Phase Transfer Catalysis:Oxidation of 2-Methyl-1-butanol
2014-07-25SankarshanaYadagiriMurthy
T.Sankarshana*,E.Yadagiri,J.S.N.Murthy
Catalysis,Kinetics and Reaction Engineering
Phase Transfer Catalysis:Oxidation of 2-Methyl-1-butanol
T.Sankarshana*,E.Yadagiri,J.S.N.Murthy
University College of Technology,Osmania University,Hyderabad 500007,India
A R T I C L EI N F O
Article history:
Aliquat336
Phase transfer catalysis
Potassium permanganate
2-Methyl-1-butanol
Enhancement factor
In liquid-liquid systems,the substrates in the liquids are inaccessible to each other for the reaction.By adding a small quantity of phase transfer catalyst,the reaction can be made accessible and accelerated.The present study involves the phase transfer catalyzed oxidation of 2-methyl-1-butanol by quaternary ammonium permanganate (tricaprylyl methyl ammonium permanganate).The attempt was to compare the kinetics under homogeneous and heterogeneous conditions.Experiments were conducted in a batch reactor to determine the kinetics under homogeneous conditions.A baff l ed borosilicate agitated reactor was used to f i nd the enhancement factor and the kinetics under heterogeneous conditions.The rate constants determined under both homogeneous and heterogeneous conditions agreed very well.The oxidation was found to be f i rst order with respect to each of the reactants,quaternary ammonium permanganate and the alcohol,resulting in an overall second order rate expression.Aliquat336(tricaprylylmethylammonium chloride)was found to be the best compared with the other catalysts tested(triethylbenzylammonium chloride,tetrabutylammonium bromide,tetrabutylammonium iodide and tetrabutylammonium hydrogen sulfate)and it gave an enhancement factor of 9.8.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Phasetransfercatalysisprovidesmanygoodbenef i tsprimarilyrelatedtoreducingthecostofmanufactureoforganicchemicals.Forthepast four decades,extensive work has been reported using this technique.It accelerates the reaction between the compounds present separately in two immiscible liquid phases.Many reactions like substitution, dichlorocarbene reaction,oxidation,and polymerization involving aqueous-organic systems have been carried out using phase transfer catalysts(PTCs).Oxidation reaction of various organic compounds by extractingpermanganateionintotheorganicphasefromanaqueousreservoir of potassium permanganate has been reported by many investigators using various PTCs like triethylbenzylammonium chloride(TEBAC), tetrabutylammonium bromide(TBAB),cetyltrimethylammonium bromide(CTMAB)and tricaprylylmethylammonium chloride(Aliquat336). Herriott and Picker[1]studied the extractability of permanganate ion from aqueous solution into benzene.Gibson and Hosking[2]reported the phase transfer catalytic oxidation of various organic compounds by extracting permanganate ion from an aqueous reservoir of potassium permanganate.Sam and Simmons[3]found that PTC,dicyclohexyl-18-crown-6,could solubilize solid potassium permanganate in benzene to the extent of about 0.6 mol·L−1.The resulting purple benzene solution was used to oxidize a number of organic substrates in good to excellent yields.Thetwophasepermanganateoxidationofpiperonyltopiperonylic acid was reported by Menger et al.[4].The effect of different solvents on the catalyst performance and structure-activity relationship of catalyst was investigated by carrying out oxidation reactions of benzaldehyde and benzyl cyanide by potassium permanganate using different phase transfer catalysts[5].Sankarshana et al.[6]investigated the kinetics of the oxidation of 2-ethyl-1-hexanol,n-heptanol and n-hexanol under homogeneous conditions.Pentaf l uro-pentanol was converted to pentaf l uro-pentanoic acidusingaqueoussodiumpermanganatesolution employing tetraethylammonium hydrogen sulfate as the PTC by Arshad Mohamood et al.[7].Tang et al.