石墨烯纳米片担载Pt-Pd双金属纳米球作为甲醇氧化的电催化剂
2014-09-02杨子娟赵梦溪
杨子娟,赵梦溪,陈 森,樊 阳
(信阳师范学院 化学化工学院,河南 信阳 464000)
石墨烯纳米片担载Pt-Pd双金属纳米球作为甲醇氧化的电催化剂
杨子娟,赵梦溪,陈 森,樊 阳*
(信阳师范学院 化学化工学院,河南 信阳 464000)
利用简便的无表面活性剂的方法合成了石墨烯担载的Pt-Pd双金属纳米球. 首先由Na2PdCl4与氧化石墨烯发生氧化还原反应生成Pd晶种,然后诱导Pt纳米粒子的生长,得到Pt-Pd双金属纳米球. 采用扫描电子显微镜、透射电子显微镜和X射线粉末衍射仪表征了合成的Pt-Pd/GR催化剂的结构,并测定了其作为甲醇氧化电催化剂的性能. 结果表明,Pt-Pd/GR催化剂对甲醇氧化反应表现出高催化活性和稳定性,甲醇氧化电流密度为51.8 mA·cm-2.
石墨烯纳米片;担载Pt-Pd纳米球;甲醇氧化;电催化剂
Graphene-based electrocatalysts have shown promising catalytic performance for fuel cells reactions[1-20]. In these electrocatalysts systems, graphene nanosheets serve as a support to disperse catalytic nanoparticles (NPs) and the electron-transfer medium at the interface of electrode and reactants[21]. The remarkable enhancement in the catalytic activities of graphene-based electrocatalysts can be ascribed to the high specific surface area and conductivity of the graphene nanosheets[22-23]. Furthermore, graphene nanosheets provide a unique two-dimensional (2D) surface for the growth and assembly of catalysts NPs with controlled morphology, orientation and dimensionality[24-27]. Pt-Pd bimetallic electrocatalysts with designed morphologies such as nanotubes, nanowires and nanospheres have attracted much attention, because they are considerably less expensive than pure Pt catalysts and exhibits unique characteristics[28-32]. For example, Pt-Pd bimetallic catalyst exhibits high CO-poisoning tolerance owing to the presence of Pd oxide species, and it has significantly improved catalytic performance as compared with pure Pt catalyst[3,16,18].
Herein, we report a facile surfactant-free route forinsitugrowth of Pt-Pd bimetallic nanospheres on graphene nanosheets. Briefly, Pd NPs were nucleated on graphene oxide nanosheetsviathe redox reaction between [PdCl4]2-and graphene oxide. Pt NPs were then grown and self-assembled around the Pd seeds in association with the reduction of graphene oxide affording corresponding graphene supported catalyst. With this approach, Pt-Pd bimetallic nanospheres can be directly formed on the surface of graphene oxide without the presence of any surfactant molecules. In as-obtained graphene supported Pt-Pd electrocatalyst, the Pt-Pd nanospheres can act as pillars to efficiently separate the graphene sheets from each other thereby preventing the agglomeration of graphene layers[33-34]. Therefore, the characteristics of individual graphene sheets can be retained in the hybrid material. This article reports the synthesis of the title hybrid material with unique structure as well as its high catalytic activity and long-term stability towards methanol oxidation.
1 Experimental
1.1 Reagent and apparatus
Graphite powder (spectrographic purity) and H2PtCl6·6H2O (w(Pt) ≥ 37%) were purchased from Sinopharm Chemical Reagent Co. Ltd., Ascorbic acid (analytical grade reagent) and Na2PdCl4(w(Pd)=36.4%) were purchased from Aladdin Chemistry Co. Ltd., Pt/C (w(Pt)=20% on carbon black, Johnson Matthey) was obtained from Alfa Aesar. Nafion (w= 5%) was obtained from Sigam-Aldrich. Water used throughout all experiments was purified with the Millipore system.
