Hydrolysis Kinetics of 2-Pyridinecarboxamide,3-Pyridinecarboxamide and 4-Pyridinecarboxamide in High-Temperature Water☆
2014-07-25JieFuHaomingRenJuZhuXiuyangLu
Jie Fu,Haoming Ren,Ju Zhu,Xiuyang Lu..,*
Catalysis,Kinetics and Reaction Engineering
Hydrolysis Kinetics of 2-Pyridinecarboxamide,3-Pyridinecarboxamide and 4-Pyridinecarboxamide in High-Temperature Water☆
Jie Fu1,Haoming Ren1,Ju Zhu2,Xiuyang Lu..1,*
1Key Laboratory of Biomass Chemical Engineering of Ministry of Education,Department of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China2School of Biological and Chemical Engineering,Zhejiang University of Science and Technology,Hangzhou 310023,China
A R T I C L EI N F O
Article history:
High-temperature water
Hydrolysis
Pyridinecarboxamide
Kinetics
Activation energy
A kinetic study is reported here on hydrolysis of three pyridinecarboxamides in high-temperature water in the temperature range of 190-250°C at 8 MPa.2-Pyridinecarboxamide,3-pyridinecarboxamide and 4-pyridinecarboxamide hydrolyze to corresponding picolinic acids.2-Picolinic acid is further decarboxylated to pyridine.Experiments at different temperatures show that the f i rst-order rate constants display an Arrhenius behavior with activation energies of(110.9±2.3),(70.4±2.1)and(61.4±1.8)kJ·mol−1for 2-pyridinecarboxamide,3-pyridinecarboxamide and 4-pyridinecarboxamide,respectively.These kinetic parameters for pyridinecarboxamide hydrolysis are more reliable and accurate than those from the consecutive hydrolysis of cyanopyridines.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
High-temperaturewater(HTW),liquidwaterabove180°C,isagreen medium for chemical reactions.The properties of HTW are very different from those of ambient water.It has acid-or base-catalytic ability,good solubility for organic compounds,and wide tunability of physical properties[1-7].In recent years,the research on hydrolysis of nitriles in HTWattractsmuchattentionforpotentialapplicationsintreatmentofindustrialwastestreams[8]andproductionofcarboxylicacids[9-11],since HTW provides a green strategy without strong acid or base catalyst. Amide is the intermediate product of nitrile hydrolysis.A better understanding on the hydrolysis of amides in HTW would be important to useHTWforwastecleanupandcarboxylicacidproduction.Moreover,hydrolysis of amides in HTW may occur in the hydrothermal processing of proteincontainingbiomass[12].Therefore,thestudyonhydrolysisofamides could be useful for transformation of biomass to chemicals or fuels.
There is a considerable amount of literature on the hydrolysis of amide in HTW,but concentrating on aliphatic and aromatic amides[8, 12-23].ThehydrolysisofheterocyclicamidesinHTWisrarelyreported. In our previous work[24],the hydrolysis kinetics of 2-cyanopyridine, 3-cyanopyridine and 4-cyanopyridine in HTW were studied.The rate constants for the hydrolysis of 2-cyanopyridine,3-cyanopyridine, 4-cyanopyridine,2-pyridinecarboxamide,3-pyridinecarboxamide and 4-pyridinecarboxamide were obtained at each temperature by the equations of f i rst-order consecutive hydrolysis,and their activation energies were obtained by the Arrhenius equation.A model was established for the consecutive hydrolysis of the three cyanopyridines. However,thepredictionforhydrolysisofthethreepyridinecarboxamides with the model does not f i t the experimental data well,indicating that the kinetic parameters for hydrolysis of the three pyridinecarboxamides arenotsatisfactory.Thepyridinecarboxamidesareintermediateproducts in the consecutive reactions[24],and their measurement and data correlation may be diff i cult.More reliable kinetic work is needed for the hydrolysis of pyridinecarboxamides.
In this study,the hydrolysis kinetics of 2-pyridinecarboxamide, 3-pyridinecarboxamide,and 4-pyridinecarboxamide in HTW are investigated.Fig.1 shows the reaction pathways for hydrolysis of the three pyridinecarboxamides(2-picolinic acid can be decarboxylated subsequently).Withthekineticparameters,amodelisestablishedforthehydrolysis of these three pyridinecarboxamides.Moreover,the effects of substituent position and heterocyclic N on hydrolysis of heterocyclic amides in HTW are provided.