[8]synthesized 2-ethyl-1-hexanoic acid from 2-ethyl-1-hexanol using potassium permanganate in aqueous solution under basic conditions in the presence of quaternary ammonium compounds as PTC.Li demonstrated the enhancement of trichloroethylene degradation by aqueous permanganate solution in the presence of hexadecyl-trimethyl-ammonium bromide and observed a pseudo f i rst order reaction kinetics with respect to potassium permanganate in the presence of free trichloroethylene[9].The kinetics of oxidation ofD-fructose by permanganate was studied in cationic micelles of cetyltrimethyl ammonium bromide by Andrabi et al.[10].Their results indicated a very strong partitioning of the permanganate in favor of the micellar pseudo phase.The oxidation of benzyl alcohol in benzene to benzaldehyde with solid potassium permanganate using the PTC, 18-crown-6,was carried out by Jose et al.[11].They observed that the initial rate increasedwith concentration of thecatalyst and benzyl alcohol,and developed a homogeneous rate equation.Lele et al.[12]investigated the effect of PTC on some fast and very slow reactions and reported enhancement factors of 200 in the case of fast reactions and 7 in the case of slow reactions.Enhancement factor is def i ned as the ratio of rates of reaction with and without PTC.Similarly in the reaction of diphenyl chlorophosphate with sodium phenolate to give triphenylphosphateusingAliquat336,theenhancementfactorwasfoundtobe90 by Kumar and Sharma[13].The enhancement factor of 4 to 9 was obtained by Sankarshana and Rao[14]in the oxidation of higher alcohols employing different types of PTC.
It is evident that the conventional reactions involving heterogeneous phases can be performed easily at accelerated rates and at normal conditions.In the present study,the reaction involves the permanganate oxidation of 2-methyl-1-butanol under homogeneous and heterogeneous conditions.Enhancement factor was determined using different PTCs under heterogeneous conditions. The rate constants obtained under homogeneous and heterogeneous conditions are compared.For this purpose,Aliquat336 was the catalyst employed.The advantage of using Aliquat336 is its insolubility in the aqueous phase.It can be recycled along with the unconverted substrate in the organic phase.Also,the product thus formed can be easily separated from the aqueous phase by acidif i cation.
2.Experimental
2.1.Kinetics under homogeneous conditions
The experimental set-up consisted of a 0.25×10−3m3cylindrical borosilicate glass reactor.The reactor was provided with a stirrer and was placed in a temperature bath.The oxidation reaction of 2-methyl-1-butanol(RCH2OH)with tricaprylyl methylammonium permanganate (QMnO4)was carried out in the reactor and the reaction is represented as follows.

For each homogeneous kinetic run a solution of RCH2OH of known concentration in benzene was prepared.QMnO4solution was prepared bydissolvingthePTC,tricaprylylmethylammoniumchloride(Aliquat336, quaternary ammonium chloride,QCl).QCl of known quantity in benzene was brought into contact with potassium permanganate(KMnO4)solution and thoroughly mixed.The benzene solution containing QCl became purpleindicatingtheformationofQMnO4accordingtothereactiongiven below:
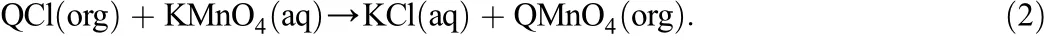
QCl is insoluble in the aqueous phase.Hence,the reaction occurs at theinterface.Purple benzenei.e.quaternary ammoniumpermanganate (QMnO4)solution was separated and the concentration was determined by measuring its absorbance in a UV-spectrophotometer (Shimadzu 160,Japan).The absorbance values were converted to the concentration(kmol·m−3)from the calibration curve.The calibration was made as follows.As mentioned above purple benzene(QMnO4) was prepared by taking a known quantity of QCl in benzene and excess of KMnO4in aqueous phase.A sample solution of theQMnO4was diluted with benzene and made suitable for measurement of absorbance in the UV-spectrophotometer.