1.2 Structure characterization
Scanning electron microscopy (SEM) images were obtained with a Hitachi S-4800 field emission scanning electron microscope. SEM samples were prepared by dispersing in ethanol and sonicating for several minutes, followed by drop-casting onto a silicon wafer. Transmission electron microscopy (TEM), high-resolution TEM (HRTEM), and energy dispersive X-ray (EDX) spectrometry analyses were conducted with a JEOL JEM-2010 transmission electron microscope. Specimens for all of these TEM experiments were prepared by diluting the catalyst ink with ethanol, sonicating for 2 min in order to ensure adequate dispersion of the nanostructures, evaporating of one drop of the solution onto a 300 mesh Cu grid, and coating with a lacey carbon film. X-ray diffraction (XRD) analyses were carried out with a Bruker D8/Advance X-ray diffractometer with Cu Kαradiation (λ= 0.154 06 nm).
1.3 Electrochemical measurements
All electrochemical experiments were performed with a CHI 850C electrochemical analyzer (CH Instruments). A conventional three-electrode system was used for all electrochemical experiments,and it consists of a platinum wire as counter electrode, a standard calomel electrode (SCE) as reference electrode, and a glassy carbon (GC) electrode (3 mm diameter) coated with the catalyst as working electrode. The catalyst ink was prepared by dispersion of catalyst powder in a mixed solution of ethanol (1 mL) and Nafion (10 μL) under sonication to form a homogeneous black suspension (5 g·L-1). 5 μL of the catalyst ink was cast on the freshly-polished GC electrode surface and dried under an infrared lamp. Before measurements, the working electrode was first activated with cyclic voltammetry (CV, 0-1.0 V, 50 mV·s-1) in a N2-saturated 0.5 mol·L-1H2SO4solution until a steady CV was obtained.
1.4 Preparation of Pt-Pd/GR catalyst
Graphene oxide was prepared by the modified Hummers method[35]. In the growth of Pt-Pd bimetallic nanospheres on graphene sheets, Pd NPs were first nucleated on graphene oxide sheetsviathe redox reaction between [PdCl4]2-and graphene oxide[8]. Pt NPs were then grown and self-assembled around the Pd seeds, with the reduction by ascorbic acid. In a typical procedure, 1 mL of Na2PdCl4aqueous solution (5 g·L-1) was added to 100 mL of a homogeneous graphene oxide suspension (0.5 g·L-1), and the mixture was stirred in an ice-water bath for 30 min. After addition of ascorbic acid (50 mg), the mixture was gradually heated to 90 ℃. Then, 1 mL of H2PtCl6·6H2O solution (16 g·L-1) was rapidly injected into the flask, and the mixture was stirred at 90 ℃ for 3 h. The product, denoted as Pt-Pd/GR, was collected by centrifugation, washed with water, and vacuum-dried at 50 ℃ for 6 h. The reference sample of graphene supported Pt NPs, denoted as Pt/GR, was prepared under the same conditions but without the addition of Na2PdCl4.
2 Results and discussion
The crystallographic structure of as-synthesized Pt-Pd/GR catalyst was characterized by powder XRD. As shown in Fig.1a, the diffraction peaks at 2θvalues of 40.1°, 46.7°, 68.0°, 82.1° and 86.5° can be indexed as the (111), (200), (220), (311) and (222) crystalline planes, which indicates that Pt and Pd NPs exhibit typical face-centered cubic (fcc) crystal structure[1-5]. Due to the similarity of the crystalline structures of Pt and Pd (JCPDS card No. 04-0802 (Pt) and No. 46-1043 (Pd)), the peak positions of Pt and Pd show little difference[3-4]. As to the XRD pattern of graphene oxide (Fig.1b), the (002) diffraction peak corresponding to the hexagonal graphite structure shifts to 10.1°. In contrast, it moves to higher angle of 21.9° in that of Pt-Pd/GR, which indicates that graphene oxide is reduced to graphene[6-8].
