Inf l uence of the Surface and Structural Characteristics of Activated Carbons on Adsorptive Removal of Halo-Olef i nic Impurities from 1,1,1,3,3-Pentaf l uoropropane☆
2014-07-25BoZhangChuangZhangMingquanWei
Bo Zhang*,Chuang Zhang,Mingquan Wei
Separation Science and Engineering
Inf l uence of the Surface and Structural Characteristics of Activated Carbons on Adsorptive Removal of Halo-Olef i nic Impurities from 1,1,1,3,3-Pentaf l uoropropane☆
Bo Zhang*,Chuang Zhang,Mingquan Wei
Research Institute of Industrial Catalysis,Zhejiang University of Technology,Hangzhou 310014,China
A R T I C L EI N F O
Article history:
Adsorption
Removal
Halo-olef i nic impurities
1,1,1,3,3-Pentaf l uoropropane
Activated carbon
Halo-olef i nic impurities in 1,1,1,3,3-pentaf l uoropropane(HFC-245fa)product used as blowing agents,etc.could damage the human body and must be removed.Activated carbon was treated by HCl,HNO3and NaOH,respectively.The adsorptive performance of unmodif i ed and modif i ed activated carbons for the removal of a low content of 1-chloro-3,3,3-trif l uoro-1-propene(HCFC-1233zd),1,3,3,3-tetraf l uoro-1-propene(HFC-1234ze),1-chloro-1,3,3,3-tetraf l uoro-1-propene(HFC-1224zb)and 2-chloro-1,3,3,3-tetraf l uoro-1-propene(HFC-1224xe) halo-olef i ns in the 1,1,1,3,3-pentaf l uoropropane(HFC-245fa)product was investigated.These halo-olef i nic impurities could be substantially removed from the HFC-245fa product via the adsorption over activated carbon when the adsorption temperature was under 333 K,which can be attributed to the π-π dispersion interactions between the halo-olef i ns and carbon graphite layer.The basic surface groups of activated carbon could catalyze the decomposition of HFC-245fa to form HFC-1234ze.However,the signif i cant increase in the amount of surface acidic groups of activated carbon led to a distinct decrease of adsorption capacity due to the reduction in the micropore volume of adsorbent and a decrease in the strength of the π-π dispersive interactions between haloolef i n molecules and carbon basal.The breakthrough time of halo-olef i nic impurities on activated carbon increased with the increase of molecular mass and the decrease of molecular symmetry.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
1,1,1,3,3-Pentaf l uoropropane(HFC-245fa)is a non-ozone depleting compound with an acceptable global warming potential(GWP).It has been offered recently as a substitute for trichlorof l uoromethane(CFC-11),1,1-dichloro-1-f l uoroethane(HCFC-141b)and 1,2-dichloro-1,1,2,2-tetraf l uoroethane(CFC-114)[1,2],which are used in a variety of applicationsincludingrefrigerants,propellants,blowingagentsandsolvents,and havebeenperceivedashavinganadverseeffectontheozonelayerand/or as contributing to global warming.In the preparation of HFC-245fa, various halo-olef i nic impurities are formed mainly including 1-chloro-3,3,3-trif l uoro-1-propene(HCFC-1233zd),1,3,3,3-tetraf l uoro-1-propene (HFC-1234ze),1-chloro-1,3,3,3-tetraf l uoro-1-propene(HFC-1224zb) and 2-chloro-1,3,3,3-tetraf l uoro-1-propene(HFC-1224xe).Since these halo-olef i nic impurities have potential toxicity,their presence in the HFC-245fa product even at low concentrations is of major environmental concern.Hence,the removal of these halo-olef i nic impurities from the HFC-245faproductiscriticaltoensurethesafetyoftheHFC-245faproduct application.
Impurities are generally removed from the desired product by distillation,but this method is diff i cult if the boiling point of the impurity is close to that of the desired product.Furthermore,even after distillation,it is possiblethatsmallquantitiesofundesirableimpuritieswillremain.Afterdistillation of the HFC-245fa crude product,halo-olef i nic impurities mentioned above will still be present in amounts from about 0.01%to 0.1%due to the small difference in relative volatility between these unsaturated impurities and HFC-245fa.Thus,there is a need for an alternative purif i cation method toremoveresidualhalo-olef i nicimpuritiesfromtheHFC-245faproductsequentially.Adsorptionseparationisoneofthemosteffectiveandfrequently usedprocedures fortheremoval ofpollutantsinwastewaters orthepurif ication of chemicals.The separation of olef i ns from paraff i ns using adsorption process is very challenging.Indeed,one of the most important differencesbetweenthosetwotypesofcompoundsistheelectroniccharacter of the double bonds that exist in the olef i ns.Many patents have disclosed some methods for the removal of halo-olef i ns from halo-alkane productsthroughtheadsorptiononanappropriateadsorbent.Forexample, the adsorptive separation of vinyl chloride from 1,1-dif l uoroethane can be performed on an active carbon[3],dichloroacetylene can be substantially removed from a stream of 1,1-dichloro-1-f l uoroethane over a carbon molecularsievehavingameanporesizeofabout0.42-0.45nm[4],andtheremoval of 1-chloro-2,2-dif l uoroethylene from 1,1,1,2-tetraf l uoroethane is accomplished by the adsorption on β or Y zeolites[5].However,the research reports about the inf l uences of composition,structure and naturesof above mentioned adsorbents on the adsorption performances for haloolef i ns are rarely found.In our previous study[6],it was found that HCFC-1233zdimpuritycanbesubstantiallyremovedfromtheHFC-245faproduct via the adsorption over multivalent metal cations and Cu+cation exchangedYzeolites,whichisascribedtotheformationofπ-adsorptioncomplexes between HCFC-1233zd and zeolites,rather than over alkaline metal cations exchanged Y zeolites.However,HCFC-1224zb and HCFC-1224xe impurities cannot be obviously removed from the HFC-245fa product via the adsorption,regardless of the cations introduced in Y zeolite used as an adsorbent.
