Porous Spherical Cellulose Carrier Modif i ed with Polyethyleneimine and Its Adsorption for Cr(III)and Fe(III)from Aqueous Solutions☆
2014-07-25ZhijianHeHangSongYannanCuiWeixiaZhuKaifengDuShunYao
Zhijian He,Hang Song,Yannan Cui,Weixia Zhu,Kaifeng Du,Shun Yao,*
Separation Science and Engineering
Porous Spherical Cellulose Carrier Modif i ed with Polyethyleneimine and Its Adsorption for Cr(III)and Fe(III)from Aqueous Solutions☆
Zhijian He1,2,Hang Song2,Yannan Cui2,Weixia Zhu2,Kaifeng Du2,Shun Yao2,*
1School of Chemistry and Chemical Engineering,Mianyang Normal University,Mianyang 621000,China2Department of Pharmaceutical and Biological Engineering,Sichuan University,Chengdu 610065,China
A R T I C L EI N F O
Article history:
Polyethyleneimine
Cellulose particle
Adsorption
Cr(III)
Fe(III)
An eff i cient porous spherical polyethyleneimine-cellulose(PEI-cell)absorbent was synthesized and characterized.The main inf l uencing factors and adsorption mechanism for two typical metal ions,Cr3+and Fe3+,were investigated.The adsorption performance primarily depends on the initial concentration of metal ions,pH value and temperature,and the chelation action between N atoms of PEI-cell and metal ions plays an important role.Under dynamic adsorption conditions,the saturation adsorption of polyethyleneimine-cellulose is 83.98 mg·g−1for Cr(III)and 377.19 mg·g−1for Fe(III),higher than reported data and that of unmodif i ed cellulose.The adsorption can be well described with second-order kinetic equation and Freundlich adsorption model,and ΔH,ΔG and ΔS of the adsorption are all negative.With 5% HCl as eluent,the elution ratio of Cr(III)and Fe(III)achieved 99.88%and 97.74%at 313 K,respectively. After the porous PEI-cell was reused 6 times,it still presented satisfactory adsorption performance. Above results show the advantages such as easily-acquired raw material,high eff i ciency,stable recycling performance and biodegradability.
©2014TheChemicalIndustryandEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Many industries,such as pharmacy,battery,rubbers,leather tanning,dye,paper,coating,car,aeronautic and steel generate large quantities of wastewater containingchromiumand iron[1,2].The two metal ions are readily infused in underground water and contaminate drinkingwaterbecausetheyarehighlywater-solubleanddiffusible.Effective removal of heavy metals in wastewater is of great importance.Various methods have been developed and employed,such as adsorption,neutralization,ion exchange,precipitation and biological purif i cation[3-7]. Amongthese methods,adsorption is oneof the most popular and effective techniques,which offers great f l exibility in design,operation,and regeneration in many situations.Some adsorbents reported for Cr(III) and Fe(III)include lignin[8],biochar and sugarcane pulp residue[9], bread mold fungus[10],activated carbon[11],sugarcane bagasse modif i ed with sodium hydroxide and citric acid[12],zeolite synthesized fromf l yash[13],thioureacross-linkedchitosan[14],unmodif i edraphia palm fruit endocarp[15],hazelnut hull[16],chitosan-tripolyphosphate beads[17],imprinted polymer[18],polyacrylamide grafted activated carbon[19],and so on.
As abundant renewable and biodegradable adsorbent materials,cellulose and modif i ed cellulose are widely used in wastewater treatment, chemical industry,and medication adsorption[20,21].Cellulose can interactwithadsorbatesthroughhydrogenbond,complexationandother interactions.Different porous cellulose adsorbents with high performance can be prepared by grafting functional polymers on the surface, which are effective to remove heavy metals[22].The solid adsorption materials can well combine the functionality of polymers and excellent properties of porous cellulose,such as low cost,high specif i c area,environmentally friendly,strong mechanical property,and good chemical and thermal stability.
Polyethyleneimine(PEI)is a water-soluble polyamine and its aqueoussolutionisalkalinewithalotofaminogroupsonitsmacromolecular chains.Commercial PEI is a branched macromolecule and the ratio of primary,secondary and ternary amine groups is approximately 1:2:1 [23],which is well known for its metal chelation potentiality.In this study,PEI is grafted onto the surface of porous cellulose particles to obtainasolidadsorbentPEI-cell,whichisusedtoremoveCr(III)andFe(III) ions from their aqueous solutions.Compared with some adsorbents reported[24-26],the raw material is relatively more available and cheap,and the two-step synthesis process is simple.The PEI-cell adsorbent has an intense adsorption capability for Cr(III)and Fe(III)due to the strong chelation between nitrogen atoms and metal ions.This is apromising method to graft functional polymer on porous cellulose to prepare eff i cient adsorbents for the removal of heavy metal ions from industrial wastewater.
