. Catal Lett, 2009,132: 104-108.
[9]SEIFI M, SHEIBANI H. High surface area MgO as a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media [J]. Catal Lett, 2008, 126(3/4): 275-279.
[10]靳通收,王爱卿,张建设,等. 水中“一锅法”合成2-氨基-3-氰基-4-芳基-7, 7-二甲基-5-氧代-4H-5, 6, 7, 8-四氢苯并[b]吡喃[J]. 有机化学, 2004, 24(12): 1598-1600.
[11]史达清,张 姝,庄启亚. 水介质中2-氨基-4-芳基-7,7-二甲基-5-氧代-4 H-5,6,7,8-四氢苯并[b]吡喃-3-羧酸酯的有效合成[J]. 有机化学, 2005,25(12): 1570-1574.
[12]张 慧,曹卫国,任仲皎. 无溶剂研磨条件下四氢苯并毗喃衍生物的一锅法合成[J]. 有机化学, 2006, 26(8): 1018-1021.
[13]BALALAIE S, SHEIKH-AHMADI M, BARARJANIAN M. Tetra-methyl ammonium hydroxide: An efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media [J]. Catal Commun, 2007, 8: 1724-1728.
[14]SALVI P P, MANDHARE A M, SARTAPE A S, et al. An efficient protocol for synthesis of tetrahydrobenzo[b]pyrans using amino functionalized ionic liquid [J]. C R Chimie, 2011, 14: 878-882.
[15]XU Dazhen, LIU Yingjun, SHI Sen, et al. A simple, efficient and green procedure for Knoevenagel condensation catalyzed by [C4dabco][BF4]ionic liquid in water [J]. Green Chem, 2010, 12(3): 514-517.
[16]SHAABANI A, SAMADI S, RAHMATI A. One-pot, three-component condensation reaction in water. An efficient and improved procedure for the synthesis of pyran annulated heterocyclic systems [J]. Synth Commun, 2007, 37(3): 491-499.
[17]XU Jiangcheng, LI Wanmei, ZHENG Hui, et al. One-pot synthesis of tetrahydrochromene derivatives catalyzed by lipase [J]. Tetrahedron, 2011, 67(49): 9582-9587.
[18]ZHI Huizhen, LV Chunxu, ZHANG Qiang, et al. A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzofurans [J]. Chem Commun, 2009, 20: 2878-2880.
[19]MOSADDEGH E, HASSANKHANI A, MANSOURI G. An efficient, simple and green Zn(Phen)2Cl2complex catalyzed synthesis of 4-H-benzo[b]pyrans in water at ambient temperature [J]. E-J Chem, 2011, 8(2): 529-534.
[20]PATRA A, MAHAPATRA T. Synthesis of tetrahydrobenzo[b]pyran derivatives catalysed by Aliquat 336 in water under microwave irradiation [J]. J Chem Res, 2010, 34(12): 689-693.
[21]HASANINEJAD A, SHEKOUHY M, GOLZAR N, et al. Silica bondedn-propyl-4-aza-1-azoniabicyclo[2.2.2]octane chloride (SB-DABCO):a highly efficient, reusable and new heterogeneous catalyst for the synthesis of 4H-benzo[b]pyran derivatives [J]. Appl Catal A: Gen, 2011, 402(1/2): 11-22.
[22]GAO S, TSAI C H, TSENG C, et al. Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media [J]. Tetrahedron, 2008, 64(38): 9143-9149.
[23]KHAN A T, LAL M, ALI S, et al. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst [J]. Tetrahedron Lett, 2011, 52(41): 5327-5332.
[24]FAN Xuesen; HU Xueyuan, ZHANG Xinying, et al. Ionic liquid-promoted Knoevenagel and Michael reactions [J]. Aust J Chem, 2004, 57(11): 1067-1071.
Abstract: A novel naphthalene-based fluorescent Hg2+sensor was designed and synthesized. The selective recongnition ability of as-synthesized Hg2+sensor towards Hg2+, Li+, Na+, K+, Zn2+, Co2+, Ni2+, Cu2+, Fe2+, Mn2+, Cr3+and Fe3+was evaluated by fluorescent spectra titration experiments. Results indicate that as-synthesized Hg2+sensor exhibits good selectivity towards Hg2+over Li+, Na+, K+, Zn2+, Co2+, Ni2+, Cu2+, Fe2+, Mn2+, Cr3+and Fe3+in H2O-DMSO with a buffered pH value of 7. 4. The fluorescent sensor and Hg2+form a 1∶1 complex, thereby leading to fluorescent quenching with a binding constant of (9.07 ± 0.41) × 104.
Keywords: fluorescence; chemical sensor; Hg2+; design; synthesis
Synthesisofatetrahydrobenzo-pyrananditsderivatives
inaqueousmedium
GAO Jie1, LIU Shuo2, ZHOU Hongying3, ZHU Wanli1, LIU Lu1, YING Anguo1,4*
(1.SchoolofPharmaceuticalandChemicalEngineering,TaizhouUniversity,Taizhou318000,Zhejiang,China;
2.SchoolofChemicalEngineeringandTechnology,TianjinUniversity,Tianjin300072,China;
3.ZhejiangJianyeOrganicChemicalCompanyLtd.,Hangzhou311604,Zhejiang,China;
4.InstituteofAppliedChemistry,TaizhouUniversity,Taizhou318000,Zhejiang,China)
1,4-diazabicyclo[2.2.2]octane (denoted as DABCO) based ionic liquid was synthesized. As-obtained DABCO-based ionic liquid was adopted as a catalyst to catalyze the one-pot three-component condensation reaction for synthesizing 4H-benzo[b]pyran derivatives in aqueous media affording target compound 2-amino-3-cyano-4-aryl-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran. The effects of reaction time, catalyst dosage, catalyst-adding time, and reaction temperature on the yield were investigated, and the optimal reaction condition was established. Moreover, various aromatic aldehydes and active methylene compounds were adopted as starting materials to synthesize a series of tetrahydrobenzo-pyran compounds, and possible reaction mechanisms were discussed. Results indicate that as-established synthesis route has the advantages of mild reaction condition, short reaction time, high yield and easy post-treatment, while the catalyst retains nearly unchanged activity after 4 times of recycle. In terms of the reaction mechanisms, as-prepared DABCO-based ionic liquid catalyst exhibits dual catalytic activity.
DABCO; ionic liquid; 4H-benzo[b]pyran; derivative; aqueous phase synthesis
DesignandsynthesisofanewfluorescentHg2+sensor
ZU Fuli, LI Qian, SONG Pan, WANG Chenjuan, XU Kuoxi*
(CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
O 621.25DocumentcodeAArticleID1008-1011(2014)03-0254-06
2013-12-26.
国家自然科学基金项目(No. 21106090),浙江省低碳脂肪胺工程技术研究中心开放基金项目(2012E10033),台州学院校立学生科研项目(13XS26).
高 洁(1992-),女,本科生,研究方向为药物合成.*
,E-mail: agying@tzc.edu.cn, yinganguo@163.com.
O 626.31
A
1008-1011(2014)03-0247-07