Activated carbons are very effective adsorbents for the removal of organic and inorganic pollutants from gaseous or aqueous media [7-9].To the present,activated carbon remains to be one of the most important microporous adsorbents from an industrial view of point. However,the removal of a low content of halo-olef i nic impurities from the HFC-245fa product via the adsorptive separation over an activated carbon adsorbent has not been systematically investigated so far. Activated carbonhas very complex surfacecharacteristics(porosityand surface chemistry),with pore size ranging from micropores(less than 2 nm)to macropores(more than 50 nm),and has a variety of surface groups,impurities and irregularities.In many applications,the adsorption properties of activated carbons may be correlated with the chemical nature of the carbon surface rather than with the surface area and the porosity of the carbon[10-12].Carbons can be treated by acids, bases,or oxidizing agents to produce favorable chemical and physical properties for different applications[13-15].In this paper,the adsorptive performances of activated carbons treated by HCl,HNO3or NaOH, respectively,forthe removalof a lowcontentof halo-olef i nic impurities from the HFC-245fa product were investigated.
2.Experimental
2.1.Preparation of activated carbon adsorbents
A coconut activated carbon(AC0)was purposed from Suyang Shuangyuan Corporation(Jiangxi,China).20 g of AC0sample was treated with 80 ml of 5 mol·L−1HCl,5 mol·L−1HNO3and 5 mol·L−1NaOH solutions for 4 h at 333 K,respectively.After the treatment,the samples were washed with distilled water and dried in air at 373 K overnight.The carbon samples treated by acids and base were then referred to as AC-HCl,AC-HNO3and AC-NaOH.The ash content was determined by burning off the carbon at 1023 K.The ash mass content of all samples was below 0.2%.
2.2.Characterization of activated carbon adsorbents
The textural properties of the activated carbon samples were determinedfromnitrogenadsorption-desorptionisothermsobtainedat77K on a Micromeritics ASAP 2020 instrument.Prior to the measurements, the samples were degassed at 523 K for 5 h under a high vacuum.The specif i c surface areas(SBET)were calculated by using the multipoint BET method in the relative pressure(P/P0)range of 0.01-0.1.The average pore diameter(Dave)and the total pore volume(Vtotal)were calculated from theamount of gas adsorbed at therelated pressure of 0.995-0.999.The micropore area(Smicro—pore width less than 2 nm)and the micropore volume(Vmicro)were calculated using the t-Plot method.
The surface functional groups were investigated by transmission infrared spectra obtained from a Nicolet 370 FT-IR spectrophotometer using KBr pellet technique.The spectra were recorded from 4000 to 400 cm−1.The contents of acidic and basic sites on the surface of samples were determined according to Böehm titration[16].1 g of carbon sample was placed in 50 ml of the following solutions:0.1 mol·L−1sodium hydroxide,0.1 mol·L−1sodium carbonate,0.1 mol·L−1sodium bicarbonate,and 0.1 mol·L−1hydrochloric acid.The vials were sealed and shaken for 24 h and then f i ltered;5 ml of the f i ltrate was pipetted, and the excess base or acid was titrated with HCl(0.1 mol·L−1)or NaOH(0.1 mol·L−1),respectively.The number of acidic sites was determined under the assumption that NaOH neutralizes carboxylic,lactonic, and phenolic groups;and Na2CO3neutralizes carboxylic and lactonic groups;and NaHCO3neutralizes carboxylic groups.The number of basic sites was calculated from the amount of hydrochloric acid that reacted with the carbon.
The pHpzc of the activated carbon sample was determined from acid-base titration.The procedure of pHpzc determination is described as follows[17]:aliquots with 50 ml of 0.01 mol·L−1NaCl solution were prepared in different f l asks.Their pH values were adjusted to the value between2 and 12 withthe addition of 0.01 mol·L−1solutions of HCl or NaOH.When the pH value was constant,0.15 g of the activated carbon sample was added to each f l ask and it was shaken for 48 h.The f i nal pH wasmeasuredusingpHmeterpHS-3Cafter48h.ThepHpzcvalueisthe point where the curve pHfinalversus pHinitialcrosses the line pHfinal= pHinitial.
2.3.Adsorption experiments
The main halo-olef i ns impurities contained in the HFC-245fa productafterdistillation,kindlyprovidedbyZhejiangLantianEnvironmental Protection Hi-Tech Co.,Ltd.(Hangzhou,Chia),are HCFC-1233zd,HCFC-1224zband HCFC-1224xe,HFC-1234ze and their contents of whichand that of HFC-245fa are listed in Table 1.
Adsorption experiments of the HFC-245fa product feed mentioned above on various activated carbon adsorbents were performed in gas phase using a f i xed bed continuous f l ow reactor(10 mm i.d.)with a thermocouple in the center of the adsorbent bed at 283 K to 453 K and atmospheric pressure.5.0 g of adsorbent(0.4-0.6 mm)was loaded into the adsorbent bed.Prior to the feeding,the HFC-245fa product was gasif i ed at 323 K and was used as the feed without dilution.The adsorbent was pre-treated in situ at 573 K for 5 h under a nitrogen f l ow of 30 ml·min−1before starting an adsorption run.Then HFC-245fa product was passed through the adsorbent bed at the desired temperature.The space velocity of HFC-245fa product,WHSV(weight-hourly space velocity,g of HFC-245fa product per g of adsorbent per hour),was maintained at 0.5 h−1by controlling the f l ow rate of HFC-245fa product.After running 30 min, the concentrations of components in the eff l uents were analyzed on an Agilent 6890 N gas chromatograph equipped with a FID and a DB1301 column.
Breakthrough experiments were also performed in a f i x-bed reactor under the experimental conditions described above except for a f i xed adsorption temperature of 303 K.
