Separation of Primary Alcohols and Saturated Alkanes from Fisher-Tropsch Synthesis Products
2014-07-25SuqiaoLiZhongliTangFujunZhouWenbinLiXigangYuan
Suqiao Li,Zhongli Tang*,Fujun Zhou,Wenbin Li,Xigang Yuan
Separation Science and Engineering
Separation of Primary Alcohols and Saturated Alkanes from Fisher-Tropsch Synthesis Products
Suqiao Li,Zhongli Tang*,Fujun Zhou,Wenbin Li,Xigang Yuan
State Key Laboratory for Chemical Engineering and School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
A R T I C L EI N F O
Article history:
Fisher-Tropsch synthesis
Primary alcohol
Saturated alkane
Fractional distillation
Extraction
Azeotropic distillation
AmethodforseparatingprimaryalcoholsandsaturatedalkanesfromtheproductsofFisher-Tropschsynthesisis developed.The separation scheme consists of three steps:(1)the raw material is pre-separated by fractional distillation into four fractionsaccording to normal boilingpoints;(2)appropriate extractants are selected to separate the primary alcohols from the saturated alkanes in each fraction;(3)the extractants are recovered by azeotropic distillation and the primaryalcohols inthe extract phase are purif i ed.Based on the proposed method, the total recovery rates of the primary alcohols and the saturated alkanes are 86.23%and 84.62%respectively. ©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Although natural gas is a limited resource,the associated gas in the offshore oilf i eld is usually vented or burned directly,wasting resources andcontaminatingtheenvironment.Recently,theconversion ofassociated gas into linear chain primary alcohols and saturated alkanes via Fisher-Tropsch synthesis has been reported[1-6].With a Co-based catalyst applied because of its high activity,high selectivity for long chain paraff i ns and a low water gas shift activity[7,8],the Fisher-Tropsch synthesis produces a product with primary alcohols:hydrocarbon weightratiocloseto1:1andthecontentof waxyhydrocarbonsless than 5%,demonstrating great industrial potential.
The hydrocarbons in the Fisher-Tropsch product can be used as various fuels according to the number of carbon atoms,such as, liquef i ed petroleum gas(C3-C4),gasoline(C5-C12),diesel fuel (C13-C22),and light waxes(C23-C33).The primary alcohols in the product also have many industrial applications.For instance,higher alcohols,which contain more than six carbon atoms[9],are the primary material in synthesizing surfactants,washing agents,plasticizers and many other f i ne chemicals.In addition,derivatives of these higher alcohols have been widely used in spinning,papermaking, foodstuffs,pharmaceuticals,leather,building,mining,metallurgy, machinery and agriculture[10].
Separation of alcohols and alkanes has been extensively studied. Bonthuys et al.[11,12]studied the separation of alkanes and alcohols with supercritical f l uids.Krishna and Van Baten[13]developed a method to separate linear alkanes and alcohols by using cage-type zeolites according to different saturation capacities.Mathys et al.[14]used pervaporation to separate long-chain 1-alkanols from the correspondingn-alkanes.Although thesenovel methodspossessmanyadvantages, such as high recovery rate and high product purity,they are not matureenough to be applied to industrial production.Therefore,a combined process of extraction and azeotropic distillation[15,16]for simultaneous recovery of primary alcohols and saturated alkanes from Fisher-Tropsch synthesis products is presented herein.This process can be readily converted to an industrial scale.