2.Experimental
2.1.Materials
Medical degrease cotton(100%cotton f i ber)was provided by Hualu Hygienic Material Co.Ltd.,Shandong,China.PEI(99%purity)was provided by Hangjia Biological Medicine Co.Ltd.,Sichuan,China.1,5-Diphenyl carbazide and phenanthroline(purity>98%)was purchased from Chengdu Kelong Reagent Co.Ltd.,Sichuan,China.Other reagents were all of analytical grade.
2.2.Two-step synthesis of porous polyethyleneimine-cellulose particle
(1)The cotton was infused with 5 mol·L−1NaOH aqueous solution for 2 h and aged at 25°C for 2 days.1.5 mol·L−1NaOH was added to get liquid glue after sulfonation with CS2.The brilliant yellow solution and nano-CaCO3were stirred eff i ciently with the shaker.Then the mixture was transferred to a large round bottom f l ask containing transformer oil and stirred persistently at 90°C for 2.5 h.After the mixture was cooled to room temperature,the porous cellulose particles were rinsed with ultrapure water several times and external nano-CaCO3was removed with HCl.The porous cellulose particlesweresievedwithsizerangefrom100to 200 mesh.Finally,the resultant was infused in 20%ethanol at 4°C.
(2)5 g of wet porous cellulose reacted with 5 ml epichlorohydrin and 10 ml of 2 mol·L−1NaOH in a three-neck round bottom f l ask at 40°C for 2.5 h,followed by adding 3.0 ml PEI,and the mixture was stirred at 90°C for 9 h under nitrogen gas.The product was washed with distilled water and dried at 40°C for 6 h in vacuum.
2.3.Characterization of cellulose particles and porous PEI-cell
The morphology of cellulose particle was f i rst observed by a JEM-100CX-II scanning electron microscope(SEM)(JEOL,Japan).Fourier transform infrared spectra,obtained with a NEXUS 670 spectrometer (Thermo Nicolet,USA)using potassium bromide pellets,were applied to characterize the porous PEI-cell.The amount of amino groups on the PEI-cell was determined by the titration method with hydrochloric acid according to the following procedure:0.2 g of dry PEI-cell was mixed with 15 ml of 0.3 mol·L−1HCl solution and the mixture was placed on the shaking table for 8 h.5 ml of sample in supernatant was diluted with 50 ml of redistilled water and 3 drops of phenolphthalein were added into the sample as the indicator. The mixture was demarcated with 0.15 mol·L−1NaOH solution until the color changed from colorless to pink.The content of amino groups (Y)is calculated by

where C1and C2(mol·L−1)are the concentrations of HCl and NaOH solutions,respectively,V1and V2(L)are the volume of HCl and NaOH solutions,respectively,and G(g)is the mass of sample.
2.4.Adsorption kinetics for Cr(III)and Fe(III)
100mlof Cr(NO3)3and FeCl3solutionswithinitialconcentrationsof 100 and 1250 mg·L−1was placed in two conical f l asks,and 0.2 g of porous PEI-cell particles was added into the solution separately.The twoconicalf l askswereplacedinashakerat30°C.0.5mlofsupernatant liquid was taken out from the f l ask at intervals.The concentrations of Cr(III)and Fe(III)were determined at 540 nm and 510 nm,respectively,by a TU1720 UV-visible spectrophotometer(Puxitongyong Instrumental Co.Ltd.,China)after the analytes were mixed with 1,5-diphenyl carbazide and phenanthroline.The standard curves of Cr(III)and Fe(III)are determined with stand solutions with gradient concentrations as y=0.00672+0.63298x(Rc=0.9992)and y=0.01381+0.19314x(Rc=0.9995),respectively.The adsorption amount is calculated by

where Q(mg·g−1)is the adsorption amount,Co(mg·L−1)is the initial concentration of Cr(NO3)3or FeCl3solution,C(mg·L−1)is the f i nal concentration,Vk(L)is the volume of metal salt solution, and m(g)is the mass of porous PEI-cell.
2.5.Isothermal adsorption experiment

where Virepresents the solution volume(L)and m represents the absorbent mass(g).