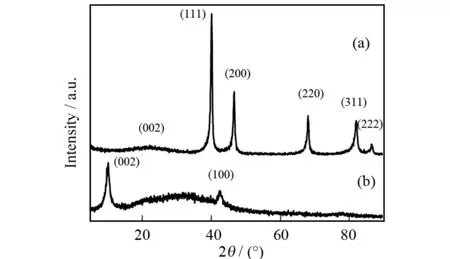
Fig.1 XRD patterns of Pt-Pd/GR (a) and GO (b)
The morphology and microstructure of Pt-Pd/GR catalyst were investigated with SEM and TEM. As shown in Figs. 2a and 2b, a number of Pt-Pd nanospheres with the size ranging from 50 nm to 80 nm are evenly distributed on graphene sheets. Despite of the layering of graphene sheets owing to solvent evaporation, the presence of Pt-Pd nanospheres keeps these layers sufficiently exfoliated. Hence, the characteristics of individual graphene nanosheets can be retained in the hybrid material. Moreover, the sandwiching of Pt-Pd nanospheres between graphene layers produces a highly porous nanostructure, which could facilitate the transport of reactant molecules within the bulk catalyst. In thisinsitugrowth process, the oxygen-containing functional groups on the basal planes of graphene oxide provide the reactive sites for the heterogeneous nucleation of Pd NPs[10]. After the growth of Pd nuclei particles, most of the nucleation sites on graphene surface may be occupied. Therefore, the subsequent growth of Pt NPs preferentially occurs around the pre-anchored Pd NPs, resulting in the formation of Pt-Pd bimetallic nanospheres. The TEM image (Fig.2c) shows that the nanospheres are composed of interconnected fine NPs, which confirms that the Pt-Pd nanospheres are constructed from the self-assembly of primary NPs building blocks. The graphene-supported Pt-Pd nanospheres were also analyzed by EDX (Fig.3), and the results reveal the presence of Pd, Pt and C. The HRTEM image in Fig.2d shows good crystallinity of the nanospheres with well-defined lattice fringes. The lattice fringes with adspacing of 0.23 nm correspond to the typical (111) plane offccPt[12,15-16].
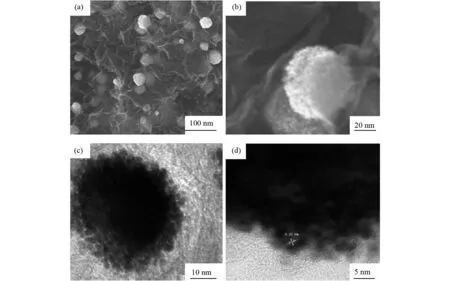
Fig.2 SEM (a and b), TEM (c) and HRTEM (d) images of Pt-Pd/GR
The electrochemical properties of the Pt-Pd/GR catalyst were investigated and compared with those of Pt/GR and Pt/C catalysts. Fig.4 shows the cyclic voltammograms (CVs) of different catalyst-coated GC electrodes in N2-saturated 0.5 mol·L-1H2SO4solution. The CV profiles show typical potential regions of hydrogen adsorption-desorption (-0.2 V to 0.15 V), double-layer capacitance (0.15 V to 0.3 V) and metal oxidation-reduction (above 0.3 V). It is noteworthy that the reduction peak of metal oxide positively shifts to higher potential on Pt-Pd/GR (0.52 V), in comparison with that on Pt/C (0.45 V) and Pt/GR (0.47 V). This suggests that the metal oxide layer is more readily reduced on the Pt-Pd nanospheres to activate the methanol oxidation reaction[36]. The electrochemically active surface area (ECSA) was estimated by integrating the voltammograms corresponding to hydrogen adsorption from the electrode surface. It is found that the ECSA value of Pt-Pd/GR (70.5 m2·mg-1) is higher than that of Pt/C (51.5 m2·mg-1) and Pt/GR (41.3 m2·mg-1) catalysts. This indicates that more active sites for electrocatalytic reaction and electron transfer are available on the Pt-Pd/GR catalyst.