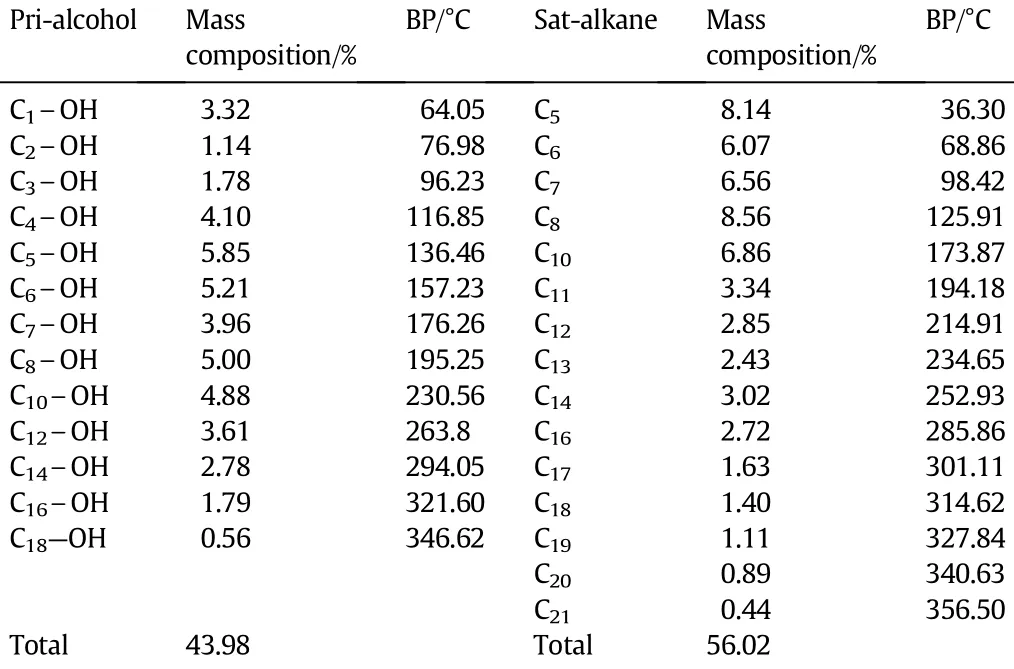
Table 1Composition and normal boiling points of the components in the raw material

Fig.1.Separation process of the experiment.
2.Raw Material and Separation Scheme
2.1.Raw material characteristics
TherawmaterialwasthemixtureofproductsfromaFisher-Tropsch synthesis with a Co-based catalyst.The qualitative and quantitative analyses for the raw material were performed with the gas chromatographic method.The composition of the raw material and the normal boiling point[17]of each component are listed in Table 1.The primary alcohols account for a weight ratio of 43.98%and the saturated alkanes account for 56.02%of the raw material.The lowest normal boiling point(36.3°C)is for n-pentane and the highest(356.5°C)is for n-heneicosane,accountingfor thewideboilingrangeoftherawmaterial.The saturated alkanes are non-polar,whereas the primary alcohols are polar or weakly polar.Methanol has the strongest polarity and the polarity of alcohols decreases as the number of carbon atoms increase. The polarity of C18-alkyl alcohols is so weak that it can form a stable and homogeneous solution with the non-polar saturated alkanes.
2.2.Separation scheme
Distillation is the most commonly used method to separate liquid mixtures,but it is not suitable for complete separation of mixed alcohols and mixed alkanes due to their similar volatilities.A hybrid separation scheme is proposed here to accomplish the separation.It includes three steps:(1)the raw material is pre-separated by fractional distillation into four fractions according to normal boiling points;(2)appropriate extractants and operating conditions are selected to separate the primary alcohols from the saturated alkanes and then extraction is performed on each fraction;and(3)the extractants are recovered by azeotropic distillation and the primary alcohols in the extract phase are purif i ed.The separation process is given in Fig.1.