For a particular reaction QMnO4solution was adjusted to the required concentration by adding pure benzene.0.075×10−3m3of this solution was mixed with 0.075×10−3m3of known concentration of RCH2OH in benzene at the same temperature.At this point the reaction commenced as per Reaction(1).Samples were taken from the reactor at different time intervals as the reaction proceeded,to determine the fall in concentration of QMnO4by measuring the absorbance.
In all the experiments,the concentration,of RCH2OH was maintained in large excess,so that its change during the reaction is insignif i cant resulting in a pseudo order situation.MnO2in the reaction can be considered as negligible.The conditions for these homogeneous kinetic runs were as follows.Concentration of QMnO4,CQ0= 1.13 to 9.03×10−4kmol·m−3.Concentration of RCH2OH,CA0was varied between 0.093 and 0.278 kmol·m−3.Temperature,T,was set at 303.15 K.To study the temperature effect,runs were made in the range of 297.15 to 323.15 K,keeping CA0and CQ0constant at 0.278 and 3.39×10−4kmol·m−3respectively.
2.2.Kinetics under heterogeneous conditions and enhancement factor
The setup consisted of a glass reactor of 0.08 m diameter,0.1 m height and 0.5×10−3m3capacity with four baff l es and a stirrer for mixing.The speed of the stirrer was controlled by means of a regulator. The reactor was kept in a temperature bath.
2.3.Comparison of enhancement factor
To determine the enhancement factor,runs were made with and without phase transfer catalyst under otherwise similar conditions. Aliquat336,TEBAC,TBAB,tetra butyl ammonium iodide(TBAI)and tetra butyl ammonium hydrogen sulfate(TBAHS)were the 5 catalysts used for the oxidation of RCH2OH.For the experiments with no PTC, 0.05×10−3m3of benzene solution with 0.925 kmol·m−3concentrationofRCH2OHwasstirredwith0.1×10−3m3ofaqueoussolutioncontaining 3.164×10−2kmol·m−3of KMnO4.The temperature and the speed ofagitation were308.15K and450 r·min−1respectively.Sample was collected from the aqueous phase to determine the concentration of KMnO4.Similarly,the runs were made using f i ve different PTCs.In these runs the initial concentration of each of the PTC was maintained at 2.26×10−3kmol·m−3and the sample was taken at the intervals of 1800 s.
2.4.Heterogeneous kinetics
The kinetics of the phase transfer catalytic oxidation of RCH2OH by permanganate ion in heterogeneous system consisting of organic and aqueous phases was determined using Aliquat336 as the PTC.This was found to be the best among the catalysts studied.The rate constant khtwas determined by varying(a)the concentration of Aliquat336,CQCl(b)theconcentrationofRCH2OH,CA,and(c)thetemperature,T.Known weightsofAliquat336andRCH2OH weredissolvedin 0.05×10−3m3of benzene solution.This solution was mixed with 0.1×10−3m3aqueous phase containing known concentration of KMnO4,CK,and stirred.At a particular time,agitation was stopped and the contents in the reactor were allowed to separate into two layers.Then,a sample was withdrawnfromtheaqueouslayertodetermineCK.Thecombinedoperation took a total time of about 45 to 50 s.During this time any reaction may be neglected.After the sample was taken,the experiment was continued by restarting the agitation.
The conditions at which these runs were made are as follows.CK0was 3.164×10−2kmol·m−3in aqueous phase.CQCl0was changed from 2.71 to 6.78×10−3kmol·m−3.CA0was varied from 0.74 to 1.30 kmol·m−3.All the experiments were carried out at 303.15 K.To study the temperature effect on the reaction,few runs were made from 302.15 to 323.15 K.
