Chemical Fixation of Carbon Dioxide by Zinc Halide/PPh3/n-Bu4NBrNBr
2014-08-06YangHaiJianHuangHailiPengJingYangHongwei
Yang HaiJian, Huang Haili,Peng Jing, Yang Hongwei
(College of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China)
Nowadays, with the increase of CO2emissions, the greenhouse effect has become a major problem[1]. The fixation of CO2has been a challenge for synthetic chemistry and has recently attracted much interest in view of the green chemistry[2]. As one of the effective routes for chemical fixation of CO2, the synthesis of cyclic carbonates by the coupling reactions of epoxides and CO2have been extensively investigated[3]. Consequently, various kind of catalysts and catalytic systems have been developed, including phosphonium iodide, transition metal complexes, supported metal complexes, electroreduction, and ionic liquids[4]. Hence, it is desirable to develop new types of active and stable catalysts that are highly active for the coupling reaction of epoxides and CO2, but almost inert to the decomposition reactions of cyclic carbonates.
Herein, we reported an extremely simple, solvent-free, air-insensitive, cheap catalyst with shorter reaction time and high selectivity and ecologically safer route to cyclic carbonate from the reactions of epoxide with CO2in the presence of catalytic amounts of Zinc halide, PPh3andn-Bu4NBr.
1 Experimental
1H NMR spectra were recorded on a Bruker Al-400 MHz instrument using trimethylsilane (TMS) as an internal standard. All spectra were recorded at room temperature. All the known compounds were identified by comparison of their physical and spectral data with those in previous reports. The epoxides were distilled from CaH2.
General procedure for synthesis of cyclic carbonates: A stainless steel autoclave (250 mL) dried in vacuo containing catalysts was linked to CO2cylinders. A prescribed amount of epoxide was added with a hypodermic syringe. The catalysts were successively charged into the reactor without using any additional solvent. The reactor vessel was placed under a constant pressure of CO2for 20 min to allow the system to equilibrate and then heated to the desired temperature under stirring. After cooling to ambient temperature, the result mixture was transferred to a 50 mL round bottom flask. The unreacted propylene oxide was removed in vacuo, the product was obtained as a colorless liquild. All the cyclic carbonates were identified by GC/MS (HP6890/5973) and 400 MHz NMR.
2 Results and Discussion
During our investigation of the cycloaddition between CO2and PO catalyzed by ZnX2,in the presence of quaternary salts and PPh3(Fig.1),it turned out that the propylene carbonate could be obtained in 98.1 % yield without using any co-solvents (Tab.1, entry 22).

Fig.1 Synthesis of cyclic carbonates from epoxides and CO2图1 环氧烷与CO2合成环状碳酸酯

Entry[a]Catalytic systemT/℃P/MPat/hYield/%[b]1ZnCl2/PPh3/n-Bu4NBr1303593.42ZnCl2/PPh3/n-Bu4NCl1303560.83ZnCl2/PPh3/n-Bu4NI1303573.84ZnCl2/PPh3/Et4NBr1303545.95ZnCl2/PPh3/DMAP1303533.76n-Bu4NBr1303530.87ZnCl21303512.68ZnCl2/n-Bu4NBr1303574.69ZnCl2/PPh3130359.510PPh3130355.611PPh3/n-Bu4NBr1303521.112ZnCl2/PPh3/n-Bu4NBr1103566.013ZnCl2/PPh3/n-Bu4NBr903544.214ZnCl2/PPh3/n-Bu4NBr703514.915ZnCl2/PPh3/n-Bu4NBr8354.616ZnCl2/PPh3/n-Bu4NBr130319.117ZnCl2/PPh3/n-Bu4NBr1303373.818ZnCl2/PPh3/n-Bu4NBr1303785.519ZnCl2/PPh3/n-Bu4NBr1301532.620ZnCl2/PPh3/n-Bu4NBr1305537.321ZnCl2/PPh3/n-Bu4NBr1307533.522ZnBr2/PPh3/n-Bu4NBr1303598.123ZnI2/PPh3/n-Bu4NBr1303597.024ZnEt2/PPh3/n-Bu4NBr1303554.325Zn(CH3COO)2/PPh3/n-Bu4NBr1303589.926Zn/PPh3/n-Bu4NBr1303579.4
[a]Reaction conditions: propylene oxide (15 mL, 12.44 g, 0.214 mol),r(ZnBr2︰PPh3︰n-Bu4NBr︰PO) = 10︰1︰2︰1783, the selectivity to products are all more than 99 %;[b]Isolated yields
2.1 The effect of CO2 pressure
The effect of CO2pressure on the catalytic activity was studied at 130 ℃ and 5 h under the pressure of 1.0~7.0 MPa, as can be seen in Fig.2, the product propylene carbonate (PC) yield increased to 1.0~3.0 MPa, which probably because PC is in its liquid form under the adopted reaction conditions, the increase of CO2pressure should be a favorable factor for PO conversion[5]. However, when the pressure reached to 3.0~7.0 MPa, the yield decreased immidiately (Tabl.1, entries 1, 19~21), which could be attributed to the decrease in polarity of CO2with an increase in pressure. The polarity change resulted in the solubility decrease of the catalyst in CO2[6].