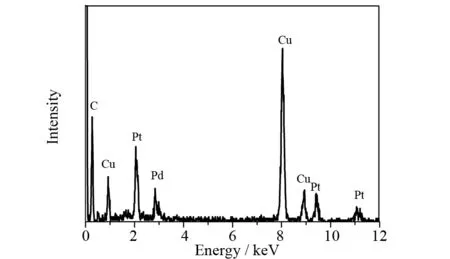
Fig.3 EDX spectrum of Pt-Pd/GR
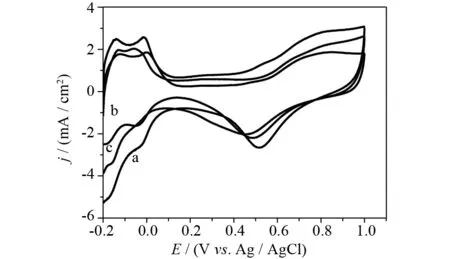
Fig.4 CVs of Pt-Pd/GR (a), Pt/GR (b) and Pt/C (c) catalysts modified electrodes in N2-saturated 0.5 mol·L-1 H2SO4 solution at a scan rate of 50 mV·s-1
The catalytic performance of Pt-Pd/GR catalyst towards methanol oxidation was investigated in 0.5 mol·L-1H2SO4solution containing 1.0 mol·L-1methanol. Two typical oxidation peaks appear in the CV curves of these catalysts (Fig.5), which arise from the oxidation of methanol in the forward scan and the oxidation of COads-like species in the backward scan[8-10]. The peak potential for methanol oxidation at Pt-Pd/GR (0.64 V) is considerably lower than that on Pt/C (0.74 V) and Pt/GR (0.72 V), indicating decreased overpotential at the Pt-Pd/GR catalyst. The peak current density is 51.8 mA·cm-2for Pt-Pd/GR, and it is much higher than those on Pt/GR (29.0 mA·cm-2) and Pt/C (22.9 mA·cm-2) catalysts. This means that Pt-Pd/GR catalyst exhibits remarkably improved catalytic activity for methanol oxidation. The CO-poisoning tolerance of Pt-based catalysts is generally evaluated by the peak current ratio of forward to backward scans (If/Ib)[18-20]. The calculatedIf/Ibratio on Pt-Pd/GR (1.2) is higher than that of Pt/C (0.8) and Pt/GR (1.0). The increasedIf/Ibratio of Pt-Pd/GR indicates less accumulation of COadsspecies on the catalyst surface and more complete methanol oxidation reaction thereon.
The stability and durability of the Pt-Pd/GR catalyst were evaluated by chronoamperometry tests. As shown in Fig.6, Pt-Pd/GR catalyst exhibits a much higher initial current density than the other catalysts, because of the presence of a larger number of catalytic active sites. The final current density for Pt-Pd/GR after holding the cell potential at 0.7 V for 3 000 s is 10.0 mA·cm-2, and it is about 1.7 and 2.8 times as much as that of Pt/C (5.8 mA·cm-2) and Pt/GR (3.6 mA·cm-2), respectively. It is noteworthy that Pt/GR also exhibits higher initial current density, however, it decays rapidly and becomes lower than that of Pt/C after 700 s. This may be ascribed to the aggregation of Pt NPs and graphene layers under the acidic and oxidative conditions. In contrast, the current density of Pt-Pd/GR remains higher throughout the whole process. This suggests that graphene supported Pt-Pd nanospheres remain stable in the electrocatalytic reaction.