3.Results and Discussion
3.1.Kinetics under homogeneous conditions
The rate equation for the disappearance of QMnO4in Reaction(1)is represented as
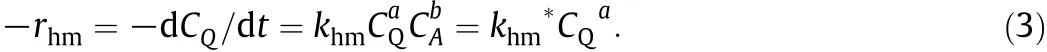
Since the initial CAin Eq.(3)was large,it can be essentially consideredto remain atitsinitialconcentration value of CA0,duringthecourse of the reaction i.e.CA0=CA.Hence the product khmCA0b,can beconsidered constant and equal to khm*.Assuming the reaction to be f i rst order with respect to QMnO4,and upon integration,it gives
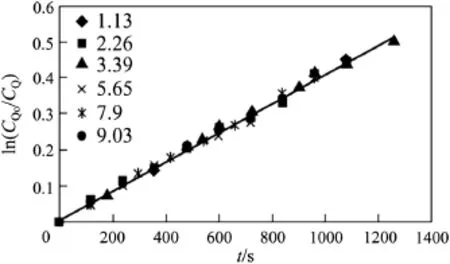
Fig.1.Effect of QMnO4concentration at CA0=0.278 kmol·m−3,T=303.15 K.

Fig.2.Effect of alcohol concentration at CQ0=3.39×10−4kmol·m−3,T=303.15 K.
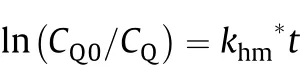
where CQ0is the initial concentration of QMO4at time t=0.If the experimental data when plotted as ln(CQ0/CQ)vs.t,and if it results in a straight line with a positive slope passing through the origin,the assumption that the oxidation of RCH2OH is f i rst order with respect to CQis true,and the slope is khm*,a pseudo f i rst order rate constant.
Figs.1 and2 give theresults for variousinitialCQandCA.Inthese f i gures the plot ln(CQ0/CQ)vs.t followed a straight line,indicating that the order ofreaction a is equal to 1.In Fig.1 itcan beseen that all thepoints lie on a single line for different concentrations of CQCl.Values of khm* were obtained from these data and the corresponding values of CA0in a log-log plot,if linear will result in the slope equal to b,since khm*=khmCA0b.The data f i t a straight line with a slope of 0.96.This indicates that the reaction is also f i rst order with respect to CAand hence,the overall order of oxidation reaction of RCH2OH is two. The calculated values of khmare given in Table 1.The average value of khmis calculated to be 1.5×10−3m3·kmol−1·s−1and the maximum deviation of individual values from the average value is found to be±3.2%.For the experiments conducted,the accuracy and the reproducibility of the data were considered good.The correlation coeff i cient was above 0.992 at 95%conf i dence level.
Fig.3 gives the effect of temperature on the rate constant khm.The corresponding values of khmare given in Table 1.The plot of ln khmagainst temperature was observed to follow the Arrhenius expression as shown in Fig.4.The frequency factor,k0,and the activation energy, E,obtained are 9.83×107m3·kmol−1·s−1and 62.76 kJ respectively.
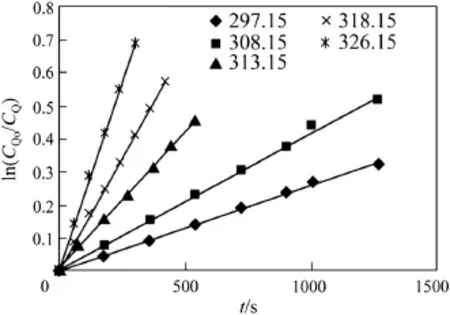
Fig.3.Effect of different temperatures,CAO=0.278 kmol·m−3,CQO=3.39× 10−4kmol·m−3.
3.2.Kinetics under heterogeneous conditions and enhancement factor
Effect of stirrer speed was made by taking 0.1×10−3m3volume of KMnO4solution with a concentration of 3.164×10−2kmol·m−3and 0.05×10−3m3of benzene solution containing 0.93 kmol·m−3of RCH2OH and 2.26×10−3kmol·m−3of Aliquat336 in the reactor and stirred.The temperature was 308.15 K.The duration of stirring was 1800 s.At different speeds of 100,200,300,400,450,550 and 800 r·min−1,it was observed that the conversion was constant beyond 400 r·min−1indicating that the mass transfer effects are negligible.CQin the organic phase was found to be equal to the initial CQCl(=2.26×10−3kmol·m−3)within less than 250 s.For speeds of 100 to 300 r·min−1the concentration of QMnO4in the organic phase also reached a constant value after longer times.This constant value was also equal to the initial CQCl.
