Novel Gas-assisted Three-liquid-phase Extraction System for Simultaneous Separation and Concentration of Anthraquinones in Herbal Extract☆
2014-07-25XingfuYangXiangfengLiangLiangrongYangFengPanFuliDengHuizhouLiu
Xingfu Yang,Xiangfeng Liang*,Liangrong Yang,Feng Pan,Fuli Deng,Huizhou Liu*,3
Separation Science and Engineering
Novel Gas-assisted Three-liquid-phase Extraction System for Simultaneous Separation and Concentration of Anthraquinones in Herbal Extract☆
Xingfu Yang1,2,Xiangfeng Liang*,1,Liangrong Yang1,Feng Pan1,Fuli Deng1,2,Huizhou Liu*,1,3
1Key Laboratory of Green Process and Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China2University of Chinese Academy of Sciences,Beijing 100049,China3National Key Laboratory of Biochemical Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
A R T I C L EI N F O
Article history:
Three-liquid-phase extraction
Gas-assisted solvent extraction
Separation
Anthraquinones
Gas-assisted three-liquid-phase extraction(GATE),which has the advantages of both three-liquid-phase extraction and solvent sublation,is a novel separation technique for separation and concentration of two organic compounds into different phases in one step.This highly effective and economically applicable method has been developed for separating emodin and rhein from herbal extract.In a GATE system composed of butyl acetate/ PEG4000/ammonium sulfate aqueous solution,inf l uence of various parameters including gas f l ow rate,f l otation time,saltconcentration,initialvolumeofPEGandbutylacetatewasinvestigated.Within50minof30 ml·min−1nitrogen f l ow,removal ratio of emodin and rhein from aqueous phase could be over 99%and 97%,respectively. Mass fraction of emodin in the BA phase and rhein in the PEG phase could reach 97%and 95%,respectively.It is demonstrated that gas bubbling is effective for partitioning of emodin and rhein into butyl acetate and PEG phase respectively,and dispersed PEG and butyl acetate could be captured from the aqueous solution.ExperimentalresultsshowthatGATEcouldbeaneffectiveandeconomicaltechnologyforconcentrationandseparation of co-existed products in medicinal plants.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Anthraquinones are the major biologically active ingredients of some most popular traditional medicinal herbs,e.g.Rhubarb and Polygonum cuspidate.Previous pharmacology studies have demonstrated that anthraquinones have various bioactivities,such as antitumor,antifungal,antiplasmodial,antiviral and virucidal activity[1-5].Recently, various technologies have been reported for the separation of anthraquinones from medicinal plant,such as ultrasonic extraction,supercritical CO2extraction,high-speed countercurrent chromatography,and aqueous two-phase extraction[6-11].Although some exciting results have been achieved with these methods,some inevitable shortcomings limit their further applications,including sophisticated equipment, harshcondition,highenergyconsumptionandcumbersomeprocedure. Besides,few studies have been reported concerning the separation of co-existing anthraquinones from each other.
Three-liquid-phase extraction system(TES),composed of three coexisting liquid phases,is a promising alternative to the traditional solvent extraction because of its outstanding advantages[12,13].The physicochemical propertiesof threeco-existingphases in TESaretunable bymanipulatingvarious factors and itiseasytoscale-up,so that TES provides potential to separate two or more target compounds selectively through one-step process[14].TES has been successfully applied to concentration of natural products[15,16],antibiotic separation[17,18],phenolic wastewater treatment[19-22]and multimetal separation[23-30]. However,in a TES process,the intensive agitation leads to loss of organic solvent and polymer and creates secondary contamination for aqueous solutions,which may reduce the separation eff i ciency of objectivechemicalcompounds.Inourpreviousreport,TESwasimproved by combination with solvent sublation,and the new technique was named gas-assisted three-liquid-phase extraction(GATE)[31].With the assistance of ascending gas stream,GATE could reduce the consumption of organic solvents and polymers and give higher concentration coeff icient comparing with TES.Therefore,it is more eff i cient,environment friendly and economically applicable in the extraction of natural product.
In this work,GATE is applied to isolate two coexisting anthraquinones in the simulated herbal extract.Because of their good surface activity,highly similar structures and properties(Fig.1),emodin and rhein are selected as the target compounds for separation and concentration.InaGATEsystemcomposedofbutyl acetate/PEG4000/ammonium sulfate aqueous solution,inf l uence of various parameters including gas f l ow rate,f l otation time,salt concentration,initial volume of PEG and butyl acetate is investigated.In the GATE process,organic phase,polymer phase and salt aqueous solution phase coexist in the column equipment as shown in Fig.2.As the ascending of gas bubbles,emodin and rhein dissolved in the aqueous phase can be extracted and concentrated into the organic phase and the polymer phase,respectively.