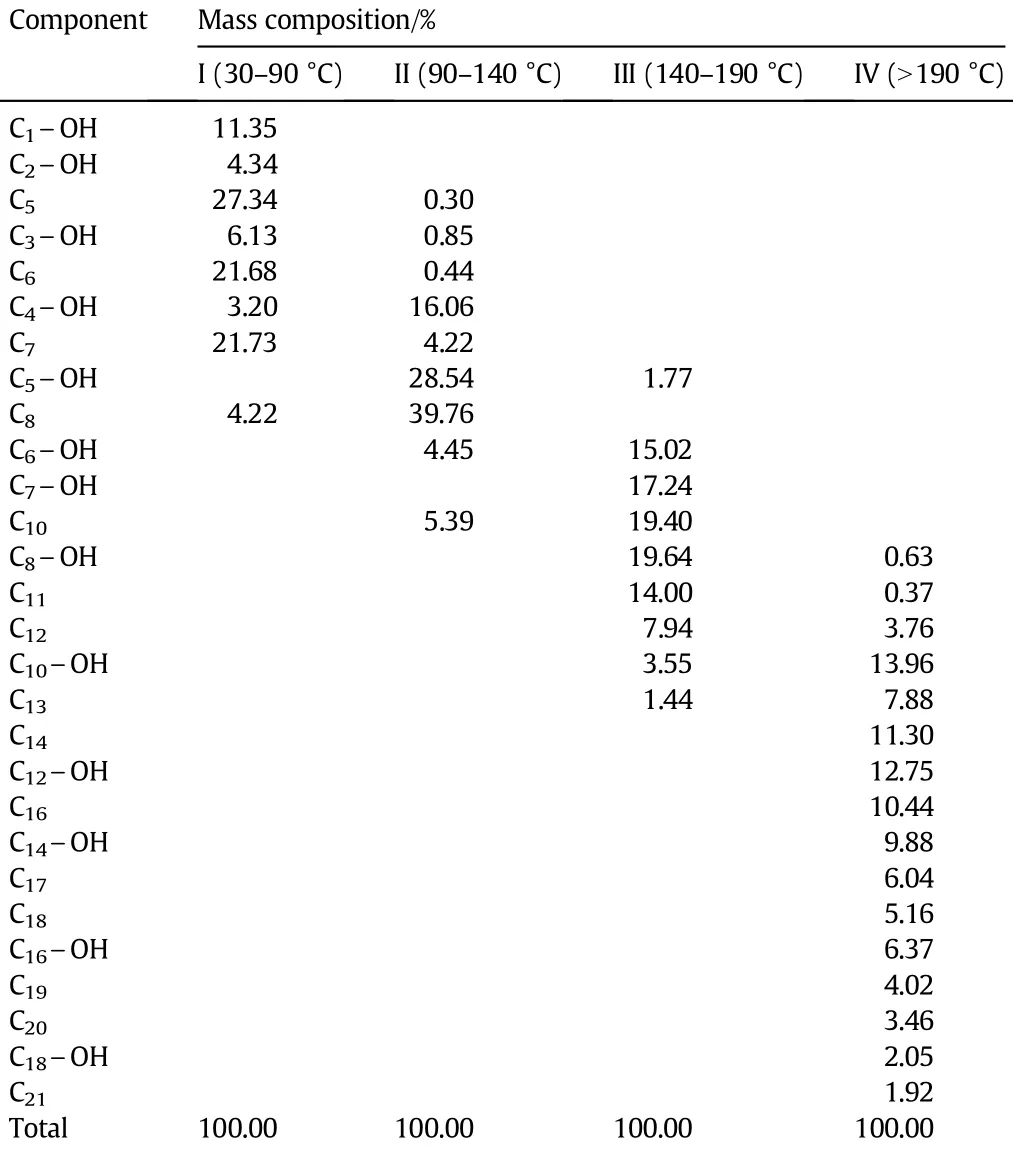
Table 2Compositions of the distillation fractions
2.3.Experimental equipment
A packed column with 3-mm-diameter Dixon packings was used in this separation process.The height and diameter of the column were 1200 mm and 25 mm,respectively.Liquid-liquid extraction was performed in a 500-ml separatory funnel.An Agilent Technologies 7890A GC System was employed to perform qualitative and quantitative analyses for each component in the distillation and extraction experiments.The gas chromatographic conditions are as follows: column of HP-5MS,30 m×0.25 mm,f i lm 0.25 μm;f l ame ionizationdetector;carrier,N2at 25 cm·s-1;oven,40°C for 1 min,40-240 at 5°C·min-1;and injection,split 280°C,split ratio 1:100.