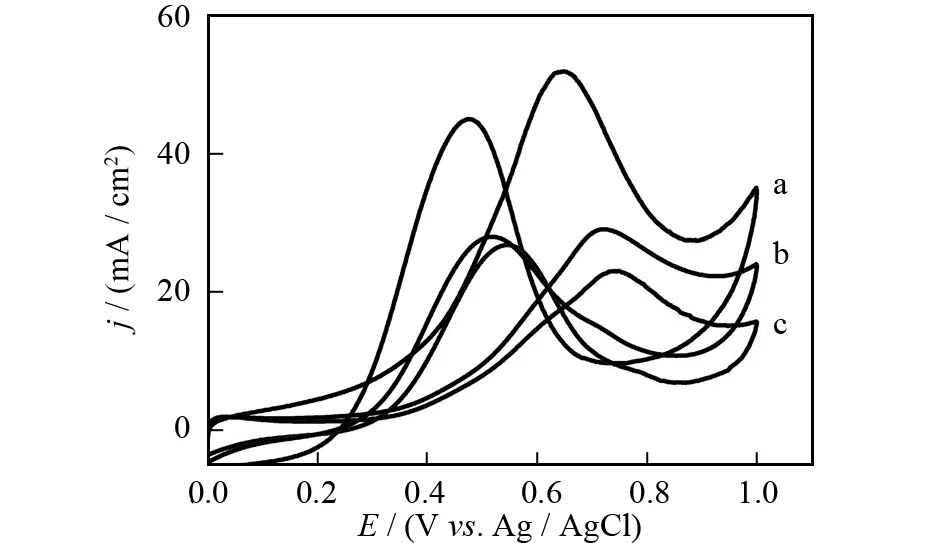
Fig.5 CVs of Pt-Pd/GR (a), Pt/GR (b) and Pt/C (c) catalysts modified electrodes in N2-saturated 0.5 mol·L-1 H2SO4 + 1.0 mol·L-1 methanol solution at a scan rate of 50 mV·s-1
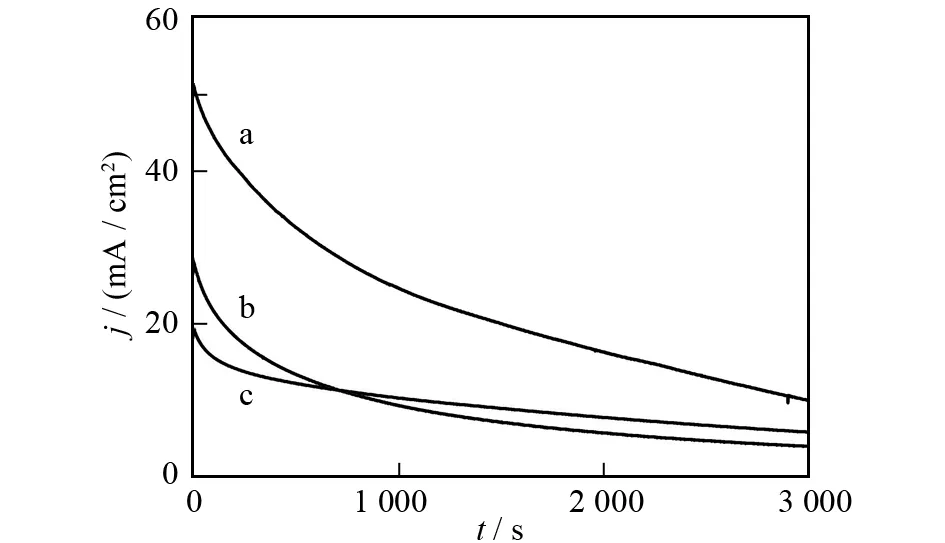
Fig.6 Chronoamperometric curves of Pt-Pd/GR (a), Pt/GR (b) and Pt/C (c) catalysts modified electrodes recorded at 0.7 V in N2-saturated 0.5 mol·L-1 H2SO4 solution containing 1.0 mol·L-1 methanol
3 Conclusions
In summary, we have developed a facile surfactant-free method to synthesize Pt-Pd bimetallic nanospheres on graphene nanosheets.As-prepared hybrid material exhibits a superior catalytic activity and long-term stability for methanol oxidation as compared with commercial Pt/C catalyst and Pt/GR catalyst. In one word, Pt-based NPs with nanospheres structure can be constructed through designedinsitugrowth process on graphene oxide nanosheets, which is promising in improving the catalytic performance towards fuel cell reactions.
[1]YOO E, OKATA T, AKITA T,et al. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface [J]. Nano Lett, 2009, 9(6): 2255-2259.
[2]LI Yueming, TANG Longhua, LI Jinghong. Preparation and electrochemical performance for methanol oxidation of Pt/graphene nanocomposites [J]. Electrochem Commun, 2009, 11(4): 846-849.
[3]GUO Shaojun, WEN Dan, ZHAI Yueming, et al. Platinum nanoparticle ensemble-on-graphene hybrid nanosheets: one-pot, rapid synthesis, and used as new electrode material for electrochemical sensing [J]. ACS Nano, 2010, 4(7): 3959-3968.