Fig.1.Reaction pathway for hydrolysis of pyridinecarboxamides.
2.Experimental
4-Pyridinecarboxamide(98%)was obtained from ACROS Organics,Belgium.3-Pyridinecarboxamide(99.5%)and 3-picolinic acid(99.5%)were obtained from Chengdu Kelong Chemical Co. Ltd.,China.2-Pyridinecarboxamide(99%)was obtained from TCI Chemical Co.Ltd.,China.4-Picolinic acid(99%)was obtained from Sinopharm Chemical Reagent Co.Ltd.,China.2-Picolinic acid(99%)was obtained from J&K Chemical Ltd.,China.They are all of analytical reagent gradeandwereusedasreceived.Deionizedwaterwaspreparedinhouse.
The reactor,experimental procedure,sampling,and analysis were described in our previous work[24].
Product yield was calculated as the moles of product divided by initial moles of pyridinecarboxamide loaded in the reactor.The mole balance was calculated as the total moles of products and pyridinecarboxamide in the reactor divided by the initial moles of pyridinecarboxamide.
3.Results and Discussion
3.1.Hydrolysis of 2-pyridinecarboxamide
Fig.2 shows the hydrolysis of 2-pyridinecarboxamide at 220°C, 8 MPa,and an initial 2-pyridinecarboxamide concentration of 0.5 g·L−1. 2-Pyridinecarboxamide decreased with time,while 2-picolinic acid and pyridine increased.The yield of 2-picolinic acid did not increase after 150minbuttheyieldofpyridineincreasedfurther,signalingthereaction pathway of 2-pyridinecarboxamide→2-picolinic acid→pyridine.The total moles in the reaction system were around 100%,indicating that 2-picolinic acid and pyridine are the only products.In the hydrolysis of 3-pyridinecarboxamide and 4-pyridinecarboxamide under the same conditions,pyridine was not found in the reaction systems.The total moles of these two reaction systems were also around 100%,which indicates that no by-product is yielded.
3.2.Effect of temperature on hydrolysis of 2-pyridinecarboxamide
Fig.3 shows the effect of temperature on hydrolysis of 2-pyridinecarboxamideataninitial2-pyridinecarboxamide concentration of 0.5 g·L−1and reaction pressure of 8 MPa.Pyridine is a product and its yield increases with time,so decarboxylation occurs in the system.The yield of 2-picolinic acid shows a characteristic of intermediate product,with the maximum value of 8%,6%and 4%at 230,240 and 250°C,respectively.Curves in Fig.3(a)correspond to the f i rst-order reaction model.

Fig.2.Hydrolysis of 2-pyridinecarboxamide at 220°C.
3.3.Effect of temperature on hydrolysis of 3-pyridinecarboxamide
Fig.4 shows the effect of temperature on hydrolysis of 3-pyridinecarboxamide at an initial 3-pyridinecarboxamide concentration of 0.5 g·L−1and reaction pressure of 8 MPa.3-Picolinic acid is the only product.Curves in Fig.4(a)correspond to the f i rst-order kinetic model.
3.4.Effect of temperature on hydrolysis of 4-pyridinecarboxamide
Fig.5 shows the effect of temperature on hydrolysis of 4-pyridinecarboxamide at an initial 4-pyridinecarboxamide concentration of 0.5 g·L−1and reaction pressure of 8 MPa.4-Picolinic acid is the only product.Curves in Fig.5(a)correspond to the f i rst-order kinetic model.
3.5.Kinetics of hydrolysis
It has been shown that hydrolysis of amides follows f i rst-order kinetics[8,12].Thus we f i t the data with ln(1−conversion)vs.time at each temperature using Trendline of Excel.The slope of the straight line represents the rate constant of hydrolysis at a certain temperature. Table 1 presents the results.The uncertainty is obtained by the LINEST function of Excel and expressed as standard deviations.The curves in Figs.3-5 show that the f i rst-order kinetics f i t the experimental data well.The variation of rate constants with temperature follows the Arrhenius equation.The Arrhenius parameters determined by linear regression of lnk vs.1/T are shown in Table 1.The kinetic data for hydrolysis of 2-pyridinecarboxamide,3-pyridinecarboxamide,and 4-pyridinecarboxamidefromtheconsecutivehydrolysisof2-cyanopyridine,3-cyanopyridine and 4-cyanopyridine[24]are also presented in Table 1,in which the rate constants of pyridinecarboxamides were obtained by f i tting the conversion of cyanopyridine,yield of pyridinecarboxamide,picolinic acid and even pyridine with the equations of f i rst-order consecutive reaction,and the errors in experiments and f i tting were accumulated.Although the kinetic parameters for hydrolysis of cyanopyridines and pyridinecarboxamides can be obtained simultaneously[24],the result is unsatisfactory.In this work, pyridinecarboxamides are used as reactants and the rate constants are obtained by directly f i tting the conversion of pyridinecarboxamides, so that experimental data and f i tting results for hydrolysis of pyridinecarboxamides are more reliable.