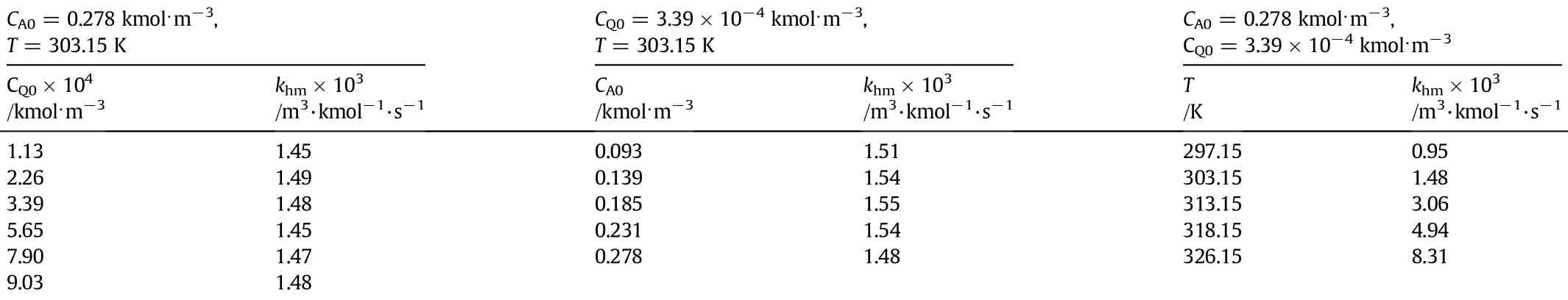
Table 1Reaction rate constants under homogeneous conditions
3.2.1.Enhancement factor
DifferenttypesofcatalystsusedwereTBAB,TEBAC,Aliquat336,TBAI and TBAHS.The mode of anion transfer between these catalysts and KMnO4dependsuponthecatalystsolubilityineitherphase.ThecatalystAliquat336 is insoluble in the aqueous phase and exchanges its anion at the interface to form quaternary ammonium permanganate, QMnO4.This is represented in Reaction(1).TBAB and TBAI and its corresponding permanganate salts are soluble in both organic and aqueous phase and hence the permanganate salt divides itself into these two phases depending on the distribution coeff i cient.The anion exchange occurs in the bulk of the aqueous phase.TEBAC and TBAHS are insoluble in benzene but these are soluble in water and in RCH2OH.Hence,these catalysts distribute into aqueous phase and organic phase to the extent of their solubility in the RCH2OH present in the benzene phase.
Experiments with the following conditions were performed.Vaq= 0.1×10−3m−3,Vorg=0.05×10−3m−3,CK0=3.164×10−2kmol·m−3, CA0=0.925 kmol·m−3,T=307.15 K,speed=450 r·min−1,t= 1800 s but the concentration of the all the catalysts was maintained at 2.26×10−3kmol·m−3.The average rate of reaction,−rhtcalculated without the catalyst was 0.68×10−5kmol·m−3·s−1.Enhancement factors were calculated with−rhtvalues obtained using different catalysts and are given in Table 2.The PTC,Aliquat336 gave the highest enhancement factor of 9.84.The effectiveness of these catalysts is more or less consistent with the observations made by Herriott and Picker[15].

Fig.4.Effect of temperature at CA0=0.278 kmol·m−3,CQ0=3.39×10−4kmol·m−3.