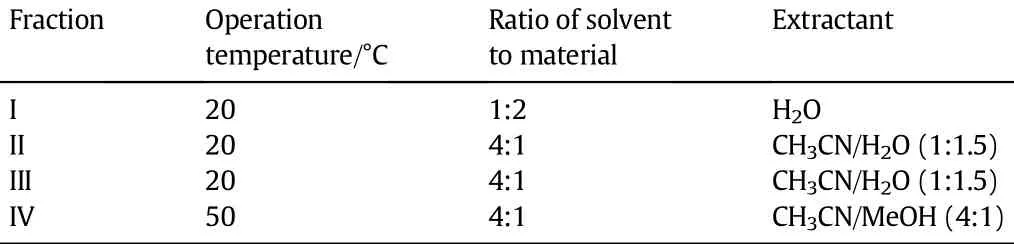
Table 3Extractant and operation parameters
3.Results and Discussion
3.1.Preparation of distillation fractions
The raw material was distilled in the packed column and four distillation fractions were obtained according to normal boiling points. They are:Fraction I(30-90°C)that mainly contains C1-C3alcohols and C5-C7alkanes,Fraction II(90-140°C)that mainly contains C4-C5alcohols and n-octane,Fraction III(140-190°C)that mainly includes C6-C8alcohols and C10-C12alkanes,and Fraction IV(>190°C)that mainly includes C10-C18alcohols and C12-C21alkanes.The composition of each fraction is shown in Table 2.The polarities of the primary alcohols in each fraction are similar and the 50-60°C boiling range of the fractions is convenient for choosing the utilities in designing distillation system.
3.2.Separation of alcohols and alkanes
Extraction[18,19]was applied to each fraction in order to separate the alcohols and alkanes.The extraction conditions for each fraction were optimized by using an orthogonal experimental design[20-22]. The extractant and operational parameters for each fraction are listed in Table 3.Water was used as the extractant to recover methanol, ethanol and propanol in Fraction I.For Fractions II,III and IV,mixed extractantsacetonitrile/waterandacetonitrile/methanolwereemployed to separate the alcohols and alkanes.
Acetonitrile is strongly polar and miscible with low molecular weight primary alcohols,but it is nearly immiscible with alkanes[23]. As the number of carbonatomsincreases,thesolubility of primary alcohols in acetonitrile decreases.Thus methanol is adopted to enhance the extraction eff i ciency in Fraction IV.Since the density of acetonitrile is close to that of the saturated alkanes,water is added to facilitate a two-phase separation.In addition,water as an entrainer can help to recover acetonitrile in the subsequent azeotropic distillation.
The extraction procedure is as follows:the extractant and corresponding fraction are mixed in the desired proportions in a separatory funnel.After standing for 30 min,samples from both phases are analyzed by gas chromatography.The compositions of the aqueous (A)and oil(O)phases for the extraction of the four fractions are shown in Table 4.
For Fraction I,the recovery rates of methanol and ethanol in the aqueous phase and the alkanes in the oil phase were over 95%.Only a trace amount of alkanes was detected in the aqueous phase,indicating that water has a high selectivity for alcohols.Since the recovery rate of butanol is only 56.7%,perhaps a more polar extractant is needed.
ForFractionII,exceptforhexanol,highrecoveryrateswereobtained for all of the alcohols in the aqueous phase,and the hydrocarbons and alcohols were well separated.A similar situation occurred for Fraction III when CH3CN-H2O was used to extract the alcohols.The primary alcohols accounted for about 10%of the aqueous phase and the average recovery of alcohols was 84.94%.
Compared with Fraction II,the extractant had a poorer ability to extractalcoholsfromFractionIII,loweringtherecoveryrateoftheprimary alcohols and increasing the amount of saturated alkanes in the extract phase.