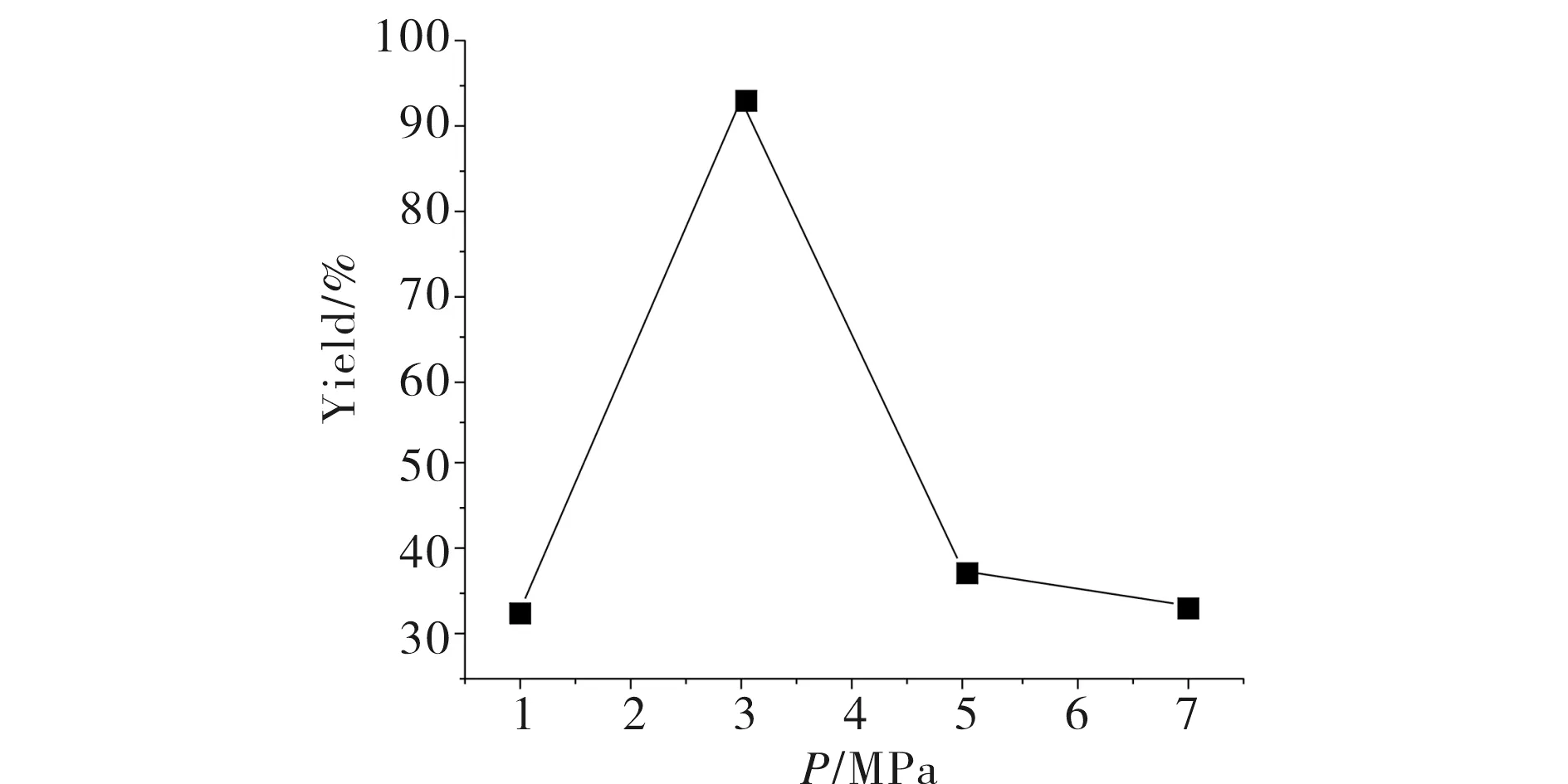
Fig.2 Effect of CO2 pressure on the PC yield图2 CO2压力对PC产率的影响
2.2 The effect of reaction time
The effect of reaction time on the catalytic activity was studied at 130 ℃ and 3 MPa in 1~7 h (Fig.3). The PC yield increased rapidly within 5 h, but the yield decreased at 7 h. (Tab.1, entries 1, 16~18). It can be explained that the contact between the catalytically active site and the reaction substrates became more and more difficult along with the increase of PC amount in the reaction system, which could be attributed to the increasing visicoty[7].