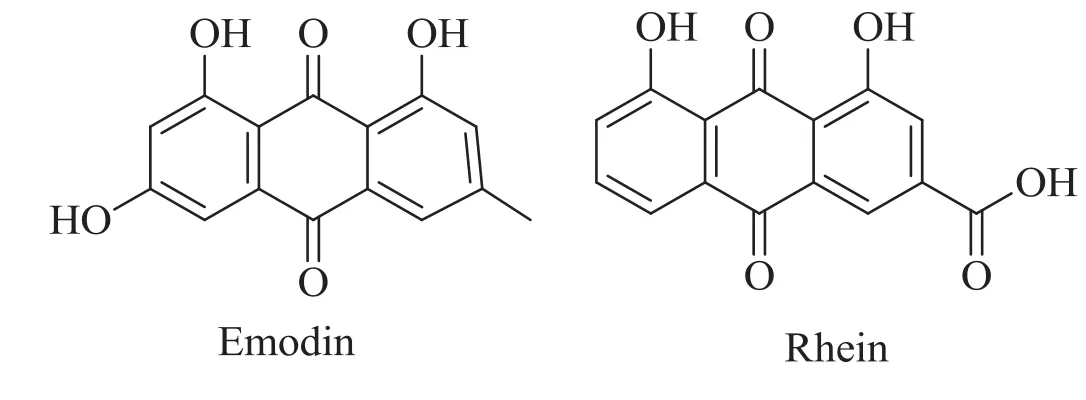
Fig.1.Chemical structures of emodin and rhein.
2.Materials and Methods
2.1.Chemicals and apparatus
Polyethylene glycol(PEG)4000 was purchased from China National Pharmaceutical Group Corporation.Emodin and rhein crude powder were purchased from Shaanxi Sciphar Hi-tech Industry Co.Ltd.,China, and the standards of emodin and rhein(purity>97%by HPLC)were purchased from National Institute for Food and Drug Control,China. Water was of HPLC grade.Butyl acetate(BA),ammonium sulfate and other chemicals were of analytical reagent grade purchased from Beijing Chemical Reagent Co.,Ltd.,China.
A pH meter(pH211,HANNA,Italy)was used to adjust the pH of the solution.For transfer convenience,stocksolutionsof ammonium sulfate with mass concentration of 40%and polymers with mass concentration of 50%were prepared.Simulated herbal stock solutions were prepared with purchased crude powder of emodin and rhein.Concentrations of emodin and rhein were approximately 30 ppm and were determined by HPLC(HP1100 AgilentTechnologies,U.S.)with ultraviolet spectrometer at 254 nm as detector.The analysis was performed with a C18 Zorbax ODS column(4.6 mm I.D.×150 mm,5 μm,Agilent,U.S.)at 20°C in thermostat.The mobile phase consisted of methanol and 1.0% aqueous acetic acid solution anda 20μl sample wasejected into thecolumn with a gradientelution of 75%-80%methanol at 0-15 min ata f l ow rate of 1.0 ml·min−1.Both calibration curves showed satisfactory linearity over the concentration range of(1-100)×10−6with correlation coeff i cients≥0.9999.
Fig.2 is the schematic diagram of the experimental device.The glass column isequippedwithaG4sinteredglasssparger(poresize3-4μm). The inner diameter of the column is 36 mm and the length is 450 mm. Three ports are open at different parts along the column to obtain samples of the three phases.
2.2.Optimization of different parameters
Based on our preliminary study,the optimum pH of the separation of emodin and rhein is 8.0.PEG 4000 is the most favorable polymer and BA isthebestorganicextractantconsideringthetoxicity,volatilityandcrosssolubility.Therefore,PEG 4000 with 50%(mass)water(for transfer convenience)and BA was used to construct the polymer phase and organic solvent phase in this study.The initial pH of all the systems was set at 8.0.Concentrations of emodin and rhein were determined by HPLC.VariousparametersofGATEsuchasgasf l owrate,gasbubblingtime,concentration of ammonium sulfate,initial volume of PEG phase and BA phase were optimized.All the initial volumes of aqueous phase were 320 ml, and all the separation processes were carried out at room temperature.
2.3.Separation procedure
The aqueous phase of the GATE was prepared by mixing of 40% (mass)ammonium sulfate solution,simulated herbal extract and water in a 500 ml beaker.Under magnetic stirring,solution pH was adjusted to 8.00±0.05 by adding sulfuric acid and sodium hydroxide solution.
In order to quantify the separation and concentration eff i ciency,the removalratiooftargetcompoundfromaqueousphase(Ei)andmassfraction of compound i in the three different phases(Wi,p)are def i ned as

Fig.2.Schematic diagram of gas-assisted three-liquid-phase extraction system.