2.6.Investigation of major factors affecting adsorption capacity
The amount of absorbent,temperature and pH value are important factors in adsorption processes.pH values of solutions were adjusted with HCl or NaOH and measured by a pHS-25 digital pH meter (Leica Instrumental Co.Ltd.,China)to determine their effect on the adsorption capacity of porous PEI-cell.The adsorption experiments were implemented with different amounts of PEI-cell particles for a given amount of metal.The inf l uence of temperature(20,30,40, 50,60°C)was also explored for the adsorption capacity of porous PEI-cell particles.
2.7.Continuous adsorption and desorption experiments
2.02 g of PEI-cell was f i lled in a glass column with an inner diameter of 1 cm by a wet packing mode.The concentrations of Cr(III)and Fe(III)were 100 and 1250 mg·L−1,respectively, and the f l ow rate was controlled at 9.42 bed volume per hour (BV·h−1).Metal concentrations in the eff l uent were monitored with spectrophotometry at regular time intervals.The saturated adsorption amount can be calculated with obtained concentrations and above equations.
The elution experiments were performed with 5%HCl as an eluting reagent,and the f l ow rate of elution was controlled at 16.35 BV·h−1.The metal concentrations of eluent were determined and the elution curve was plotted.
After the desorption process,the adsorbent was regenerated, and the adsorption with the same metal concentrations and f l ow rate was repeated.With continuous adsorption and desorption on the PEI-cell adsorbent,the reusability for the two metal ions was evaluated.
在论坛同期举办的塑化电商联盟第一次全体会议和塑化电商联盟一届一次理事会议上,通过了联盟工作规程,选举产生联盟领导机构和秘书处等组成人员,安排了年度工作计划。
3.Results and Discussion
3.1.Cellulose particle and optimal preparation conditions of PEI-cell
The size distribution of cellulose particles is shown in Fig.1(a).Most particles are between 0.15 and 0.075 mm(78.51%),followed by those smaller than 0.075 mm(13.10%).The cellulose particles have relatively larger speci fi c surface and the particle size exhibits a normal distribution,as shown in Fig.1(b).SEM images are used to observe the morphology.Fig.1(c)shows that the sphericity of the cellulose particle is better and particle size is more uniform.In Fig.1(d),cellulose particles present relatively smooth surface and particle size is larger,indicating that amorphous region of cellulose is larger.Usually,the interaction fi rst occurs in these regions,sotheaccessibility ofobjects will behigher.
Preliminary experiments showthat themosteffective conditionsfor the preparation of PEI-cell are time(A,h),temperature(B,°C),concentration of NaOH(C,mol·L−1)and volume of PEI(D,ml).L9(34)orthogonal experiments are designed to optimize these parameters,and the content of-NH2is selected as the index.Table 1 indicates that the R (Xmax−Xmin)order is RD(0.76)>RB(0.75)>RC(0.72)>RA(0.33). The square of deviance of PEI volume(1.131),NaOH content(0.904) and temperature(0.867)is much larger than that of time(0.167),so the former three are the major in fl uencing factors.The optimized preparation conditions are as follows:time=8 h,temperature=90°C, NaOH content=2.0 mol·L−1,and PEI volume=3 ml.As a result,the maximum content of amino groups was obtained as 3.85%.
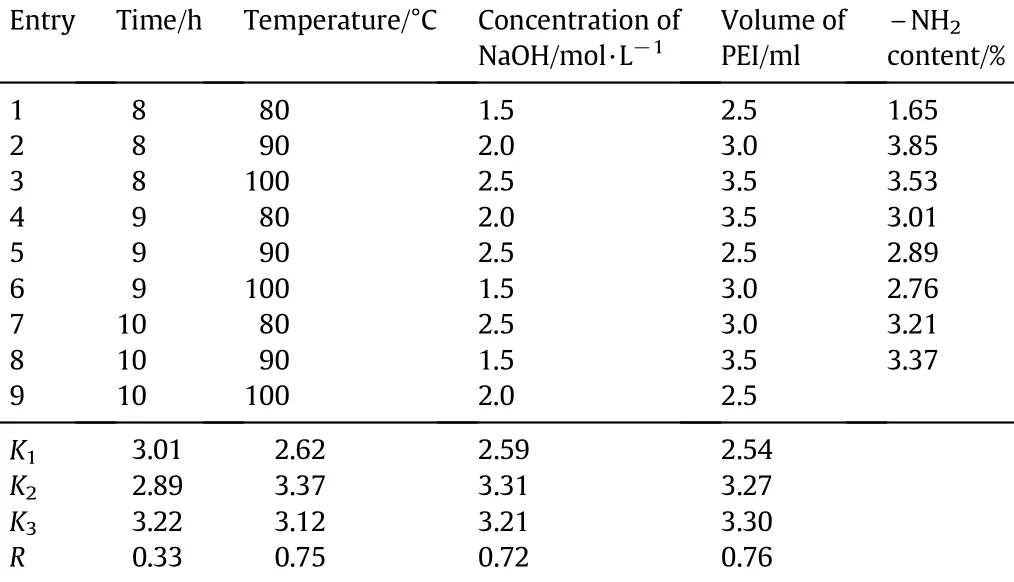