When one adsorption bed reached a saturated adsorption,the regeneration of the spent activated carbon was performed in situ at 523 K for 10 h under a nitrogen f l ow of 30 ml·min−1.Then breakthrough experiments were carried out for the regenerated adsorbents under the experimental conditions mentioned above again.
3.Results and Discussion
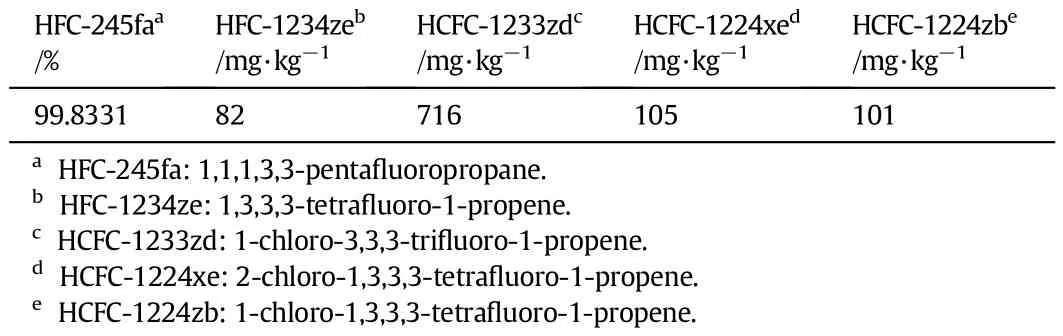
Table 1The contents of halo-olef i ns and HFC-245faain HFC-245faaproduct after distillation
3.1.Characterizations of activated carbon adsorbents
The surface functional group is an important characteristic of the activated carbons since it determines the surface properties of the carbonsand has a signif i cant implication on their behavior.Fig.1 shows the FT-IR spectra of AC0,AC-HCl,AC-HNO3and AC-NaOH.All spectra show a wide adsorption band at about 3100-3650 cm−1with a maximum at about 3420 cm−1and a wear sharp adsorption band at 3734 cm−1.The former can be assigned to the O-H stretching mode of hydroxyl groups and adsorbed water[18].The latter may be ascribed to isolated O-H groups [19].A broad band at 1300-1000 cm−1is assigned to C-O stretching in acid,alcohols,phenols,prone,ethers and eaters[20].The presence of a relatively strong band around 1560 cm−1can be attributed to the conjugated systems such as diketone,keto-ester and keto-enol structures,carboxylic groups and quinone structure[7,21,22].This band also appears in all the samples.The spectra of AC0and AC-HCl are in some similar.After the NaOH treatment the intensities of the bands at around 1560 cm−1and 1300-1000 cm−1somewhat decrease,which can be attributed to the reduction in the amount of surface acidic groups caused by the neutralization of NaOH.A distinct adsorption of around 1710 cm−1assigned to a specif i c peak for the carboxylic acid group or attributed to the vibration of C=O band in carboxylic acids,ketones,aldehydes,lactones or esters and a weak peak at 1750 cm−1assigned to carboxyl groups appearsinthespectrumofAC-HNO3only[23,24].ItindicatesthatanotableamountofcarboxylicacidgroupsisformedonthesurfaceofAC-HNO3comparedwithAC0,AC-HClandAC-NaOHasaresultofnitricacidoxidation.These surface functional groups are generally classif i ed as acidic, basic or neutral.Carboxylic,anhydride and lactone are acidic groups, andphenolicandquininegroupsareneutralorweaklyacidic,whilefunctionalities like pyrone,chromene,ether and carbonyl are responsible for basic properties of the carbon surface.Table 2 presents the Boehm titration results.The amount of groups is reported in terms of surface concentration(μmol·g−1).For both carbons after the HCl and HNO3treatments, the number of acidic groups mainly including carboxylic and phenolic groups increase with a decrease in the number of basic groups.These changes are more signif i cant for AC-HNO3than for AC-HCl.Reversely, the modif i cation with NaOH results in an increase in the number of basic groups at the expense of acidic groups.The sum of acidic and basic surface group concentrations is the largest for AC-HNO3,followed by AC-NaOH,AC-HCl,and the smallest for the initial carbon(AC0).
pHPZCis the pH at which the net surface charge of an adsorbent is zero,which can be considered an excellent reference index for correlatingchanges in thesurface acidity/basicity ofcarbons.Thevalues of pHPZCfor various carbon samples are also shown in Table 2.It is clear that the untreated activated carbon(AC0)and AC-NaOH show a basic character.The basicity of AC-NaOH is slightly higher than that of AC0. AC-HCl is neutral and AC-HNO3is acidic.It is in agreement with the resultsofBoehmtitration.Generally,theacid-basepropertiesofactivated carbon may have two separate origins:chemical composition of ashes and surface functional groups.Here,theresults ofash contentdetection indicate that all adsorbents contain a very low amount of mineral matter,below0.2%.Thecasesuggeststhatacid-basepropertiesarenotcontrolled by ashes but by surface functional groups.
Table3showsthetexturalparametersofAC0,AC-HCl,AC-HNO3and AC-NaOH.As shown,theoriginal activatedcarbon(AC0)has a high surface area and a well-developed porosity,and the type of the treatment greatlyaffectsthetexturalpropertiesofthematerials.Noevidentdifference in the textural properties is noticed between AC0and AC-HCl.A small decrease in the SBET,Smicro,Vtotaland Vmicrois observed following the NaOH treatment.However,the HNO3treatment results in a clear fall in the SBET,Smicro,Vtotaland Vmicroand a slight increase in the Dave. As expected,the change in the textural properties in AC-HNO3and AC-NaOH compared with AC0is attributed to the presence of a higher amount of surface oxygen functional groups in pores caused by the NaOH or HNO3treatment that may block some pore entrances.