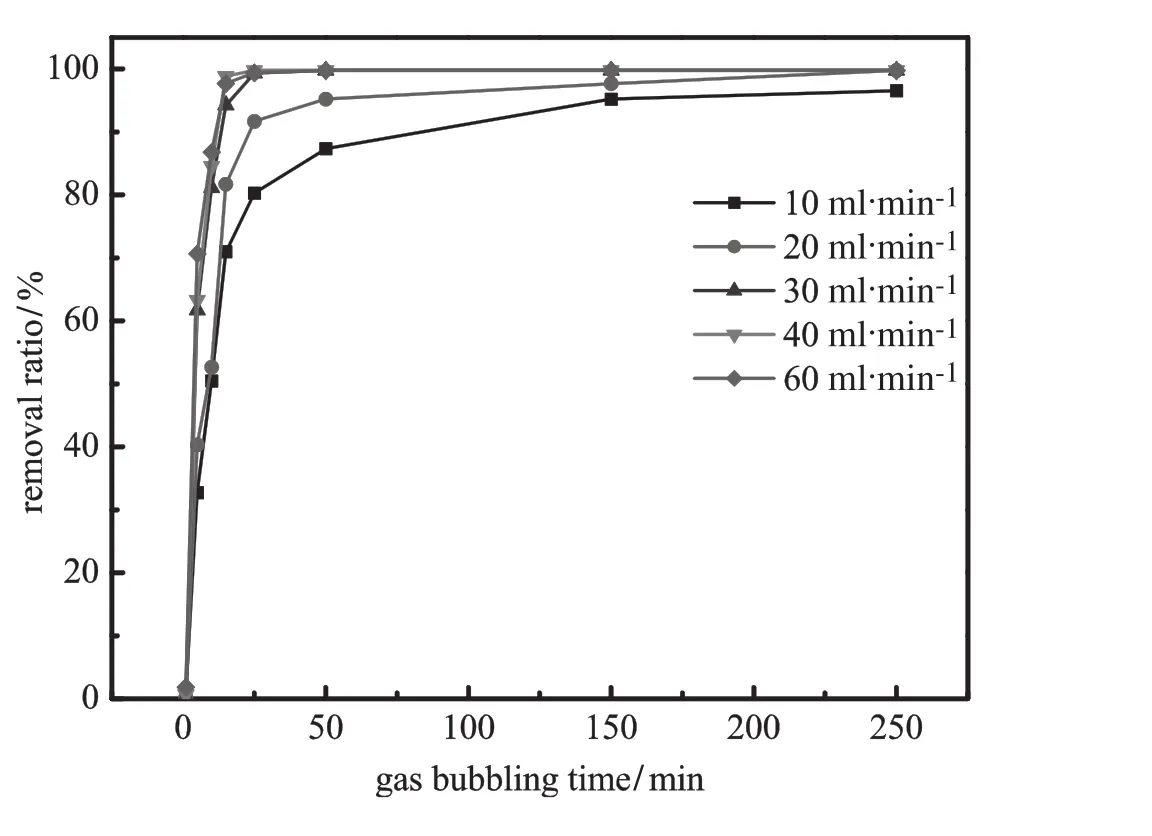
Fig.3.Effect of gas bubbling time on the removal ratio of emodin from aqueous phase (pH=8.0;salt concentration=20%;VPEG=40 ml;VBA=40 ml).

Fig.4.Effect of gas bubbling time on the removal ratio of rhein from aqueous phase (pH=8.0;ammonium sulfate 20%;VPEG=40 ml;VBA=40 ml).
The distribution ratio of emodin or rhein between two phases in the top,middle or bottom phase(Di,p1/p2),separation factor between two phases(Sp1/p2)and concentration coeff i cient of emodin or rhein in top and middle phases(αi,p)are def i ned as

2.4.Gas-assisted three-liquid-phase extraction process
In traditional operation of three-liquid-phase extraction,mechanical stirring or magnetic agitation is usually applied to accelerate mass transfer of target compounds between aqueous salt phase,polymer phase and organic phase.However,vigorous mixing also promotes the partition equilibrium of bulk phases and inevitably brings about the loss of phase-forming polymer and organic extractant.
The operation in GATE process follows a totally different mode.Instead of mechanical or magnetical agitation,solutes with surface activity are absorbed on or dissolved in the surface of ascending bubbles in theaqueousphase.Whenbubblesenterthepolymerphase,solutesrapidly transfer between the bubbles and polymer phase,and one kind of solute is strongly retained by the polymer phase.Then the bubbles go uptotheinterfaceofthepolymerphaseandorganicphase,andanother targetcompoundis primarily dissolved in the organic phase.Finallythe bubbles burst into droplets,and the droplets fall back into the polymer phase.
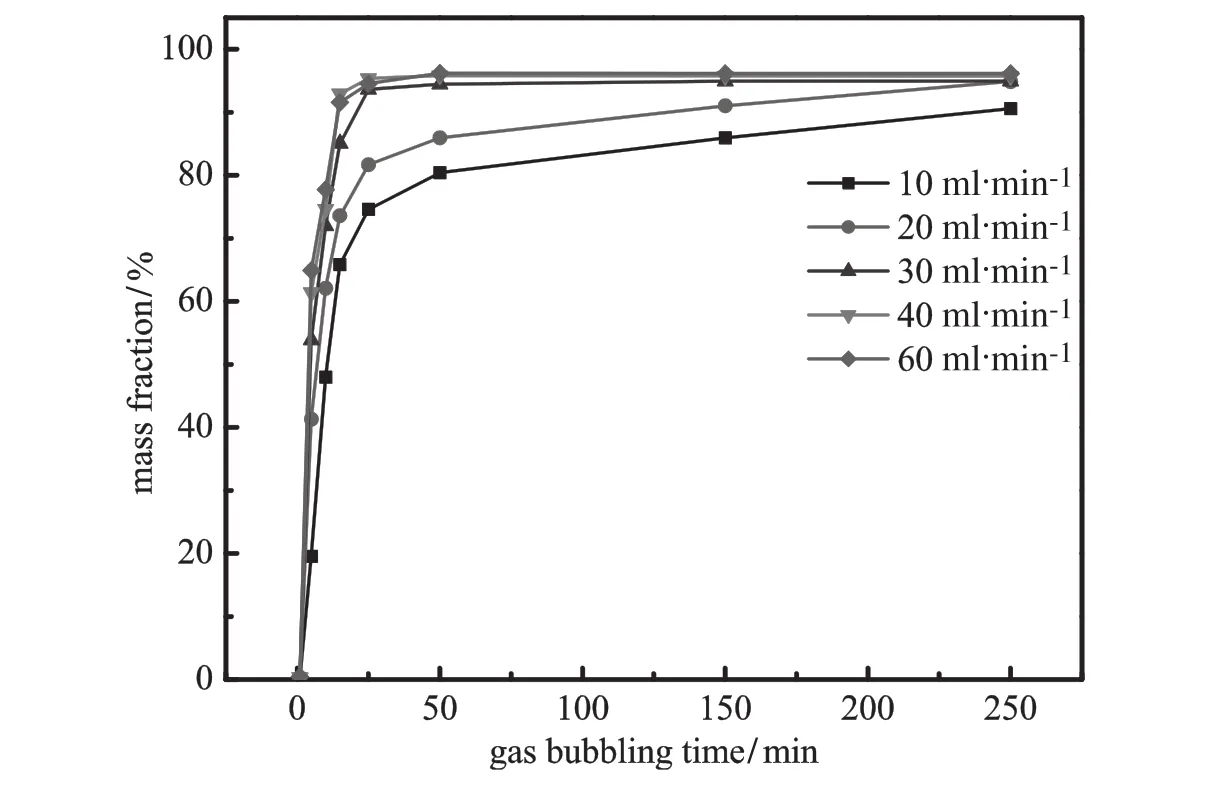
Fig.6.EffectofgasbubblingtimeonthemassfractionofrheininthePEGphase(pH=8.0; ammonium sulfate 20%;VPEG=40 ml;VBA=40 ml).