Table 1Design and results of orthogonal experiment over PEI-cell particle
3.2.FTIR spectra of porous cell-PEI
The infrared spectra of cellulose,epoxy-cellulose and PEI-cell are shown in Fig.2.After modif i cation of porous cellulose with epichlorohydrin,the adsorption peaks belonging to the stretching vibration of O-H and C-H bonds at 3429,2918 and 2852 cm−1become wider and higher in Fig.2(a).The change indicates the occurrenceof reaction between-Clof epichlorohydrin and-OHof cellulose and the increase of-CH2and-CH3groups on the surface of cellulose. Compared with the absorbance of epoxy-cellulose,the relative absorbance at 3438 cm−1increases obviously in Fig.2(b),which is assigned to the stretching vibration of N-H in amine groups.These evidences prove that PEI polymer is grafted on a cellulose surface and the adsorbent of PEI-cell is successfully synthesized.
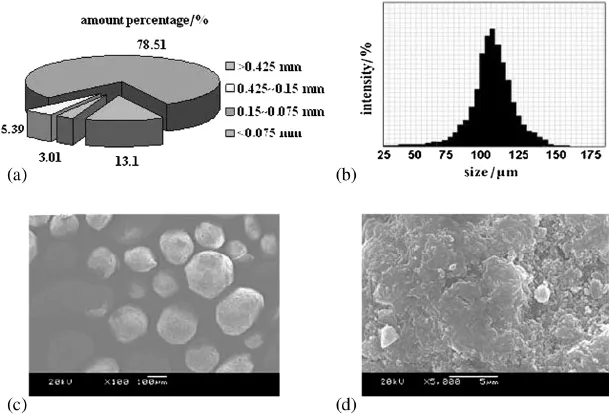
Fig.1.The size distribution of cellulose particle(a,b)and SEM images of PEI-cell(c,d).
Fig.2.Infrared spectra of cellulose,epoxy-cellulose and PEI-cell.
3.3.Adsorption mechanism of PEI-cell
According to the structure of porous PEI-cell,a single PEI chain has more than one attachment site,lowering the rigidity of cross-linked amine groups.Therefore,PEI cross-linked with the cellulose is superior on the capacity and selectivity towards metal ions.
3.3.1.Adsorption kinetics
The kinetic curves of the PEI-cell towards Cr(III)and Fe(III)are shown in Fig.3.The adsorption rate of the adsorbent is fast,and the increase in absorbed amount for Cr(III)and Fe(III)is not obvious after1 h. Therefore,thePEI-cell adsorbent could capture the two metal ions from theiraqueoussolutionseff i ciently.Theexperimentaldataarecorrelated with the f i rst and second order kinetic models.Table 2 shows that the second order model is more suitable to describe the adsorption for twometalions,sotheadsorptionrateisrelatedwiththeconcentrations of metal ions and the major interaction mechanism can be ascribed to chemical adsorption.
3.3.2.Adsorption isotherms
The adsorption isotherms of PEI-cell for Cr(III)and Fe(III)at 30,40 and 50°C are shown in Fig.4.Equilibrium adsorption amounts of the two metal ions increase quickly with the increase of equilibrium concentration and then decrease,demonstrating that the adsorption process reaches saturation.The composite material of PEI-cell has a very strong adsorption capacity for Cr(III)and Fe(III),so there exists high affi nity between metals and PEI-cell particles(chelation and hydrogen bond interactions).The interactions can be proved by comparing IR spectra for PEI-cell and PEI-cell adsorbed with metal ions.After the adsorptionofCr(III)andFe(III),boththeintensityandwavenumberofabsorption peaks at 3438 and 1641 cm−1decrease in different degrees. The stretching and bending vibrations of amino groups are restricted forhydrogenbondandchelationinteractionswithmetalions.Infurther investigation,both Langmuir and Freundlich isotherms are used to correlate the equilibrium data,and regression coef fi cients of the latter are larger than those of the former,so the Freundlich adsorption model is more suitable to describe the adsorption.The linear regression equations and coef fi cients of Cr3+and Fe3+are summarized in Table 3.