Fig.3 Effect of reaction time on the PC yield图3 反应时间对PC产率的影响
2.3 The effect of reaction temperature
Reaction temperature is a very important factor for the cycloaddition of CO2with epoxides, different temperature even would lead to different product. Herein, the reaction time effect was studied at 5 h and 3 MPa under 60~130 ℃(Tab.1, entries 1, 12~15). As can be seen from Fig.4, the PC yield is nearly proportional to reaction temperature and the reaction almost complete at 130 ℃, which indicated that the reaction reacted most during this interval[8].

θ/℃Fig.4 Effect of reaction temperature on the PC yield图4 反应温度对PC产率的影响
2.4 The effect of various catalytic systems
Basing on the above results, the effect of various catalytic systems for the coupling reaction of PO and CO2were tested at 130 ℃, 5 h and 3 MPa, the results were also summarized in Tab.1. It’s well known that the Lewis base is the most common used co-catalyst, especially quaternary salts. Four kinds of quaternary salts (n-Bu4NBr,n-Bu4NCl,n-Bu4NI and Et4NBr) were investigated as the co-catalyst, and it turned out that highest PC yield was obtained withn-Bu4NBr as co-catalyst under the same conditions (Tab.1, entries 1~4). This could be explained considerably that the catalytic activity of the halide is determined by a balance between its nucleophilicity and leaving-group ability. A good nucleophile would favour the initial opening of the epoxide ring, but it would also be a worse leaving group in the last step of the reaction, i.e. in the back-biting reaction leading to the formation of cyclic carbonate[9]. 4-Dimethylaminopyridine (DMAP) was also a co-catalyst for the coupling reaction. As an organic base, DMAP could activate CO2to form an organic base-CO2intermediate, which was different from quaternary ammonium salt[10]. However, DMAP showed much lower catalytic activity than quaternary ammonium salts (Tab.1, entry 5), which indicated that the reaction mechanism ofn-Bu4NBr was different from the system with DMAP as the co-catalyst.
Since the catalytic system was multi-compounded, it was supposed that a synergistic effect should exist[11]. To confirm the proposal, some catalytic systems were also tested without quaternary ammonium salt, ZnCl2and PPh3severally, and the component ZnCl2,n-Bu4NBr, PPh3was solely used as a single catalyst for this reaction as well. The PC yield dropped for all the tested system as expected (Tab.1, entries 1, 6~11), which indicated that there′s some inhibitory effect betweenn-Bu4NBr and PPh3.
2.5 Proposed mechanism
From the results above, a proposed mechanism was deduced in Fig.5. The catalytic system has two catalytic cycles, the quaternary salts open the ring of the epoxide to produce an oxyanion species. That may be why the yield was rather low when the quaternary salt was absent (Tab.1, entry 9). While in the other cycle, it is the ZnX2(PPh3)2 formed in situ during the early phase of the coupling reaction performed in the presence of ZnCl2and triphenylphosphine (PPh3) which activeated the inert CO2[12]. Then the metal centre ZnCl2was replaced by ZnBr2, ZnI2, ZnEt2, Zn(CH3COO)2, Zn (Tab.1, entries 22~26). The results showed that the catalytic activity order was ZnBr2≥ ZnI2> ZnCl2> Zn(CH3COO)2> Zn > ZnEt2, which suggested that leaving-group ability of the anions seem to play an important role in the process of CO2inserting[13].

Fig.5 The plausible mechanism for the cycloaddition of CO2 with PO catalyzed by ZnX2 (X= Cl, Br, I), PPh3 and n-Bu4NBr图5 由卤化锌、三苯基膦和四丁基溴化铵催化CO2与环氧丙烷环加成反应的可能机理
2.6 Cycloaddition reaction of various epoxides and CO2
To extend the scope of the coupling reaction, six epoxides (epichlorohydrin, glycidyl isopropyl ether, 1,2-epoxyhexane, isobutylene oxide, styrene oxide, cyclohexene oxide) were used as substrates and reacted with CO2under optimized and solventless reaction conditions (130℃, 5 h, 3 MPa). The cycloaddition results shown in Tab.2 indicated that the catalyst system could convert all the general epoxides studied to the corresponding cyclic carbonates effectively under mild conditions. Among them, epichlorohydrin (Tab.3, entry 1), glycidyl phenyl ether (Tab.3, entry 2), 1,2-epoxyhexane (Tab.3, entry 3) and styrene oxide (Tab.3, entry 5) gave the relevant cyclic carbonate with more than 90% yield. However, when isobutylene oxide (Tab.3, entry 4) and cyclohexene oxide (Tab.3, entry 6) were used as substrate, the yields were dramatically lower than others. This might be due to the low reactivity of electron-rich substrates and bigger steric hindrance[14].