3.6.Effect of heterocyclic N and substituent position on hydrolysis
At 300°C and 8.7 MPa pressure,the rate constant of benzamide obtained is 0.0115 min−1[8].Based on our kinetic parameters,the rate constants at 300°C for 2-pyridinecarboxamide,3-pyridinecarboxamide, and 4-pyridinecarboxamide are 0.205,0.0162,and 0.0199 min−1, respectively.They are all greater than the rate constant of benzamide, and the rate constant of 2-pyridinecarboxamide is 18 times that of benzamide.It indicates that the heterocyclic N atom can lower the hydrothermal stability of benzamide,especially for ortho-pyridinecarboxamide. The activation energy for hydrolysis of 2-pyridinecarboxamide is much larger than those of 3-pyridinecarboxamide and 4-pyridinecarboxamide.The effect of substituent position on activation energy for pyridinecarboxamide hydrolysis is ortho>meta>para. Different from 3-pyridinecarboxamide and 4-pyridinecarboxamide, the hydrolysis of 2-pyridinecarboxamide yields a large amount of pyridine,which indicates that both ortho-pyridinecarboxamide and ortho-picolinic acid have lower hydrothermal stability.It is probably caused by the short distance between the substituents and heterocyclic Natom.Overall,ortho-pyridinecarboxamideandpicolinicacidaremore active than meta-and para-position pyridinecarboxamides and picolinic acids.
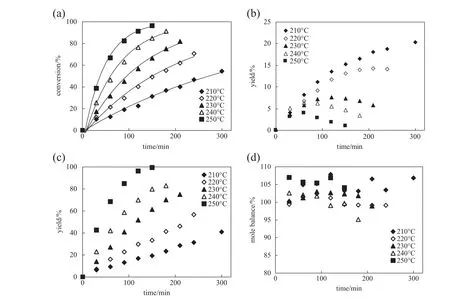
Fig.3.Conversion of 2-pyridinecarboxamide(a)yield of 2-picolinic acid(b)yield of pyridine(c)and mole balance(d)in hydrolysis of 2-pyridinecarboxamide at different temperatures.
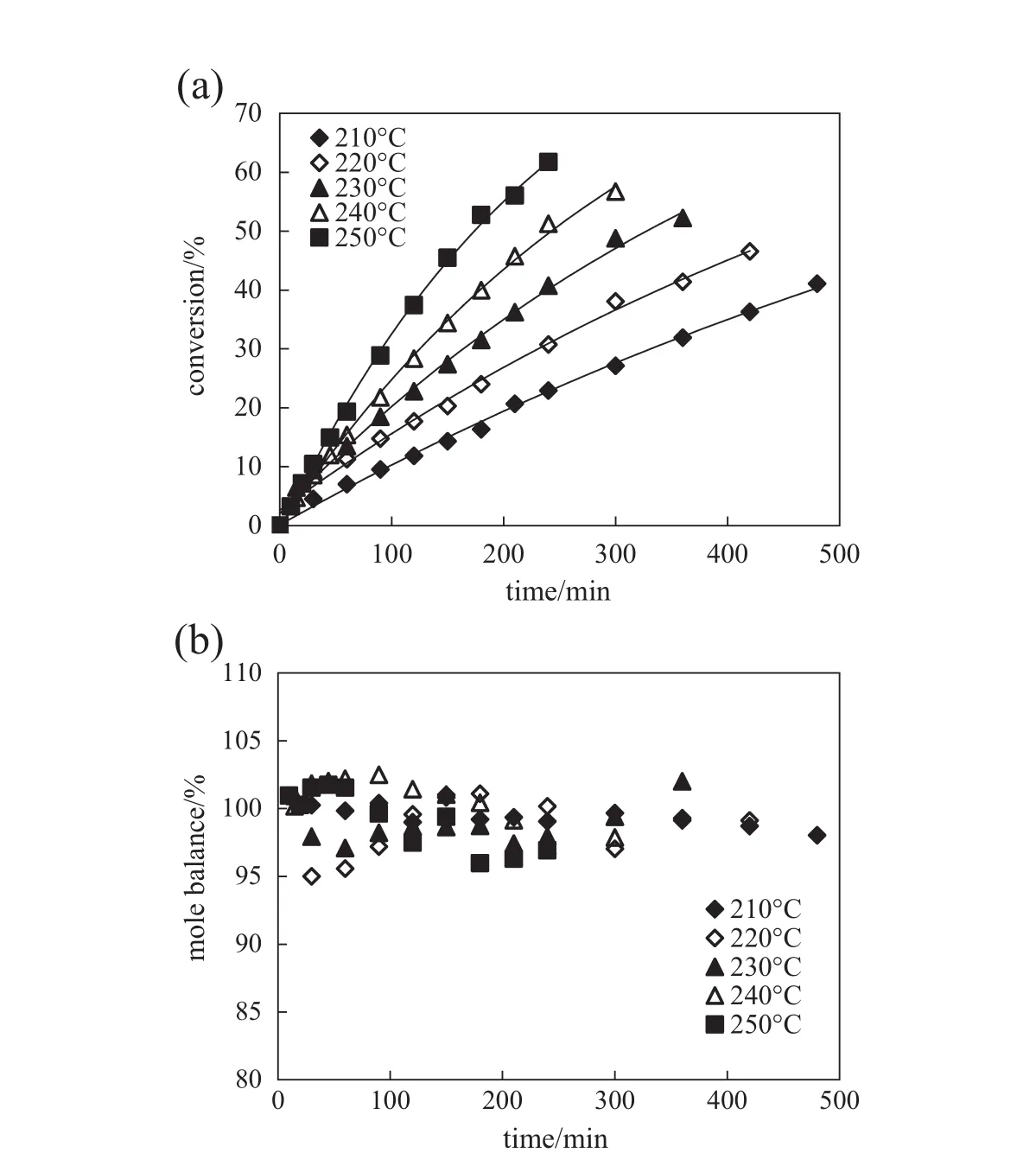
Fig.4.Conversion of 3-pyridinecarboxamide(a)and mole balance(b)in hydrolysis of 3-pyridinecarboxamide at different temperatures.