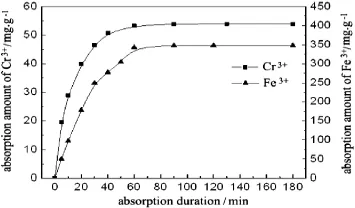
Fig.3.The kinetic curve of the PEI-cell for Cr(III)and Fe(III).
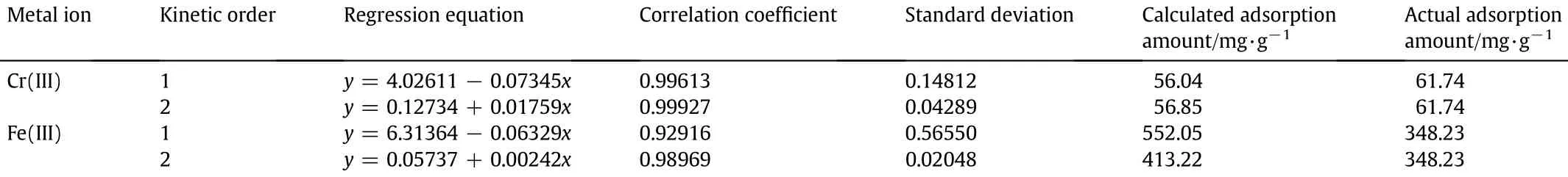
Table 2Kinetic model parameters from equation f i ttings
3.4.Factors affecting adsorption capacity
3.4.1.Effect of pH value on adsorption capacity
Adsorption capacities of the porous PEI-cell for Cr(III)and Fe(III)are differentindifferentpHvalues,asshowninFig.5(a).ThepHvaluehasa great inf l uence on the adsorption property.In basic solution,Cr(III)and Fe(III)will precipitate as Cr(OH)3and Fe(OH)3,so the adsorption at pH valueabove7isnotinvestigated.AtverylowpHvalue,theconcentration of H+is much higher than that of metal ions.The amine groups on PEI-cell surface would preferentially combine with H+to form-NH3+, which is disadvantageous for the interaction between it and metal ions. At higher pH value,the number of protonated amine groups would be less and metal ions have more opportunities to compete with H+for the combination with-NH2.The chelation becomes more signif i cant between PEI-cell and metal ions,increasing the adsorption capacity. However,Cr(III)and Fe(III)would begin to precipitate at pH valuebeyond5,asaresulttheadsorptionamountwoulddecrease.Thusweakly acidic condition(pH=5)is suitable for the adsorption of the two metal ions.
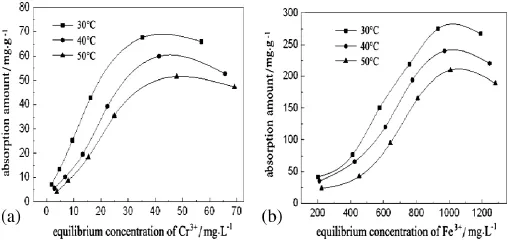
Fig.4.Adsorption isotherms of PEI-cell for Cr(III)and Fe(III)at 30,40 and 50°C.
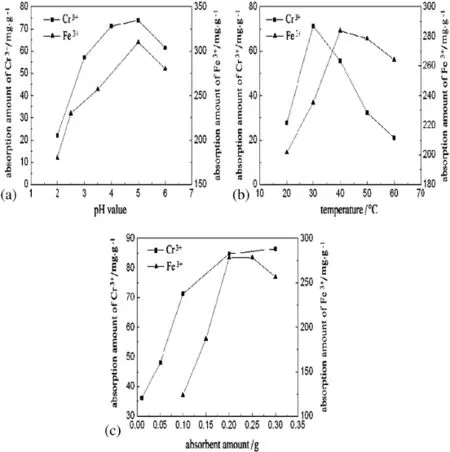
Fig.5.Effect of pH(a),temperature(b)and absorbent amount(c)on adsorption capacity.
3.4.2.Effect of temperature on adsorption capacity and thermodynamic study
Fig.5(b)shows the adsorption capacity at various temperatures with the saturation adsorption amount as the index.The adsorptioncapacity of PEI-cell particles increases with temperature at the beginning.When the temperature reaches 30°C for Cr(III)and 40°C for Fe(III),theadsorptioncapacityachieves themaximum.Furtherincrease of temperature reduces the adsorption amounts of the two metal ions obviously.