[4]WANG Lei, TIAN Chungui, WANG Huan, et al. Mass production of graphene via an in situ self-generating template route and its promoted activity as electrocatalytic support for methanol electroxidization [J]. J Phys Chem C, 2010, 114(19): 8727-8733.
[5]SHANG Naigui, PAPAKONSTANTINOU P, WANG Peng, et al. Platinum lntegrated graphene for methanol fuel cells [J]. J Phys Chem C, 2010, 114 (37): 15837-15841.
[6]LI Yongjie, GAO Wei, CI Lijie,et al. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation [J]. Carbon, 2010, 48 (4): 1124-1130.
[7]ZHOU Yige, CHEN Jingjing, WANG Fengbin, et al. A facile approach to the synthesis of highly electroactive Pt nanoparticles on graphene as an anode catalyst for direct methanol fuel cells [J]. Chem Commun, 2010, 46(32): 5951-5953.
[8]ZHANG Hui, XU Xiaoqing, GU Piao,et al. Microwave-assisted synthesis of graphene-supported Pd1Pt3nanostructures and their electrocatalytic activity for methanol oxidation [J]. Electrochim Acta, 2011, 56 (20): 7064-7070.
[9]YANG Xia, YANG Qingdan, XU Jun, et al. Bimetallic PtPd nanoparticles on Nafion-graphene film as catalyst for ethanol electro-oxidation [J]. J Mater Chem, 2012, 22(16): 8057-8062.
[10]CHEN Xiaomei, WU Genghuang, CHEN Jinmei, et al. Synthesis of “clean” and well-disperisive Pd nanoparticles with excellent electrocatalytic property on graphene oxide [J]. J Am Chem Soc, 2011, 133(11): 3693-3695.
[11]RAO C V, CABRERA C R, ISHIKAWA Y. Graphene-supported Pt-Au nanoparticles: A highly efficient anode for direct formic acid fuel cells [J]. J Phys Chem C, 2011, 115(44): 21963-21970.
[12]ZHANG Sheng, SHAO Yuyan, LIAO Honggang,et al. Polyelectrolyte-induced reduction of exfoliated graphite oxide: A facile route to synthesis of soluble graphene nanosheets [J]. ACS Nano, 2011, 5(3): 1785-1791.
[13]LIANG Yongye, LI Yanguang, WANG Hailiang, et al. Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction [J]. Nat Mater, 2011, 10(10): 780-786.
[14]GUO Shaojun, SUN Shouheng. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction [J]. J Am Chem Soc, 2012, 134 (5): 2492-2495.
[15]KOU Rong, SHAO Yuyan, MEI Donghai. Stabilization of electrocatalytic metal nanoparticles at metal-metal oxide-graphene triple junction points [J]. J Am Chem Soc, 2011, 133 (8): 2541-2547.
[16]YIN Huajie, TANG Hongjie, WANG Dan, et al. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction [J]. ACS Nano, 2012, 6 (9): 8288-8297.
[17]LI Yujing, LI Yongjia, ZHU Enbo, et al. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite [J]. J Am Chem Soc, 2012, 134(30): 12326-12329.
[18]QU Liangti, LIU Yong, BAEK J B, et al. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells [J]. ACS Nano, 2010, 4(3): 1321-1326.
[19]VINAYAN B P, NAGAR R, RAJALAKSHMI N, et al. Novel platinum-cobalt alloy nanoparticles dispersed on nitrogen-doped graphene as a cathode electrocatalyst for PEMFC applications [J]. Adv Funct Mater, 2012, 22 (16): 3519-3526.
[20]SEO M H, CHOI S M, KIM H J, et al. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition [J]. Electrochem Commun, 2011, 13 (2): 182-185.
[21]KRISHNAMURTHY S, LIGHTCAP I V, KAMAT P V. Electron transfer between methyl viologen radicals graphene oxide: reduction, electron storage and discharge [J]. J Photochem Photobiol A: Chem, 2011, 221 (2/3): 214-219.