Fig.5.Conversion of 4-pyridinecarboxamide(a)and mole balance(b)in hydrolysis of 4-pyridinecarboxamide at different temperatures.

Table 1Rate constants and activation energies for hydrolysis of pyridinecarboxamides(this work)and the consecutive hydrolysis of cyanopyridines[24]
4.Conclusions
2-Pyridinecarboxamide,3-pyridinecarboxamideand4-pyridinecarboxamide hydrolyze in hours in high-temperature water in the temperature range of 190-250°C at 8 MPa.For 3-pyridinecarboxamide and 4-pyridinecarboxamide,corresponding products are picolinic acids.2-Pyridinecarboxamide yields 2-picolinic acid and pyridine as products.The activation energies determined are(110.9±2.3),(70.4±2.1)and(61.4±1.80)kJ·mol−1for hydrolysis of 2-pyridinecarboxamide,3-pyridinecarboxamide and 4-pyridinecarboxamide,respectively.Compared to the rate constants and activation energies for pyridinecarboxamide hydrolysis obtained from the consecutive hydrolysis of cyanopyridines,those obtained directly from the hydrolysis of pyridinecarboxamides show smaller standard deviations and higher accuracies.
[1]N.Akiya,P.E.Savage,Roles of water for chemical reactions in high-temperature water,Chem.Rev.102(2002)2725-2750.
[2]M.Watanabe,T.Sato,H.Inomata,R.L.Smith,K.Arai,A.Kruse,E.Dinjus,Chemical reactions of C1 compounds in near-critical and supercritical water,Chem.Rev.104 (2004)5803-5822.
[3]H.Weingärtner,E.U.Franck,Supercritical water as a solvent,Angew.Chem.Int.44 (2005)2672-2692.
[4]A.Kruse,E.Dinjus,Hot compressed water as reaction medium and reactant properties and synthesis reactions,J.Supercrit.Fluids 39(2007)362-380.