Table 4 provides the thermodynamic parameters for Cr(III)and Fe(III)at various temperatures.As the support for the conclusion from adsorption isotherms,the data in Table 4 also indicate that the adsorptionprocessisanexothermicprocess,sohigheradsorptiontemperature is unfavorable.The values of ΔG are negative at these temperatures, conf i rming that the adsorption is spontaneous and thermodynamically favorable.Negative values of ΔS prove the decrease of randomness at the solid-solution interface during adsorption,indicating that the movement of metal ions is restricted after adsorption.
3.4.3.Effect of absorbent amount on adsorption capacity
The amount of adsorbent is another important factor on the adsorption process for metal ions.Fig.5(c)shows the adsorption performance for the two metal ions with 0.01-0.30 g adsorbents for 50 ml of 100 mg·L−1Cr(III)and 1250 mg·L−1Fe(III)solutions.The results show that the adsorption capability is the greatest with 0.2 g of PEI-cell adsorbent.
3.4.4.The saturation adsorption amount
Underoptimaldynamicadsorptionconditions,thebreakthroughexperiment of dynamic adsorption was also performed.The sample of metalsolution wascontinuously loaded inthe column of PEI-cell adsorbent,and the breakthrough point would appear when the adsorption was saturated.The steep slope of breakthrough curve indicates a rapid mass transfer,which is accorded with the static kinetic study(shown in Fig.3).At the breakthrough point,the adsorption capacity was obtained as 83.98 mg·g−1for Cr(III)and 377.19 mg·g−1for Fe(III).In order to study the contribution of PEI groups for the improvement of adsorption,unmodif i edcelluloseandPEI-cellwerecompared.Thesaturation adsorption amount of the former was only 9.78 mg·g−1for Cr(III)and 14.29 mg·g−1for Fe(III).The results indicate that a great number of attachment sites in PEI chains play an important role. Table 5 summarizes the maximum adsorption amount of similar adsorbents for Cr(III)and Fe(III).
3.5.Desorption and recycle of the porous PEI-cell
Fig.6(a)shows the elution curves of Cr(III)and Fe(III)from PEI-cell with 5%HCl screened from conventional solvents astheeluent ata f l ow rate of 16.35 BV·h−1in the column packed with PEI-cell particles.The shape of elution curve is cuspate and little tailing appears,showing relatively good elution performance.Cr(III)and Fe(III)can be eluted from the PEI-cell with desorption ratios of 98.06%and 89.51%with 15 and 40mlHCl,respectively.Whentheelutionwasoperatedat40°C,thedesorption ratio could reach 99.88%and 97.74%.Absolute values of the change in adsorption enthalpy(ΔH)are small(see Table 4),which also indicates that the desorption could be easily completed.The excellent regeneration property is meaningful to reduce the cost in adsorption application.In order to investigate and evaluate the reusability of PEI-cell,the changing trend of adsorption amount and desorption ratio for the two metal ions are shown in Fig.6(b,c),with the porous PEI-cell possessinganacceptable adsorption capacity after beingreused 6 times.No obvious change is found in its morphology.

Table 4Thermodynamic parameters for Cr(III)and Fe(III)
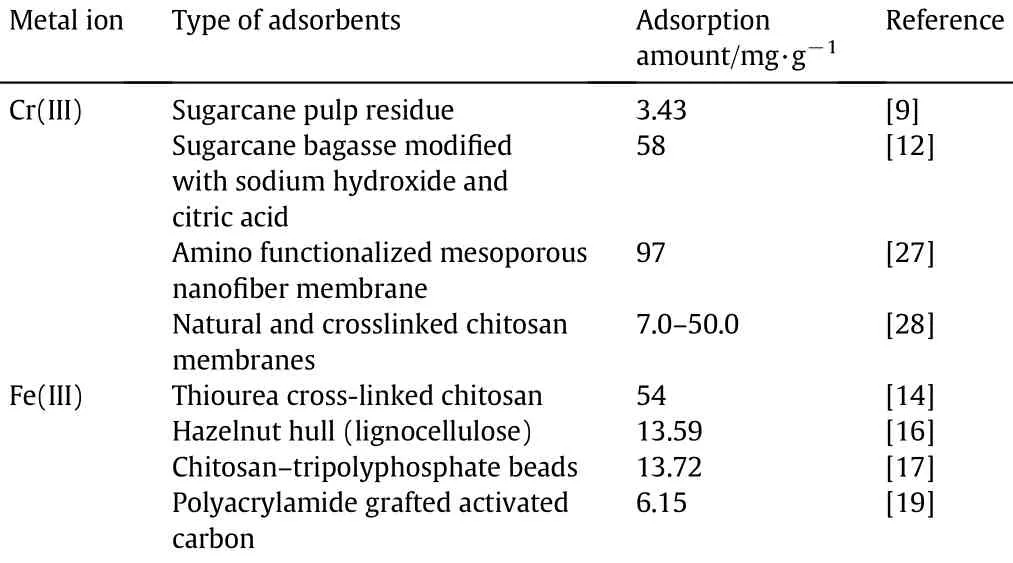