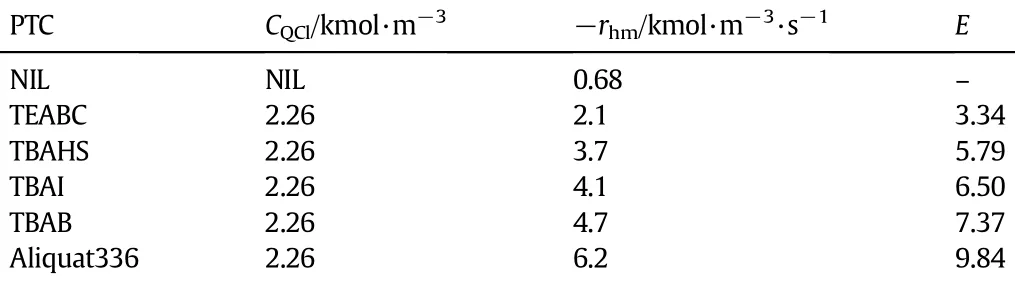
Table 2Enhancement factor with different PTCs
3.2.2.Heterogeneous kinetics
Since Aliquat336 was more effective than other PTCs,it was employed in the study of kinetics under heterogeneous conditions. The oxidation reaction occurs as follows.

The MnO4−which is transferred into the organic phase was used up in the reaction.Phase transfer of this ion from aqueous phase occurs through Q+and this process is continuous and instantaneous.At different time intervals the aqueous phase was analyzed for the depletion of KMnO4.This depletion of MnO4−in the aqueous phase during a particular time period will be equal to the amount of MnO4−that was phase transferred into the organic phase for the oxidation reaction of RCH2OH.From this data and considering the stoichiometry of Reaction(5),the rate of disappearance of QMnO4,−rhtcan be expressed as

The order of reaction is one with respect to each of the concentrationsof QMnO4,CQand RCH2OH,CA.Itis observed that CQin the organic phasereachesthesaturatedconcentrationvalue,whichistheinitialcatalyst concentration,CQCland remains at this value as long as suf fi cient KMnO4was present in the aqueous phase[14].Hence,CQin Eq.(7) can be considered to be constant and equal to CQ0.The value of CAis very large and its depletion will be negligible during the reaction,and can be considered to be equal to CA0.
Sincekhtisalsoaconstant,therighthandsideoftheaboveexpression will be constant.This suggests that the rate is independent of CQor CA, and based on this the average rate of reaction rhtand the corresponding khtare calculated for different CQCl,CA0and T are given in Table 3.It can be seen that−rhtis almost same for different reaction times,for a particular catalyst concentration,but increased linearly with CQCl.In the temperature range of 302 to 323 K,the calculated values of khtwere 1.5 to 6.98×10−3m3·kmol−1·s−1and the plot of lg khtagainst 1/T followed Arrhenius relation.The values of khmobtained under homogeneous conditions are in good agreement with khtunder heterogeneous conditions. Itistobenotedthatthesequenceofreactionstepsaredifferentunderhomogeneous and heterogeneous conditions.Aliquat336 is a cheap and effi cient PTC and owing to its insolubility in the aqueous phase,and not a hindranceintheproductrecovery,whichwillbeintheformofpotassium salt of the acid in the aqueous phase.
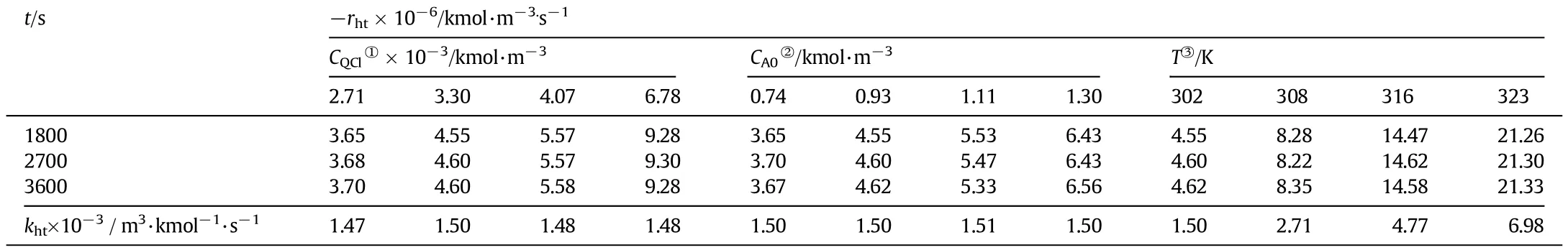
Table 3Effect of CQCl,CA0and T in heterogeneous conditions
4.Conclusions
(1)The overall reaction of oxidation by quaternary ammonium permanganate of 2-methyl-1-butanol using the phase transfer catalyst,Aliquat336 was found to be second order,while f i rst order with respect to either the concentration of quaternary ammonium permanganate or 2-methyl-1-butanol.