[5]P.E.Savage,Organic chemical reactions in supercritical water,Chem.Rev.99(1999) 603-622.
[6]G.Brunner,Near criticaland supercritical water.PartI.Hydrolyticand hydrothermal processes,J.Supercrit.Fluids 47(2009)373-381.
[7]J.Fu,P.E.Savage,X.Lu,Hydrothermal decarboxylation of pentaf l uorobenzoic acid and quinolinic acid,Ind.Eng.Chem.Res.48(2009)10467-10471.
[8]B.Izzo,C.L.Harrell,M.T.Klein,Nitrile reaction in high-temperature water:kinetics and mechanism,AICHE J.43(1997)2048-2058.
[9]V.K.Krieble,C.I.Noll,The hydrolysisof nitriles with acids,J.Am.Chem.Soc.61(1939) 560-563.
[10]G.H.Wiegand,M.Tremelling,Kinetics and mechanism of the decomposition of potassium cyanide in aqueous alkaline medium.Hydrolysis of the simplest nitrile, hydrogen cyanide,J.Org.Chem.37(1972)914-916.
[11]J.H.Hall,M.Gisler,A simple method for converting nitriles to amides.Hydrolysis with potassium hydroxide in tert-butyl alcohol,J.Org.Chem.41(1976)3769-3770.
[12]P.Duan,L.Dai,P.E.Savage,Kinetics and mechanism of N-substituted amide hydrolysis in high-temperature water,J.Supercrit.Fluids 51(2010)362-368.
[13]A.Krämer,S.Mittelstädt,H.Vogel,Hydrolysis of nitriles insupercritical water,Chem. Eng.Technol.22(1999)494-500.
[14]B.Izzo,M.T.Klein,C.LaMarca,N.C.Scrivner,Hydrothermal reaction of saturated and unsaturated nitriles:reactivity and reaction pathway analysis,Ind.Eng.Chem.Res.38 (1999)1183-1191.
[15]D.S.Lee,E.F.Gloyna,Hydrolysis and oxidation of acetamide in supercritical water, Environ.Sci.Technol.26(1992)1587-1593.
[16]M.Faisal,N.Sato,A.T.Quitain,H.Daimon,K.Fujie,Hydrolysis and cyclodehydration of dipeptide under hydrothermal conditions,Ind.Eng.Chem.Res.44(2005)5472-5477.
[17]E.Venardou,E.Garcia-Verdugo,S.J.Barlow,Y.E.Gorbaty,M.Poliakoff,On-line monitoring of the hydrolysis of acetonitrile in near-critical water using Raman spectroscopy,Vib.Spectrosc.35(2004)103-109.
[18]C.L.Harrell,J.S.Moscariello,M.T.Klein,The absence of wall effects during benzonitrile hydrolysis,J.Supercrit.Fluids 14(1999)219-224.
[19]P.Duan,Y.Wang,Y.Yang,L.Dai,Optimization of adiponitrile hydrolysis in subcritical water using an orthogonal array design,J.Solut.Chem.38(2009) 241-258.
[20]M.Sarlea,S.Kohl,N.Blickhan,H.Vogel,Valeronitrile hydrolysis in supercritical water,ChemSusChem 3(2010)85-90.
[21]M.Okazaki,T.Funazukuri,Decomposition of acetamide and formamide in pressurized hot water,J.Mater.Sci.41(2006)1517-1521.
[22]P.Duan,S.Li,Y.Yang,Z.Wang,L.Dai,Green medium for the hydrolysis of 5-cyanovaleramide,Chem.Eng.Technol.32(2009)771-777.
[23]P.Duan,S.Li,Z.Wang,L.Dai,Hydrolysis kinetics and mechanism of adipamide in high temperature water,Chem.Eng.Res.Des.88(2010)1067-1072.
[24]J.Fu,H.Ren,C.Shi,X.Lu,Hydrolysis kinetics of 2-cyanopyridine,3-cyanopyridine, and 4-cyanopyridine in high-temperature water,Int.J.Chem.Kinet.44(2012) 641-648.
30 November 2012
☆
Supported by the National Natural Science Foundation of China(21176218),and Zhejiang Key Innovation Team of Green Pharmaceutical Technology(2010R50043).
*Corresponding author.
E-mail address:luxiuyang@zju.edu.cn(X.Lu).
http://dx.doi.org/10.1016/j.cjche.2014.06.024
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 18 March 2013 Accepted 1 April 2013
Available online 30 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