Table 5Comparison among reported polysaccharide and adsorbents containing-NH2
4.Conclusions
The macromolecule polyethyleneimine was grafted on the porous cellulose particles via the introduction of epoxy group and open loop reactions,and the functional adsorbent of PEI-cell was successfully prepared.The adsorption ability of PEI-cell for Cr(III)and Fe(III)is good with hydrogen bond interaction and chelation interaction.The main factors related with the adsorption ability for Cr(III)and Fe(III)include pH value,temperatureandamountofadsorbent.Thebestadsorptioncapacity appears at pH=5,at 30°C for Cr(III)and 40°C for Fe(III).The adsorption kinetics,isotherms and thermodynamic parameters are systematically studied to explore the interaction mechanism between metal ionsand absorbent.The PEI-cell particles presenta good regeneration behavior.Thus the cellulose adsorbent grafted with functional polymer shows better adsorption and desorption performance than similar adsorbents reported and it is feasible and promising to remove Cr(III)and Fe(III)from aqueous solutions in wastewater treatment.


Fig.6.Elution curves of Cr(III)and Fe(III)and reusability of PEI-cell.
[1]V.K.Gupta,A.Rastogi,A.Nayak,Studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material,J.Colloid Interface Sci.342(2010)135-141.
[2]A.Selatnia,A.Boukazoula,N.Kechid,M.Z.Bakhti,A.Chergui,Biosorption of Fe3+from aqueous solution by a bacterial dead Streptomyces rimosus biomass,Process Biochem.39(2004)1643-1651.
[3]A.Demirbas,Heavy metal adsorption onto agro-based waste materials:a review,J. Hazard.Mater.157(2008)220-229.
[4]N.Pourreza,J.Zolgharnein,A.R.Kiasat,T.Dastyar,Silica gel-polyethylene glycol as a new adsorbent for solid phase extraction of cobalt and nickel and determination by fl ame atomic adsorption spectrometry,Talanta 81(3)(2010)773-777.
[5]X.Xu,B.Y.Gao,X.Tang,Q.Y.Yue,Q.Q.Zhong,Q.Li,Characteristics of cellulosic amine-crosslinked copolymer and its sorption properties for Cr(VI)from aqueous solutions,J.Hazard.Mater.189(2011)420-426.
[6]N.Yeddou,A.Bensmaili,Equilibrium and kinetic modelling of iron adsorption by eggshells in a batch system:effect of temperature,Desalination 206(2007) 127-134.
[7]P.G.Wightman,J.B.Fein,Iron adsorption by bacillus subtilis bacterial cell walls, Chem.Geol.216(2005)177-189.
[8]Y.Wu,S.Z.Zhang,X.Y.Guo,H.L.Huang,Adsorption of chromium(III)on lignin, Bioresour.Technol.99(16)(2008)7709-7715.
[9]Z.H.Yang,S.Xiong,B.Wang,Q.Li,W.C.Yang,Cr(III)adsorption by sugarcane pulp residue and biochar,J.Cent.South Univ.20(5)(2013)1319-1325.
[10]A.Shoaib,N.Aslam,M.M.Athar,S.Akhtar,Na fi sa,S.Khurshid,Removal of Cr(III) through bread mold fungus,Pol.J.Environ.Stud.22(4)(2013)1171-1176.
[11]S.Tangjuank,N.Insuk,V.Udeye,J.Tontrakoon,Chromium(III)sorption from aqueous solutions using activated carbon prepared from cashew nut shells,Int.J. Phys.Sci.4(8)(2009)412-417.
[12]V.C.G.D.SantosI,A.D.P.A.Salvado,D.C.Dragunski,D.N.C.Peraro,C.R.T.Tarley,J. Caetano,Highly improved chromium(III)uptake capacity in modi fi ed sugarcane bagasse using different chemical treatments,Quim.Nova 35(8)(2012),http://dx. doi.org/10.1590/S0100-40422012000800021.