(2)The performance of different catalysts is found to be consistent with the observations made by Herriott and Picker.
(3)Kinetic constants determined with both homogeneous and heterogeneous conditions agreed well with each other,though the reaction steps are different in each case.
(4)Aliquat336wasfound tobethebestamongthef i vedifferentcatalysts studied and gave the highest enhancement factor.
[1]A.W.Heriott,D.Picker,Purple benzene:permanganate oxidations using quaternary ammonium ions,Tetrahedron Lett.15(1974)1511-1514.
[2]N.A.Gibson,J.W.Hosking,Permanganate oxidations in non-aqueous solvents:I.Oxidation by triphenylmethylarsonium permanganate,Aust.J.Chem.18(1965) 123-125.
[3]D.J.Sam,H.E.Simmons,Crown polyether chemistry—potassium permanganate oxidations in benzene,J.Am.Chem.Soc.94(1972)4024-4025.
[4]F.M.Menger,J.U.Rhee,H.K.Rhee,Applications of surfactants to synthetic organic chemistry,J.Org.Chem.40(1975)3803-3805.
[5]K.Hanumanth Rao,M.Bhagvanth Rao,Studies in phase transfer catalysis,Part-I. Permanganate oxidation,J.Indian Chem.Soc.68(1991)132.
[6]T.Sankarshana,M.Bhagvanth Rao,P.S.Ramachandran,Kinetics of oxidation of alcohols:phase transfer catalysis,J.Indian Chem.Soc.79(2002)668-670.
[7]A.Mahmood,G.E.Robinson,L.Powell,Improved oxidation of an alcohol using aqueous pemanganate and PTC,Org.Process Res.Dev.3(1999)363-364.
[8]R.R.Tang,X.G.Yang,Q.Yang,B.C.Cheng,Synthesis of 2-ethylhexanoic acid by oxidation based on PTC,Huaxue Fanying Gongcheng Yu Gougyi,14(1998)153-157 (in Chinese).
[9]Z.Li,Surfactant enhanced oxidation of trichloroethylene by permanganate—proof of concept,Chemosphere,54(2004)419-423.
[10]S.M.Z.Andrabi,M.A.Malik,Z.Khan,Permanganate partitioning in cationic micelles of cetyltrimethylammonium bromide:a kinetic study ofD-fructose oxidation, Colloids Surf.,A 299(2007)58-64.
[11]N.Jose,S.Sengupta,J.K.Basu,Selective production of benzaldehyde by permanganate oxidation of benzyl alcohol using 18-crown-6 as phase transfer catalyst,J. Mol.Catal.309(2009)153-158.
[12]S.Lele,R.R.Bhave,M.M.Sharma,Fast and very slow reactions:phase transfer catalysis,Chem.Eng.Sci.38(1983)765-773.
[13]V.K.K.Kumar,M.M.Sharma,Kinetics of formation of triphenyl phosphate:phase transfer catalysis in a liquid-liquid system,Ind.Eng.Chem.Process.Des.Dev.24 (1985)1293-1297.
[14]T.Sankarshana,M.B.Rao,Potassium permanganate oxidation-PTC technique,IE(I)J CH,83(2003)51-54.
[15]A.W.Heriott,D.Picker,Phase transfer catalysis.Evaluation of catalysis,J.Am.Chem. Soc.97(1975)2345-2349.
29 November 2012
*
.
E-mail address:tsankarshana@yahoo.com(T.Sankarshana).
http://dx.doi.org/10.1016/j.cjche.2014.06.023
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 16 September 2013
Accepted 1 October 2013
Available online 3 July 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