Table 4Extraction results for Fractions I,II,III and IV
For Fraction IV,none of the primary alcohol recovery rates exceeded 90%and the recovery rate of octadecanol was only 43.66%.As thenumber of carbon atoms increases,the diff i culty in separation of alcohols and hydrocarbons increases because the polarities of the alcohols andalkanes aremoresimilar.Fortunately,the alcoholsremaining in the hydrocarbons can be also burned as fuels.
3.3.Purif i cation of the primary alcohols and recovery of extractant
Methods to separate alcohols have frequently been reportedin literature[24,25]andseparatingalcoholsfromwaterhasalsobeenreported [26].In this paper,the focus is to recover the alcohols from the extractants which contain acetonitrile,i.e.,from the aqueous extract phases of Fractions II,III and IV.
Since acetonitrile and water form a homogeneous binary azeotropic mixture at a mass composition of 85%-15%at 0.101 MPa,azeotroptic distillation was used to recover the extractant from the aqueous phases of Fractions II,III and IV and then the primary alcohols were purif i ed. The distillation equipment used to prepare the fractions was employed in this process.The rectif i cation sequence for Fractions II and III was: acetonitrile and water mixture was steamed out at azeotropic temperatureof76.5°Candacetonitrilewasremovedat81.5°C,andthentheprimary alcohols of high purity were obtained at the tower bottom.For Fraction IV,methanol was f i rst distillated at top column temperature of 64°C and then the subsequent distillate order was the same as above.The results of the azeotropic distillation are shown in Table 5.It can be seenthat the extractant andalcohols are satisfactorily separated.
4.Conclusions
Distillation and extraction were employed to separate primary alcohols and alcohols and saturated alkanes from Fisher-Tropsch synthesis products.The raw material was pre-separated by batch distillation into four fractions according to normal boiling point.Alcohols with similar polarities were contained in each fraction.Different combinations of water,acetonitrile and methanol were used as extractants to separate the primary alcohols from the saturated alkanes in each fraction.Most of alcohols were recovered in the aqueous phases,which contained few hydrocarbons.The extractants were recovered by azeotropic distillation and the primary alcohols in the extract phase were purif i ed.The total recovery rates of the primary alcohols and the saturated alkanes were 86.23%and 84.62%respectively.

Table 5Results for recovery of extractant and alcohols for Fractions II,III and IV
[1]R.B.Anderson,The Fisher-Tropsch Synthesis,Academic Press,New York,1984.
[2]A.Y.Khodakov,W.Chu,P.Fongarland,Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels, Chem.Rev.107(2007)1692-1744.
[3]A.Demirbas,Converting biomass derived synthetic gas to fuels via Fisher-Tropsch synthesis,Energy Sources 29(2007)1507-1512.
[4]J.Anfray,M.Bremaud,P.Fongarland,A.Khodakov,Kinetic study and modeling of Fisher-Tropsch reaction over a Co/Al2O3catalyst in a slurry reactor,Chem.Eng.Sci. 62(2007)5353-5356.
[5]P.C.Das,N.C.Pradhan,A.K.Dalai,N.N.Bakshi,Carbon monoxide hydrogenation over zirconia supported Ni and Co-Ni bimetallic catalysts,Catal.Lett.98(2004) 153-160.
[6]W.Zhang,A review of techniques for the process intensif i cation of f l uidized bed reactors,Chin.J.Chem.Eng.17(2009)688-702.
[7]S.Hinchiranan,Y.Zhang,S.Nagamori,TiO2promoted Co/SiO2catalysts for Fisher-Tropsch synthesis,Fuel Process.Technol.89(2008)455-459.
[8]A.M.Kazi,F.K.Bedu-Addo,J.Goodwin,G.James,CO hydrogenation over lanthana promoted cobalt catalysts,Prepr.Div.Pet.Chem.Am.Chem.Soc.37(1)(1992) 234-238.