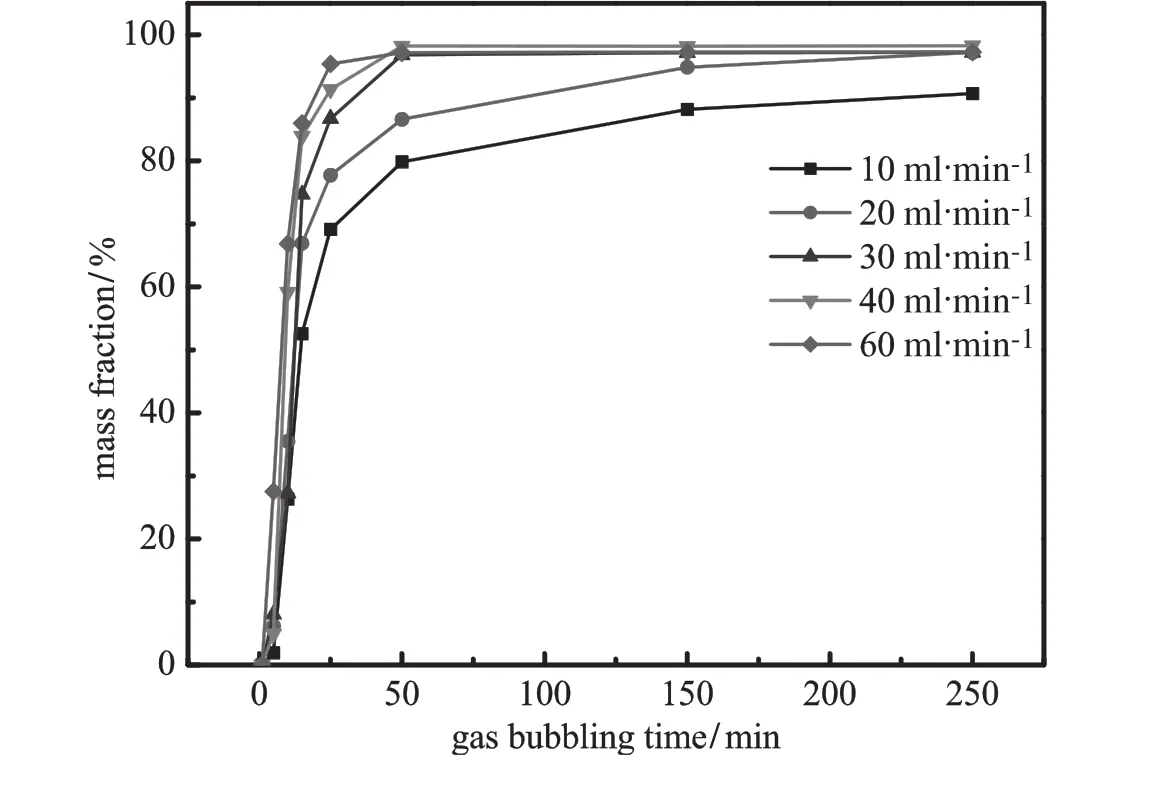
Fig.5.EffectofgasbubblingtimeonthemassfractionofemodinintheBAphase(pH=8.0; ammonium sulfate 20%;VPEG=40 ml;VBA=40 ml).
3.Results and Discussion
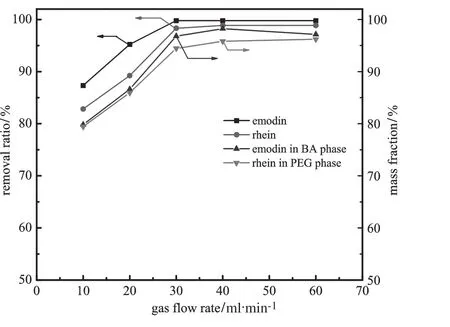
Fig.7.Effectof gasf l ow rate on theremovalratioofemodinandrheinand theirmassfractionsofintheBAphase andinthePEG phase(ammoniumsulfate20%;pH=8.0;gasbubbling time=50 min;VPEG=40 ml;VBA=40 ml).