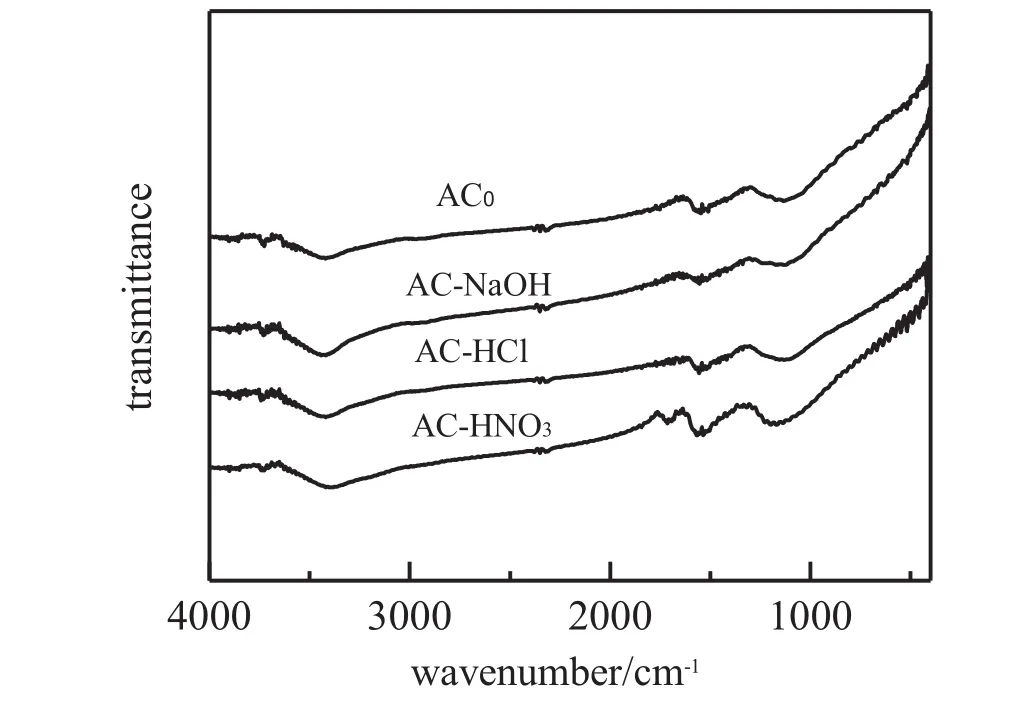
Fig.1.FT-IR spectra of unmodif i ed and modif i ed activated carbon adsorbents.
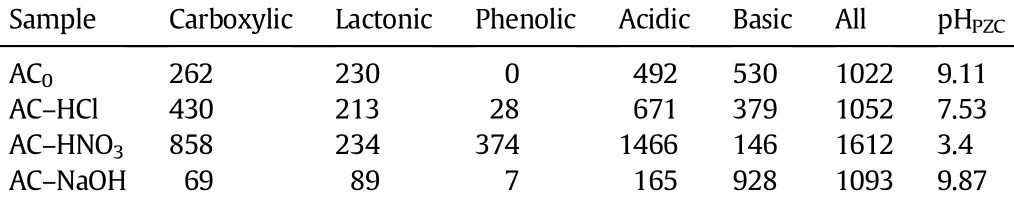
Table 2Results of Boehm titration in μmol·g−1and values of pHPZCfor activated carbon adsorbents

Table 3Pore structure characteristics of activated carbon adsorbents
3.2.Effect of adsorption temperature
Fig.2 depicts the contents of HFC-1234ze,HCFC-1233zd,HCFC-1224zb and HCFC-1224xe in the eff l uent after the adsorption of HFC-245fa product over AC-HCl adsorbent for 0.5 h asa function of the temperature from 303 to 363 K.When the adsorption temperature is under 333 K,the contents of the four halo-olef i ns in the eff l uent drop to under 5 mg·kg−1with adsorption eff i ciencies of over 95%.Above 333 K with the increase in the adsorption temperature,the contents of halo-olef i ns in the eff l uent increase,which are more pronounced for HFC-1234ze and HCFC-1233zd.This case suggests that the adsorption performance of AC-HCl for these halo-oleins from their mixture with HFC-245fa decreases with the increase of adsorption temperature.The suitable adsorption temperature for the removal of halo-olef i ns impurities from the HFC-245fa product over activated carbon is under 333 K.
The adsorption of HFC-245fa molecules on the activated carbon can take place by the dipole-dipole interactions and dispersive forces with the carbon surface groups,which depends on the dipolar moment and polarizabilityofmoleculesrespectively[25].However,intheadsorption of halo-olef i ns on the activated carbon,it is different from the adsorption mechanism of HFC-245fa in that there are dispersion interactions between the π-electrons of the halo-olef i ns and the π-electrons of carbongraphitelayerbesidesdipole-dipoleinteractionsandthedispersive forces[26].Hence,the preferential adsorption of halo-olef i nsfrom their mixture with HFC-245fa on the activated carbon can be explained in terms of the π-π dispersion interactions between the halo-olef i ns and the carbon graphite layer.Similar π-π dispersion interactions have been proposed in theadsorption of aromatic compounds on theactivated carbon[10,12].All of the dipole-dipole interaction,the dispersive force depending on the polarizability of molecular and the π-π dispersion interaction are basically in the form of the van der Waals interactions.Thus,the adsorptions of these halo-olef i ns and HFC-245fa onthe activated carbon belong to physisorption.With the increase of adsorption temperature,theseinteractionsareweakened[14]and thedifference in the interactions between halo-olef i ns and HFC-245fa with carbon surface reduces,consequently resulting in the deterioration of adsorptiveabilityofactivatedcarbonforhalo-olef i nsfromtheirmixture with HFC-245fa.