[22]MACHADO B F, SERP P. Graphene-based materials for catalysis [J]. Catal Sci Technol, 2012, 2: 54-75.
[23]SUN Yiqing, WU Qiong, SHI Gaoquan. Graphene based new energy materials [J]. Energy Environ Sci, 2011, 4: 1113-1132.
[24]XIAO Yuping, WAN Shuo, ZHANG Xing, et al. Hanging Pt hollow nanocrystal assemblies on graphene resulting in an enhanced electrocatalyst [J]. Chem Commun, 2012, 48: 10331-10333.
[25]HU Chuangang, CHENG Huhu, ZHAO Yang, et al. Newly-designed complex ternary Pt/PdCu nanoboxes anchored on three-dimensional graphene framework for highly efficient ethanol oxidation [J]. Adv Mater, 2012, 24 (40): 5493-5498.
[26]CHAI Jia, LI Fenghua, HU Yuwei, et al. Hollow flower-like AuPd alloy nanoparticles: one step synthesis, self-assembly on ionic liquid-functionalized graphene, and electrooxidation of formic acid [J]. J Mater Chem, 2011, 21: 17922-17929.
[27]CHEN Xiaomei, SU Bingyuan, WU Genghuang, et al. Platinum nanoflowers supported on graphene oxide nanosheets: their green synthesis, growth mechanism, and advanced electrocatalytic properties for methanol oxidation [J]. J Mater Chem, 2012, 22: 11284-11289.
[28]ALIA S M, ZHANG Gang, KISAILUS D, et al. Porous platinum nanotubes for oxygen reduction and methanol oxidation reactions [J]. Adv Funct Mater, 2010, 20 (21): 3742-3746.
[29]LIANG Hanpu, ZHANG Huimui, HU Jinsong, et al. Pt hollow nanospheres: Facile synthesis and enhanced electrocatalysts [J]. Angew Chem Int Ed, 2004, 43(12): 1540-1543.
[30]DING Liangxin, WANG Anliang, LI Gaoren, et al. Porous Pt-Ni-P composite nanotube arrays: highly electroactive and durable catalysts for methanol electrooxidation [J]. J Am Chem Soc, 2012, 134 (13): 5730-5733.
[31]CHEN Jingyi, XIONG Yujie, YIN Yadong, et al. Pt nanoparticles surfactant-directed assembled into colloidal spheres and used as substrates in forming Pt nanorods and nanowires [J]. Small, 2006, 2 (11): 1340-1343.
[32]LIANG Haiwei, CAO Xiang, ZHOU Fei, et al. A free-standing Pt-nanowire membrane as a highly stable electrocatalyst for the oxygen reduction reaction [J]. Adv Mater, 2011, 23(12): 1467-1471.
[33]LI Na, LIU Gang, ZHEN Chao,et al. Battery performance and photocatalytic activity of mesoporous anatase TiO2nanospheres/graphene composites by template-free self-assembly [J]. Adv Funct Mater, 2011, 21 (9): 1717-1722.
[34]XIA Baoyu, WANG Bao, WU Haobin, et al. Sandwich-structured TiO2-Pt-graphene ternary hybrid electrocatalysts with high efficiency and stability [J]. J Mater Chem, 2012, 22: 16499-16505.
[35]KOVTYUKHOVA N I, OLLIVIER P J, MARTIN B R, et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations [J]. Chem Mater, 1999, 11 (3): 771-778.
[36]WANG Shuangyin, JIANG Sanping, WHITE T J, et al. Electrocatalytic activity and interconnectivity of nanoparticles on multiwalled carbon nanotubes for fuel cells [J]. J Phys Chem C, 2009, 113 (43): 18935-18945.
date:2014-01-20.
Supported by the Science & Technology Innovation Talents in Universities of Henan Province (13HASTIT012), the Science and Technology Key Project of Henan Education Department (12A150020) and the College Outstanding Teachers Program of Henan Province (2012GGJS-128).
Biography:YANG Zijuan (1988-), graduate student, majoring in nano-electrochemistry.*
, E-mail: fanyangchem@163.com.