3.1.Effect of gas bubbling time and gas f l ow rate
Figs.3 and 4 show the effect of gas bubbling time on the removal ratio of emodin and rhein from aqueous phase at different gas velocities.In the f i rst 15 min,the removal ratios of both emodin and rhein increase rapidly at all of the gas f l ow rates.Gas f l ow rate plays animportant role in terms of separation equilibrium time.Generally, high gas f l ow rate increases the overall area of gas-liquid interface and accelerates mass transfer,reducing the time to reach separation equilibrium.With low gas f l ow rate(10 and 20 ml·min−1),it may take approximately 250 min to reach equilibrium.At gas f l ow rate higher than 30 ml·min−1,separation equilibrium can be achieved in 25 min.Because of their physiochemical property difference[32, 33],emodin is more hydrophobic than rhein and is more likely to be absorbed on the bubble surface.Therefore,emodin is removed faster than rhein,and the remanent mass fraction in the aqueous phase is less.
AsshowninFigs.5and6,gasbubblingtimeandf l owratehavea similareffectonmassfractionofemodininBAphaseandrheininPEGphase. Massfractionofsolutesescalatesrapidlyinthemiddlephaseortopphase at initial stage,and then approaches gradually staple.Increasing gas f l ow rate is conducive to reduce operation time.However,there is recognizable difference between the mass fraction of emodin in the BA phase and that of rhein in the polymer phase.The increase of mass fraction of emodinlagsbehindthatofrhein.Theanthraquinonesloadedonthebubbles enterthepolymerphaselayerand solutestransferbetweenpolymer phase and bubbles.Part of emodin is retained in the PEG phase at f i rst. Then as bubbles keep ascending,emodin in PEG phase is gradually brought into the BA phase.Therefore,rhein is naturally extracted into the middle polymer phase faster than emodin,which needs more steps to transfer into the top phase.Besides,inf l uences of f l oatage and interfacial tension jointly affect the position of rising bubbles at the interface [34].With large amount of water,the interface tension of lower two phases is much smaller than that of the upper two phases[35].Hence, atthesamegasf l owrate,bubblescouldgothroughtheaqueous-polymer interface more easily than through the polymer-organic interface.
Fig.7 shows the effect of gas f l ow rate on the removal ratios of emodin and rhein from the aqueous phase and their mass fractions in the BA phase and polymer phase.At lower gas f l ow rates,the increase of gas f l ow rate expedites the removal of the two compounds and their enrichment in the BA and polymer phase separately.At gas f l ow rateshigherthan30 ml·min−1,operationtimeisreducedwhileremoval ratio and mass fraction of two compounds change little.Moreover, high gas f l ow rate would lead to a turbulent mixing at the interfaces and bubbles accumulate at the top of f l otation column because large number of bubbles could not burst rapidly enough.Considering the balance of separation eff i ciency and operation time,bubbling 50 min at 30 ml·min−1is selected as the optimum condition.3.2.Effect of salt concentration
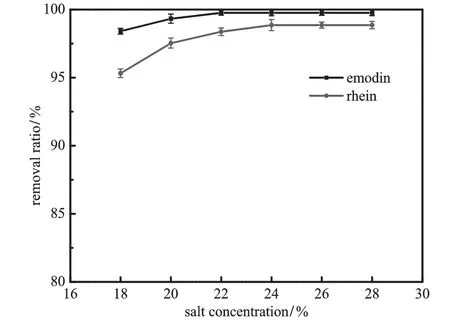
Fig.8.Effect of concentration of ammonium sulfate on the removal ratio of emodin and rhein(pH=8.0;VPEG=40 ml;VBA=40 ml;gas f l ow rate=30 ml·min−1;bubbling time=50 min).

Fig.10.EffectofvolumeofPEGphaseontheremovalratioofemodinandrhein(pH=8.0; VBA=40 ml;ammonium sulfate 24%;gas f l ow rate=30 ml·min−1;bubbling time= 50 min).
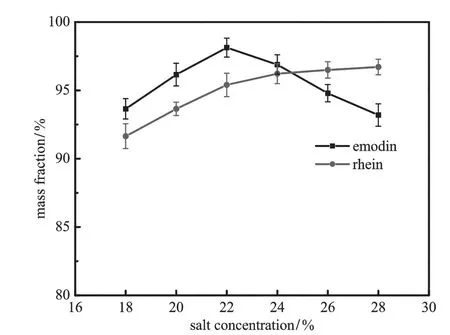
Fig.9.Effect of concentration of ammonium sulfate on the mass fraction of emodin in the BA phase and rhein in the PEG phase(pH=8.0;VPEG=40 ml;VBA=40 ml;gas lf ow rate=30 ml·min−1;bubbling time=50 min).