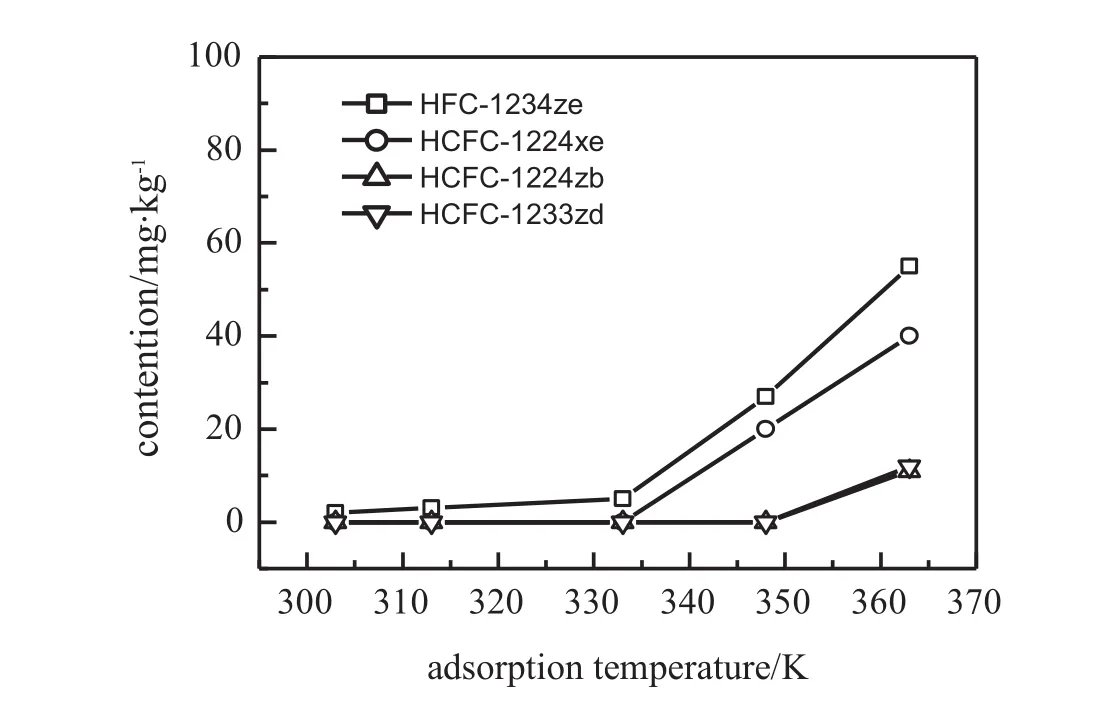
Fig.2.Adsorption of halo-olef i nic impurities in HFC-245fa product feed on AC-HCl at different temperatures.
3.3.Effect of the treatment type for activated carbon
Ithasbeenreportedthattheadsorptiveorcatalyticperformanceofactivated carbon can be inf l uenced by the treatment type.Table 4 presents the contents of HFC-1234ze,HCFC-1233zd,HCFC-1224zb and HCFC-1224xe in the eff l uent after the adsorption of HFC-245fa product over AC0,AC-HCl,AC-HNO3or AC-NaOH for 0.5 h,respectively.Comparing Table 1 with Table 4,when AC0,the activated carbon without any treatment,is used as the adsorbent,HCFC-1233zd,HCFC-1224zb and HCFC-1224xe could be effectively adsorbed from HFC-245fa product,with adsorption eff i ciencies of96%,98%and 92%,respectively,buttheadsorption effecton HFC-1234zeisnotvisible.When the activatedcarbon treatedby HCl or HNO3(AC-HCl or AC-HNO3)is used as the adsorbent,all of the above mentioned four halo-olef i ns could be substantially removed from HFC-245fa product and the content of halo-olef i ns in the eff l uent decreases to below 5 mg·kg−1.Adsorption eff i ciencies are about 96%for HFC-1234ze and exceed 98%for the other three halo-olef i ns.However, forthe activated carbontreatedwithNaOH(AC-NaOH)asthe adsorbent, the content of HFC-1234ze in the eff l uent distinctly increases and is higher than that in HFC-245fa product,although the contents of HCFC-1233zd,HCFC-1224zb and HCFC-1224xe in the eff l uent decrease to below 5 mg·kg−1and the adsorption eff i ciency exceeds 99%.The content of HFC-1234ze in the eff l uent has a positive correlation with the basicity of activated carbon adsorbents.The higher the basicity of the activated carbon,the higher the content of HFC-1234ze in the eff l uent. The appearance of HFC-1234ze in the eff l uents over AC0and AC-NaOH may be attributed to the decomposition of a small amount of HFC-245fa inwhichtheremovalofamoleculeofHFfromHFC-245faleadstotheformation of HFC-1234ze,because it is observed that the content of HFC-245fa in the eff l uents over AC0and AC-NaOH is slightly lower than that in the eff l uents over AC-HCl and AC-HNO3.These results mean that the acid treatment for activated carbon favors the removal of the four haloolef i ns from HFC-245fa product,while the base treatment for activated carbon results in the decomposition of a few of HFC-245fa.
Besides being widely used as an adsorbent,the catalytic performanceoftheactivatedcarbonhasbeeninvestigatedinhydrogenperoxide decomposition[27],oxidative dehydrogenation of isobutene[28], direct dehydrogenation of propane to propylene[29],and so on[30]. The oxygen surface chemistry and consequently the acidic or basic nature of activated carbon have been reported to have an inf l uence on the catalytic performance.The basic groups of activated carbon,such as carbonyl and quinine,are found to be the catalytic sites for the above reactions[27-30].In this work,the catalytic effect of mineral matter in AC adsorbents can be eliminated due to the very low content of ashes.Thus,it is plausible to think that the basic groups of activated carbon can catalyze the decomposition of HFC-245fa.The increase in the basicity of activated carbon adsorbent results in the enhancement in the decomposition of HFC-245fa.