[9]J.M.Zhao,Development status of higher alcohol in china,Asian Chem.Intermed.6 (2001)6-8.
[10]J.M.Zhao,High alcohol market and its development,Chem.Technol.Mark.8(2001) 10-12.
[11]G.J.K.Bonthuys,C.E.Schwarz,A.J.Burger,J.H.Knoetze,Separation of alkanes and alcohols with supercritical f l uids.Part I:Phase equilibria and viability study, J.Supercrit.Fluids 57(2011)101-111.
[12]C.E.Schwarz,G.J.K.Bonthuys,R.F.van Schalkwyk,D.L.Laubscher,A.J.Burger,J.H. Knoetze,Separation of alkanes and alcohols with supercritical f l uids.Part II:Inf l uence of process parameters and size of operating range,J.Supercrit.Fluids 58 (2011)352-359.
[13]R.Krishna,J.M.Van Baten,Entropy-based separation of linear chain molecules by exploiting differences in the saturation capacities in cage-type zeolites,Sep.Purif. Technol.76(2011)325-330.
[14]R.G.Mathys,W.Heinzelmann,B.Witholt,Separation of higher molecular weight organic compounds by pervaporation,Chem.Eng.J.67(1997)191-197.
[15]T.Huang,Z.G.Tang,Z.T.Duan,A combined process of extraction and azeotropic distillation used to separate isopropanol from dilute aqueous solution for reduction of energy cost,Fine Chem.20(5)(2003)273-279.
[16]W.Wang,L.F.Guo,S.Gao,Isolation and purif i cation of isopropyl alcohol,Yunnan Chem.Technol.38(6)(2011)58-61.
[17]N.L.Cheng,Solvent Handbook,Fourth edition,Chemical Industry Press,Beijing, 2007.
[18]J.P.De Wet,W.Jansen,Extraction of oxygenates from a hydrocarbon stream,China patent,CN1764619,2006.
[19]J.P.De Wet,Separation of oxygenates from a hydrocarbon stream,China patent, CN1468292A,2004.
[20]Y.Yadollah,S.Abolfazl,K.Mostafa,Orthogonal array design for the optimization of supercritical carbon dioxide extraction of platinum(IV)and rhenium(VII)from a solid matrix using cyanex 301,Sep.Purif.Technol.61(2008)109-114.
[21]X.Qin,X.Y.Jin,One step bioleaching of sulphide ore with low concentration of arsenic by Aspergillus niger and Taguchi orthogonal array optimization,Adv.Mater. Res.396-398(2012)2285-2288.
[22]I.Sadia,R.A.Chi,C.L.Jae,N.B.Haq,Optimization of supercritical CO2extraction of Hovenia dulcis thumb seed oil by orthogonal test methodology,Chin.J.Chem.Eng. 20(5)(2012)923-929.
[23]C.D.Keefe,Z.Istvankova,Computational study of proper and improper hydrogen bonding in methanol complexes,Can.J.Chem.89(2011)34-46.
[24]D.F.Wang,W.Ji,S.L.Liu,M.Z.Qi,Experiment study of alcohol ternary mixture separation by dividing wall column,Guangzhou Chem.Ind.39(22)(2011)42-45.
[25]Q.Ye,C.J.Qian,Z.R.Qiu,Simulation of alcohol mixture separation by divided-wall distillation column,Chem.Eng.36(2)(2008)1-4.
[26]Y.Bai,Y.H.Li,J.Q.Liu,Separation technology of the by-product in water of Fisher-Tropsch synthesis,Ph.D.thesis,Tianjin,Tianjin University.
12 December 2012
*Corresponding author.
E-mail address:zltang@tju.edu.cn(Z.Tang).
http://dx.doi.org/10.1016/j.cjche.2014.06.025
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 24 May 2013
Accepted 10 June 2013
Available online 30 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