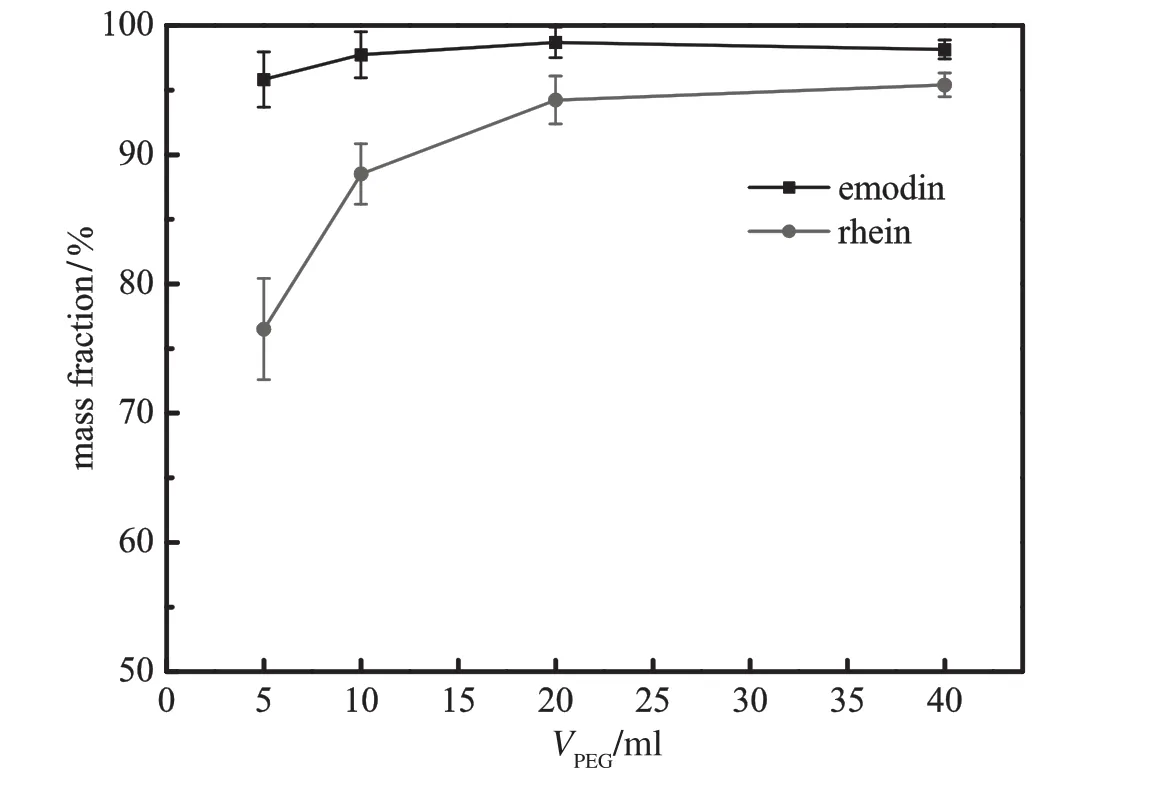
Fig.11.Effect of volume of PEG phase on the mass fraction of emodin in BA phase and rhein in PEG phase(pH=8.0;VBA=40 ml;ammonium sulfate 24%;gas f l ow rate= 30 ml·min−1;bubbling time=50 min).
In the GATE column,the three coexisting liquid phases could be viewedasthecombinationof aqueoustwo-phasesystem and traditional liquid-liquid extraction system.In this work,phase-forming salt concentration,an important parameter to maintain an immiscible twophase system[36]and affect separation eff i ciency in the aqueous twophase extraction system[37],is investigated in the GATE process.
Fig.8 shows the inf l uence of ammonium sulfate concentration on the removal ratio.With the increase of salt concentration,the removal ratio of two solutes increases.However,the separation behavior of emodin in BA phaseand rheinin PEGphaseare differentas shown inFig.9. As the ammonium sulfate concentration increases,mass fraction of emodin peaks while mass fraction of rhein keeps increasing.This could be attributedtosalting-outeffectofkosmotropicsalt.Duetothestructured water“lattice”around the ion of ammonium sulfate,the increase of its concentration would bind more water and dwindle the amount of free water[38]and therefore thesolubility of emodin and rheinin the aqueousphase.Thus theremovalratioof thetwo compounds increases with concentration.Because of their structuredifference,emodinismore hydrophobic and therefore easier to remove from aqueous phase than rhein.This explains why the removal ratio of emodin is higher than rhein over the salt concentration investigated.However,the saltingout effect of ammoniumsulfate plays differents roles in the partitioning behavior of emodin and rhein.Increasing salt content makes more solutes salt-out from aqueous phase and causes the dehydration of PEG segment,which increases the hydrophobicity in the PEG phase microenvironment.The combination of these two factors promotes the enrichment of rhein in the PEG phase.For emodin,salting-out effect increases the mass fraction of emodin in BA phase while more hydrophobic PEG microenvironment would retain more emodin and reduce the mass fraction of emodin in the BA phase.The balance of the two effects results in a maximum mass fraction of emodin.