Table 4Contents of halo-olef i ns and HFC-245fa in eff l uent after the adsorption of the HFC-245fa product over various activated carbon adsorbentsa
3.4.Breakthrough experiments
Thebreakthroughexperimentofhalo-olef i nsintheHFC-245faproduct on activated carbon sorbents was carried to estimate the breakthrough adsorption capacities of sorbents for halo-olef i ns.Fig.3(a)to (d)shows the breakthrough curves of HFC-1234ze,HCFC-1233zd, HCFC-1224zb and HCFC-1224xe in the HFC-245fa product on the ACHCl and AC-HNO3,respectively.The breakthrough adsorption capacity of AC-HCl or AC-HNO3for each halo-olef i n is calculated by integrating the area under the breakthrough curve of halo-olef i n and subtracting it from the total halo-olef i n input up to the point of breakthrough.The breakthrough time is def i ned as the point at which the halo-olef i n concentration in the HFC-245fa eff l uent reached 10 mg·kg−1.Obviously, the breakthrough time of each halo-olef i n on AC-HCl is longer than that on AC-HNO3.The breakthrough adsorption capacities of AC-HCl for HFC-1234ze,HCFC-1233zd,HCFC-1224xe and HCFC-1224zb are 0.14,1.75,0.35 and 0.43 mg·g−1,respectively.The breakthrough adsorption capacities of AC-HNO3for HFC-1234ze,HCFC-1233zd,HCFC-1224xe and HCFC-1224zb,respectively,are 0.09,1.36,0.29 and 0.35 mg·g−1,respectively.Obviously,the breakthrough adsorption capacities of AC-HCl for halo-olef i ns are higher than those of AC-HNO3.
A larger uptake of AC-HCl compared with AC-HNO3can be explained by differences in the surface groups and textural structure. From Table 2,it is observed that treating activated carbon by HCl or HNO3increases surface acidic groups while it decreases surface basic groups.However,compared with the HCl treatment,the HNO3treatmentcauses a distinct increasein theamountofsurfaceacidicfunctional groups due to the strong oxidation effect of HNO3.An introduction of acidic functional groups may cause the π-electrons to be removed from thecarbon matrix,consequently leadingto a decrease in the strength of the π-π dispersive interactions between halo-olef i n molecules and carbonbasalplanes[10-17].Thus,theπ-πdispersiveinteractionsbetween halo-olef i ns and AC-HCl are stronger than that between the haloolef i ns and AC-HNO3.On the other hand,it has been reported that the volume of micropores is an important parameter when selecting an activated carbon for the adsorption of volatile organic compounds(VOCs) such as benzene and toluene[31],because the primary adsorption ofthese VOCs takes place in the micropores where the adsorption potential is strongly enhanced.In general,the higher the volume of micropores,the higher the breakthrough time.Here,the primary adsorption of these halo-olef i ns and HFC-245fa on the activated carbon should also occur in the micropores due to their smaller molecule size. Table 3 shows that the treatment of activated carbon by HNO3results in a clear decrease in the surface area and micropore volume whiletheHCltreatmenthardlychangesthetexturalproperties,contributing thus to the reduced halo-olef i ns and HFC-245fa uptake for ACHNO3compared with AC-HCl.Hence,the higher number of surface acidic functional groups and the lower micropore volume and surface area lead to the smaller breakthrough adsorption capacities of ACHNO3for halo-olef i ns from the HFC-245fa product feed compared with AC-HCl.
Commonly,the chemical properties of adsorbates could inf l uence their adsorption behaviors.The breakthrough times of HFC-1234ze, HCFC-1233zd,HCFC-1224xe and HCFC-1224 zb on AC-HCl were 200, 290,410,490 min,respectively,indicating that the adsorption aff i nity of AC-HCl towards these halo-olef i ns increases in the order of HFC-1234ze<HCFC-1233zd<HCFC-1224xe<HCFC-1224zb.In general,the polarizability of the molecule increases with the increase of molecular mass. The molecular masses of HFC-1234ze,HCFC-1233zd,HCFC-1224xe and HCFC-1224zb are 114,130.5,148.5 and 148.5 g·mol−1,respectively. Hence,the dispersive forces of these halo-olef i ns with activated carbon, which depend on the polarizability of the molecules,should increase in the order of HFC-1234ze<HCFC-1233zd<HCFC-1224xe≈HCFC-1224zb.The dipole moment of the molecule usually increases with the decrease of the molecular symmetry.Thus the dipole-dipole interactions of the HCFC-1224zb molecules with the surface groups of activated carbon are higher than that between the HCFC-1224xe and carbon surface due to the lower symmetry of the HCFC-1224zb molecule compared withtheHCFC-1224xemolecule.Brief l y,themolecularmassandthemolecular symmetry of these halo-olef i ns have an important role in their retention time on activated time.The larger the molecular mass and the lower the molecular symmetry,the longer the retain time.

Fig.3.Breakthrough prof i les of halo-olef i nic impurities in HFC-245fa product feed at 363 K on columns packed with AC-HCl and AC-HNO3,respectively.(a)HFC-1234ze;(b)HCFC-1233zd;(c)HCFC-1224xe;(d)HCFC-1224zb.
3.5.Adsorbent regeneration
Thermal desorption is considered as an option for spent sorbent regeneration.Fig.4showsthebreakthroughbehaviorsofHCFC-1224zbon fresh AC-HCl and on the twentieth regenerated AC-HCl.Compared with the original AC-HCl,the breakthrough behaviors of HCFC-1224zb on the twentieth regenerated AC-HCl are very similar.The breakthroughbehaviorsoftheotherhalo-olef i nsontheabovementionedadsorbents(not shown here)are identical with those of HCFC-1224zb. This indicates that heating to 523 K in an inert atmosphere has no impact on the adsorptive ability of AC-HCl and the spent AC-HCl could be regenerated by thermal desorption without capacity loss.The excellentthermal regeneration behaviorofAC-HCl couldbeattributedtothe physisorption of these halo-olef i ns and HFC-245fa on it.