Tab.2 The coupling results of various epoxides and CO2
表2 其他环氧烷与CO2耦合反应结果

[a]Reaction conditions:epoxides(0.214 mol),r(ZnBr2∶PPh3∶n-Bu4NBr∶PO )= 10∶1∶2∶1783,P=3 MPa,T=130 ℃,t=5 h,the selectivity to products are all more than 99 %;
[b]Isolated yields.
3 Conclusion
The efficient catalytic system composed of ZnX2/PPh3/n-Bu4NBr was developed for the coupling reaction between epoxide and CO2. It was found that high conversion, more than 99.0% selectivity, and high PC yield (more than 90%) could be achieved in the presence of ZnX2/PPh3/n-Bu4NBr (X=Cl, Br, I) for the synthesis of cyclic carbonates under the mild conditions (130 ℃, 3 MPa, 5 h) without any organic solvents. The catalytic system was also tested for their activity with various other epoxides and was found to yield considerable amount of corresponding cyclic carbonates under similar conditions. In addition, the mechanism for the coupling reaction was also discussed which could explain the reaction results satisfactorily.
Acknowledgements
We are grateful to National Natural Science Fundation of China (No. 20607031).
[1] Najafabadi A.CO2Chemical conversion to useful products: an engineering insight to the latest advances toward sustainability [J].Int J Energy Res,2013,37(6):485-499.
[2] Clark J.Green chemistry:challenges and opportunities[J].Green Chem,1999,1(1):1-8.
[3] Darensbourg D,Holtcamp M.Catalysts for the reactions of epoxides and carbon dioxide [J].Coord Chem Rev,1996,153:155-174.
[4] Liang S,Liu H,Jiang T,et al.Highly effcient synthesis of cyclic carbonates from CO2and epoxides over cellulose/KI [J].Chem Commun,2011 ,47(7):2131-2133.
[5] Li F,Xia C,Xu L,et al.A novel and effective Ni complex catalyst system for the coupling reactions of carbon dioxide and epoxides [J].Chem Commun,2003 (16):2042-2043.
[6] Pulla S,Ramidi P,Jarvis B,et al.The role of substituents of chlorostannoxanes on the reactivity of the catalysts during the synthesis of cyclic carbonates using epoxides and carbon dioxide [J].Greenhouse Gas Sci Technol,2012,2(1):66-74.
[7] Song J,Zhang Z,Hu S,et al.MOF-5/n-Bu4NBr:an ef cient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2under mild conditions [J].Green Chem,2009,11(7):1031-1036.
[8] Pescarmona P,Taherimehr M.Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2[J].Catal Sci Technol,2012,2(11):2169-2187.
[9] Bai D,Jing H,Wang G.Cyclic carbonate synthesis from epoxides and CO2over cyanocobalamin/n-Bu4NI [J].Appl Organometal Chem,2012,26(11):600-603.
[10] Wang Y,Song X,Shao S,et al.Functionalization of carbon nanotubes by surface-initiated immortal alternating polymerization of CO2and epoxides [J].Polym Chem,2013,4(3):629-636.
[11] Castro-Gómez F,Salassa G,Kleij A W,et al.A DFT study on the mechanism of the cycloaddition reaction of CO2to epoxi2es catalyzed by Zn(Salphen) complexes [J].Chem Eur J,2013,19(20):6289-6298.
[12] Kim H S,Bae J Y,Lee J S,et al.Phosphine-Bound zinc halide complexes for the coupling reaction of ethylene oxide and carbon dioxide [J].J Catal,2005,232(1):80-84.
[13] Yang Y,Hayashi Y,Fujii Y,et al.Efficient cyclic carbonate synthesis catalyzed by zinc cluster systems under mild conditions [J].Catal Sci Technol,2012,2:509-513.
[14] Song Q W,He L N,Wang J Q,et al.Catalytic fixation of CO2to cyclic carbonates by phosphonium chlorides immobilized on fluorous polymer [J].Green Chem,2013,15(1):110-115.