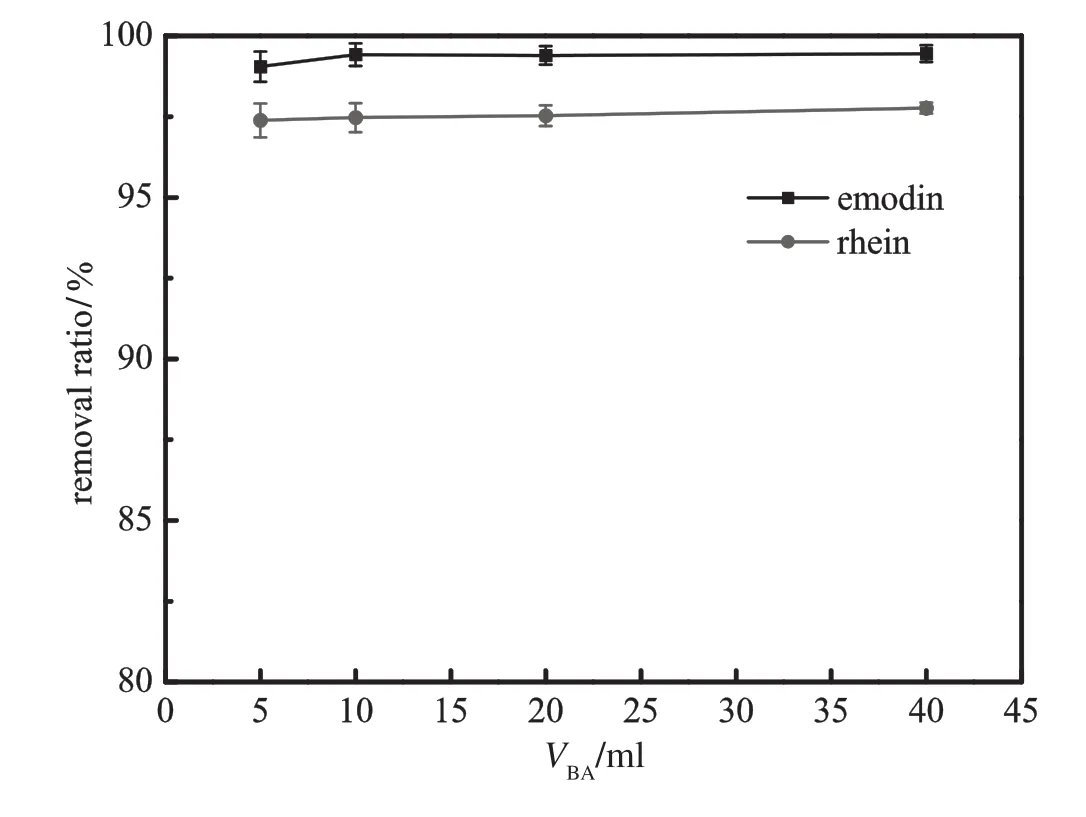
Fig.12.Effectof volume of BA phase on the removal ratio of emodinand rhein(pH=8.0; VPEG=20 ml;ammonium sulfate 24%;gas f l ow rate=30 ml·min−1;bubbling time= 50 min).
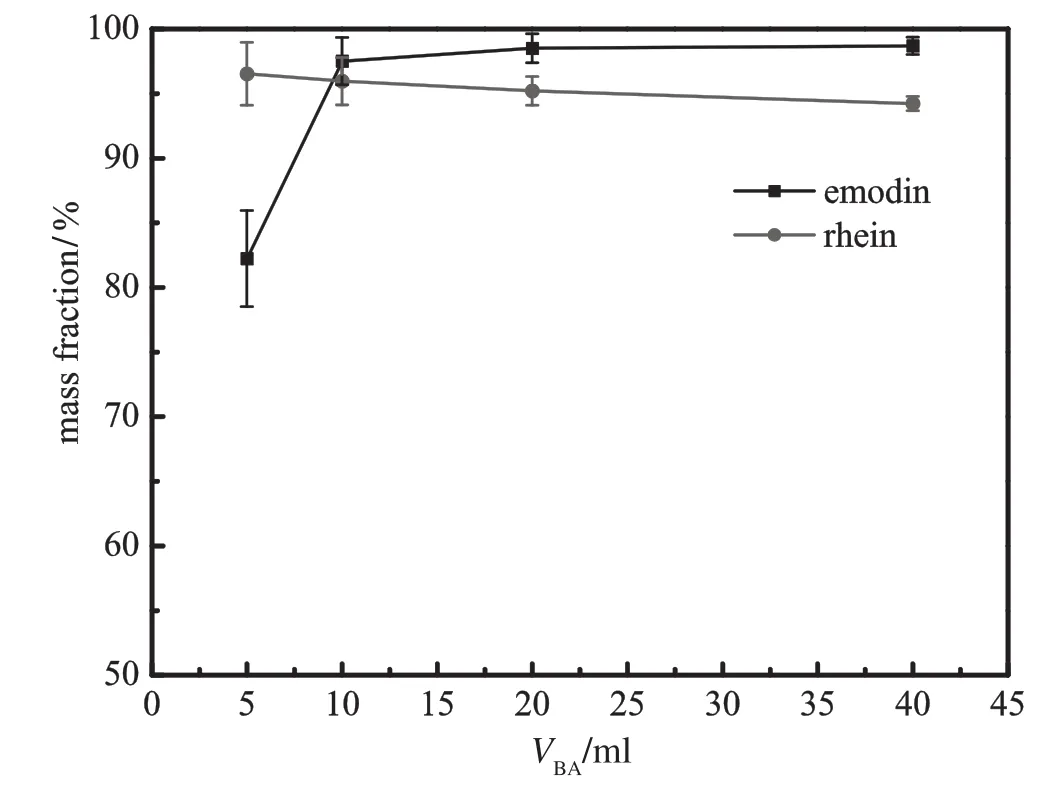
Fig.13.EffectofvolumeofBAphaseonthemass fraction of emodin inBAphase andrhein in PEG phase(pH=8.0;VPEG=20 ml;ammonium sulfate 24%;gas f l ow rate= 30 ml·min−1;bubbling time=50 min).