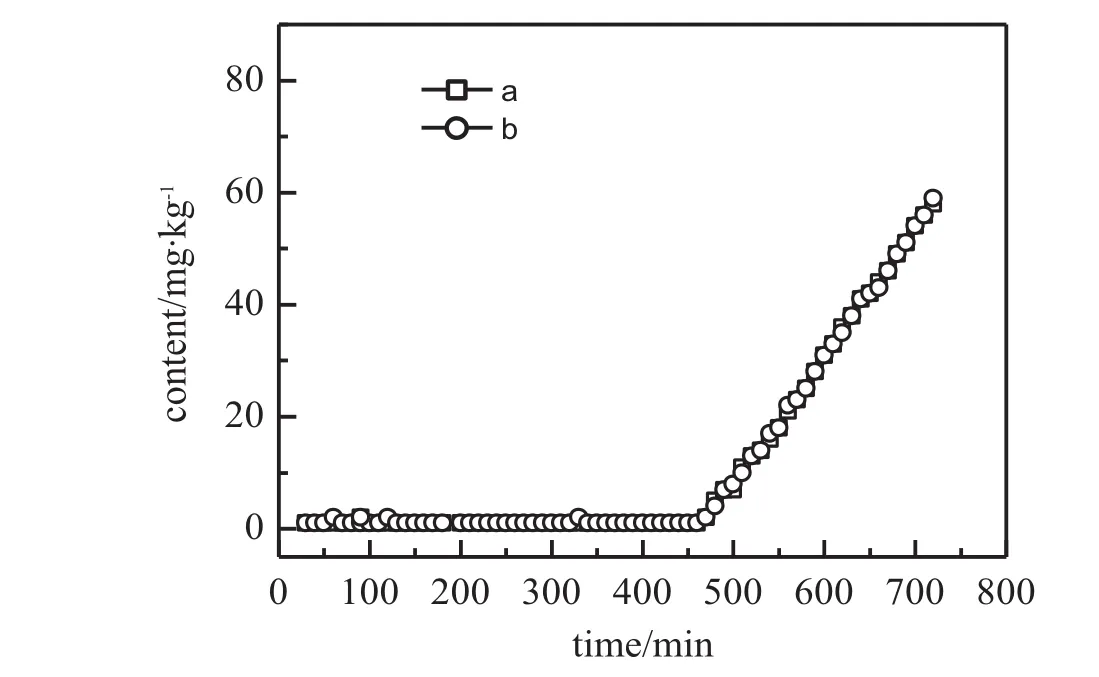
Fig.4.Breakthrough prof i les of HCFC-1224zb in HFC-245fa product at 363 K on columns packed with fresh AC-HCl(a)and the twentieth regenerated AC-HCl(b),respectively.
4.Conclusions
HFC-1234ze,HCFC-1233zd,HCFC-1224xe and HCFC-1224zb impurities can be substantially removed from the HFC-245fa product after distillation via the adsorption over activated carbon when the adsorptiontemperatureisunder333K,whichcanbeattributedtotheπ-πdispersion interactions between the halo-olef i ns and the carbon graphite layer.The surface acidities/basicities of activated carbon adsorbents have an important role in their catalytic/adsorptive behaviors.The basic surface groups of activated carbon could catalyze the decompositionofHFC-245fatoformHFC-1234ze.Thehigherthebasicityoftheactivated carbon,the higher the decomposition degree of HFC-245fa. However,thesignif i cantincreaseintheamountofacidic surfacegroups of activated carbon(HNO3treatment)leads to a distinct decrease of adsorption capacity for halo-olef i nic impurities due to the reductions in surface area and micropore volume of the adsorbent,and a decrease in the strength of the π-π dispersive interactions between halo-olef i n molecules and carbon basal planes resulting from the removal of πelectrons from the carbon matrix.The excellent thermal regeneration behavior of activated carbon adsorbent could be attributed to the physisorption of these halo-olef i ns and HFC-245fa on it.The presentinvestigation shows that the activated carbon treated by HCl is a promising low-cost adsorbent to be used in the removal of halo-olef i nic impurities from the HFC-245fa product after distillation.
The breakthrough time of halo-olef i nic impurities on the activated carbon adsorbent increases with the increase of molecular mass and the decrease of molecular symmetry.
[1]H.D.Quan,H.E.Yang,M.Tamura,A.Sekiya,Preparationof 1,1,1,3,3,-pentaf l uoropropane(HFC-245fa)by using a SbF5-attached catalyst,J.Fluor.Chem. 128(2007)190-195.
[2]V.P.Zhelezny,Y.V.Semenyuk,S.N.Ancherbak,A.J.Grebenkov,O.V.Beliayeva,An experimental investigation and modeling of the solubility,density and surface tension of 1,1,1,3,3-pentaf l uoropropane(R-245fa)/synthetic polyolester compressor oil solutions,J.Fluor.Chem.128(2007)1029-1038.
[3]P.Pascal,Process for the purif i cation of 1,1-dif l uoroethane,US Patent(1995) 5396001.
[4]Y.F.Stephen,Process for removing dicloroacetylene from 1,1-dichloro-1-fl uoroethane and/or vinylidene chloride,US Patent(1990)4940825.
[5]C.Stuart,M.J.Charles,Removal of(hydro)haloalene impurities from product stream, US Patent(2006)7084315.
[6]B.Zhang,N.F.Shi,C.L.Xu,H.F.Lu,Y.F.Chen,Z.H.Ge,Adsorptive removal of haloolef i nic impurities from 1,1,1,3,3-pentaf l uoropropane over ion-exchanged Y zeolites,J.Fluor.Chem.131(2010)554-560.
[7]Y.F.Wang,H.Z.Gao,R.Yeredla,H.F.Xu,M.Abrecht,Control of Pertechnetate sorption on activated carbon by surface functional groups,J.Colloid Interface Sci.305 (2007)209-217.