[13]C.H.Fan,Y.C.Zhang,H.R.Ma,Co-adsorption behavior of methylene blue(MB)and Cr(III)on synthesized zeolite from fl y ash:II.Competitive adsorption mechanism, Chin.J.Environ.Eng.8(3)(2014)1025-1028.
[14]J.Dai,F.L.Ren,C.Y.Tao,Adsorption behavior of Fe(II)and Fe(III)ions on thiourea cross-linked chitosan with Fe(III)as template,Molecules 17(4) (2012)4388-4399.
[15]C.Y.Abasi,A.A.Abia,J.C.Igwe,Adsorption of iron(III),lead(II)and cadmium(II) ions by unmodif i ed Raphia palm(Raphia hookeri)fruit endocarp,Environ.Res.J.5 (3)(2011)104-113.
[16]S.Ali,R.S.Masoud,A.Hamed,Removal of Fe(III)ions from aqueous solution by hazelnut hull as an adsorbent,Int.J.Ind.Chem.3(2012),http://dx.doi.org/10.1186/ 2228-5547-3-4.
[17]M.A.Correa-Murrieta,J.Lopez-Cervantes,D.I.Sanchez-Machado,R.G.Sanchez-Duarte,J.R.Rodriguez-Nunez,J.A.Nunez-Gastelum,Fe(II)and Fe(III)adsorption by chitosan-tripolyphosphate beads:kinetic and equilibrium studies,J.Water Supply Res Technol.61(6)(2012)331-341.
[18]D.K.Singh,S.Mishra,Synthesis and characterization of Fe(III)-ion imprinted polymer for recovery of Fe(III)from water samples,J.Sci.Ind.Res.69(10)(2010) 767-772.
[19]A.A.El-Zahhar,S.E.A.Sharaf El-Deen,R.R.Sheha,Sorption of iron from phosphoric acid solution using polyacrylamide grafted activated carbon,J.Environ.Chem.Eng. 1(3)(2013)290-299.
[20]H.Wang,Y.F.He,W.J.He,Y.Wang,R.M.Wang,Modif i cation of cellulose and its application in wastewater treatment,Techno.Water Treat.38(5)(2012)1-6.
[21]T.Tan,X.C.Xu,C.Yang,D.M.Yang,X.F.Zhu,Preparation of modif i ed rice straw f i bers and its adsorption of Fe3+,Cu2+,Ni2+,Zn2+in plating wastewater,Mod.Chem.Ind. 31(6)(2011)45-47.
[22]B.J.Gao,Y.B.Li,Z.P.Chen,Adsorption behavior of functional grafting particles based on polyethyleneimine for chromate anions,Chem.Eng.J.150(2009) 337-343.
[23]M.Amara,H.Kerdjoudj,Modif i cation of the cation exchange resin properties by impregnation in polyethyleneimine solutions:application to the separation of metallic ions,Talanta 60(2003)991-1001.
[24]V.K.Verma,S.Tewari,J.P.N.Rai,Ion exchange during heavy metal biosorption from aqueous solution by dried biomass of macrophytes,Bioresour.Technol.99(2008) 1932-1938.
[25]G.H.Pino,L.M.S.De Mesquita,M.L.Torem,G.A.S.Pinto,Biosorption of heavy metals by powder of green coconut shell,Sep.Sci.Technol.41(2006)3141-3153.
[26]S.Y.Kang,J.U.Lee,K.W.Kim,Biosorption of Cr(III)and Cr(VI)onto the cell surface of Pseudomonas aeruginosa,Biochem.Eng.J.36(2007)54-58.
[27]A.A.Taha,J.L.Qiao,F.T.Li,B.R.Zhang,Preparation and application of amino functionalized mesoporous nanof i ber membrane via electrospinning for adsorption of Cr3+from aqueous solution,J.Environ.Sci.24(4)(2012)610-616.
[28]E.Meneghetti,P.Baroni,R.S.Vieira,M.G.C.Silva,M.M.Beppu,Dynamic adsorption of chromium ions onto natural andcrosslinked chitosan membranes for wastewater treatment,Mater.Res.13(1)(2010)89-94.
26 April 2014
☆Supported by the National Natural Science Foundation of China(81373284, 81102344),the Project of Education Department in Sichuan(14ZB0267)and the Bureau of Science and Technology of Mianyang City of China(10Y003-8).
*Corresponding author.
E-mail address:cusack@scu.edu.cn(S.Yao).
http://dx.doi.org/10.1016/j.cjche.2014.07.001
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 12 June 2014
Accepted 14 July 2014
Available online 5 August 2014
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