[8]N.G.Asenjo,P.Álvarez,M.Granda,C.Blanco,R.Santamaría,R.Menéndez,High performance activated carbon for benzene/toluene adsorption from industrial wastewater,J.Hazard.Mater.192(2011)1525-1532.
[9]D.Richard,M.L.D.Núñez,D.Schweich,Adsorption of complex phenolic compounds on active charcoal:adsorption capacity and isotherms,Chem.Eng.J.148(2009)1-7.
[10]A.D.Marczewska,A.Swiatkowski,S.Biniak,M.Walczyk,Effect of properties of chemically modif i ed activated carbon and aromatic adsorbate molecule on adsorption from liquid phase,Colloids Surf.A Physicochem.Eng.Asp.327(2008)1-8.
[11]M.Franz,H.A.Arafat,N.G.Pinto,Effect of chemical surface heterogeneity on the adsorption mechanism of dissolve aromatics on activated carbon,Carbon 8(2000) 1807-1819.
[12]A.P.Terzyk,Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption,J.Colloid Interface Sci.268(2003)301-329.
[13]F.C.Wu,R.L.Tseng,High adsorption capacity NaOH-activated carbon for dye removal from aqueous solution,J.Hazard.Mater.152(2008)1256-1267.
[14]A.P.Terzyk,Molecular properties and intermolecular forces—factors balancing the effect of carbon surface chemistry in adsorption of organics from dilute aqueous solutions,J.Colloid Interface Sci.275(2004)9-29.
[15]S.B.Wang,Z.H.Zhu,Effects of acidic treatment of activated carbons on dye adsorption,Dyes Pigments 75(2007)306-314.
[16]I.I.Salame,T.J.Bandosz,Role of surface chemistry in adsorption of phenol on activated carbons,J.Colloid Interface Sci.264(2003)307-312.
[17]N.Wibowo,L.Setyadhi,D.Wibowo,J.Setiawan,S.Ismadji,Adsorption of benzene and toluene from aqueous solutions onto activated carbon and its acid and heat treated forms:inf l uence of surface of surface chemistry on adsorption,J.Hazard. Mater.146(2007)237-242.
[18]B.H.Hameed,I.A.W.Tan,A.L.Ahmad,Adsorption isotherm,kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon, Chem.Eng.J.144(2008)235-244.
[19]A.M.Puziy,O.I.Poddubnaya,A.Martinez-Alonso,F.Suárez-Garcia,J.M.D.Tascón, Synthetic carbons activated with phosphoric acids.I.Surface chemistry and ion binding properties,Carbon 40(2002)1493-1505.
[20]J.M.Valenta,Nabais,P.J.M.Carrote,M.M.L.Ribeiro,Carrott,J.A.Menéndez,Preparation and modif i cation of activated carbon f i ber by microwave heating,Carbon 42 (2004)1315-1320.
[21]B.K.Hamad,A.M.Noor,A.R.Af i da,M.N.MohdAsri,Highremovalof 4-chloroguaiacol by high surface area of oil palm shell-activated carbon activated with NaOH from aqueous solution,Desalination 257(2010)1-7.
[22]J.W.Yu,M.Yang,T.F.Lin,Z.H.Guo,Y.Zhang,J.N.Gu,S.X.Zhang,Effects of surface characteristics of activated carbon on the adsorption of 2-methylisobornel(MIB) and geosmin from natural water,Sep.Purif.Technol.56(2007)363-373.
[23]A.P.Terzyk,The inf l uence of activated carbon surface chemical composition on the adsorption of acetaminophen(paracetamol)in vitro.Part II.TG,FTIR,and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH,Colloids Surf.A Physicochem.Eng.Asp.177(2001)23-45.
[24]P.Chingombe,B.Saha,R.J.Wakeman,Surface modif i cation and characterization of a coal-based activated carbon,Carbon 43(2005)3132-3143.
[25]M.C.Almazán-Almazán,M.Pérez-Mendoza,M.Domingo-Carcía,I.Fernández-Morales, F.D.Rey-Bueno,A.García-Rodríguez,F.J.López-Garzón,Theroleof theporosityandoxygengroupsontheadsorptionofn-alkanes,benzene,trivhloroethyleneand1,2-dichloroethane on active carbons at zero surface coverage,Carbon 45(2007)1777-1785.
[26]S.A.Al-Muhtaseb,Role of catalyst type intheselective separation of olef i nic and paraff i nic hydrocarbons using xerogel-based adsorbents,Carbon 16(2008)1003-1009.
[27]A.Rey,J.A.Zazo,J.A.Casas,A.Bahamonde,J.J.Rodriguez,Inf l uence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen peroxide,Appl.Catal.A Gen.402(2011)146-155.
[28]J.J.D.Velásquez,L.M.C.Suárez,Oxidative dehydrogenation of iaobutane over activated carbon catalysts,Appl.Catal.A Gen.311(2006)51-57.
[29]L.Liu,Q.F.Deng,Y.P.Liu,T.Z.Ren,Z.Y.Yuan,HNO3-activatedmesoporouscarbon catalyst for direct dehydrogenation of propane to propylene,Catal.Commun.16(2011) 81-85.
[30]J.L.Figueiredo,M.F.R.Pereira,The role of surface chemistry in catalysis with carbons, Catal.Today 150(2010)2-7.
[31]M.A.Lillo-Ródenas,A.J.Fletcher,K.M.Thomas,D.Cazorla-Amorós,A.Linares-Solano, Competitive adsorption of a benzene-toluene mixture on activated carbons at low concentration,Carbon 44(2006)1455-1463.
6 November 2012
☆Supported by the Major Project of Green Chemical Industry of Zhejiang Province (2007C11043).
*Corresponding author.
E-mail address:zb10006093@zjut.edu.cn(B.Zhang).
http://dx.doi.org/10.1016/j.cjche.2014.06.020
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 11 January 2013
Accepted 17 May 2013
Available online 29 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