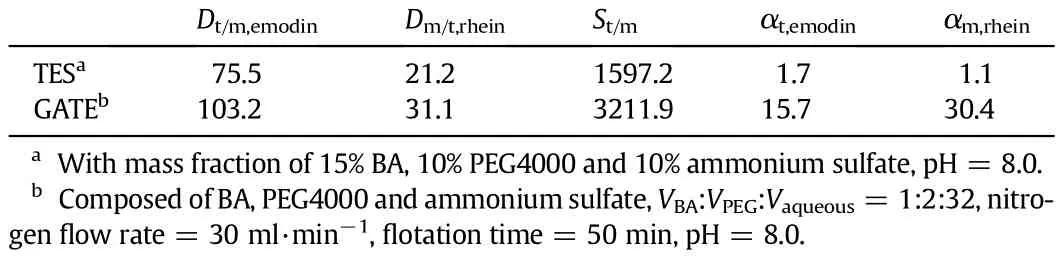
Table 1Comparison of separation and concentration between TES and GATE under the optimal operation conditions
3.3.Effect of volume of PEG and BA
Figs.10and11demonstratetheinf l uenceofinitialvolumeofPEGon the removal ratio and mass fraction of emodin and rhein,respectively. In comparison with initial volume of BA phase as shown in Figs.12 and 13,the volume of PEG phase plays a more important role in the removal of solutes from the bottom phase.Increasing PEG volume tends to promote the removal of emodin and rhein.Adding polymer phase enlarges the extraction capacity of PEG phase and the concentration of solutes in PEG decreases accordingly.The increase of the concentration gradient between the aqueous phase and the polymer phase contributes to the removal of the two compounds.Meanwhile,larger PEG volume prolongs the contact time between PEG phase and bubbles and advances the enrichment of rhein.The combination of the two factors generatesahighermassfractionasshowninFig.11.BAphaseisisolated and does not directly contact with aqueous phase,so the effect of BA volumeisnotassignif i cantasthatofPEGphase.However,whenadding a little top phase,the emodin concentration in BA phase will be very high,andpartofemodinmaydistributeintoPEGphase.Besides,itisdiffi cult to maintain interfacialstability withsucha thinlayeroftop phase. Fig.13showsthathighervolumeofBAphasedecreasesrheinmassfraction in PEGvolume.This can be explained by intrinsic distribution equilibrium of rhein between BA phase and PEG phase.Larger BA phase retains slightly more rhein in the top phase.Therefore,initial volume of 20 ml PEG and 10 ml BA is regarded as the optimal condition.
3.4.Comparison with three-liquid-phase extraction system
When using traditional liquid-liquid extraction or solvent sublation,all kinds of anthraquinones are extracted into organic phase and can not be separated from each other[39].Here we compare GATE with traditionalTES.Severalprimaryparametersforevaluatingseparationperformancesuchasdistributionratio(D),separationfactor(S)andconcentration coeff i cient(α)are def i ned and calculated for the two extraction systems.
Under the optimal operation conditions,the separation results of the two systems are compared in Table 1.The separation ratio and concentration coeff i cient of GATE are signif i cantly higher than those of TES because of higher phase ratio between aqueous phase and extractant phase.The results also demonstrate that GATE provides higher treatment capacity and consumes less PEG phase and organic phase.
4.Conclusions
GATE,a novel combination of three-liquid-phase separation and solventsublation,wasappliedtoselectivelyseparateandconcentratehighly structure-similar anthraquinones of simulated herbal extract into different phases in one step with the aid of gas bubbling.Within 50 min of 30 mL/min nitrogen f l ow,removal ratio of emodin and rhein from aqueous phase could be over 99%and 97%,respectively.Mass fraction of emodin in the BA phase and rhein in the PEG phase could reach 97%and 95%, respectively.
Compared with conventional three-liquid-phase extraction,GATE exhibits better separation eff i ciency and concentration performance with less usage of polymer and organic extractant.During GATE operation,turbulent mixing of phases is avoided and loss of BA and PEG in equilibrium aqueous solution could be reduced.Additionally,dispersed BA and PEG can be captured by rising gas f l ow with the aid of gas bubbling.Therefore,GATE could be an eff i cient,economical and highly selective technology for simultaneous separation and concentration of co-existing products with high structure similarity in medicinal plants.
[1]Q.Huang,G.Lu,H.Shen,M.Chung,C.Ong,Anti-cancerproperties ofanthraquinones from rhubarb,Med.Res.Rev.27(2007)609-630.
[2]S.Agarwal,S.S.Singh,S.Verma,S.Kumar,Antifungal activity of anthraquinone derivatives from Rheum emodi,J.Ethnopharmacol.72(2000)43-46.
[3]B.Onegi,C.Kraft,I.Köhler,M.Freund,K.Jenett-Siems,K.Siems,G.Beyer,M.F. Melzig,U.Bienzle,E.Eich,Antiplasmodial activity of naphthoquinones and one anthraquinone from Stereospermum kunthianum,Phytochemistry 60(2002)39-44.
[4]D.O.Andersen,N.D.Weber,S.G.Wood,B.G.Hughes,B.K.Murray,J.A.North,In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives,Antiviral Res.16(1991)185-196.
[5]D.L.Barnard,J.H.Huffman,J.L.B.Morris,S.G.Wood,B.G.Hughes,R.W.Sidwell,Evaluation of the antiviral activity of anthraquinones,anthrones and anthraquinone derivatives against human cytomegalovirus,Antiviral Res.17(1992)63-77.
[6]R.Liu,A.Li,A.Sun,Preparative isolation and purif i cation of hydroxyanthraquinones and cinnamic acid from the Chinese medicinal herb Rheum off i cinale Baill.by highspeed counter-current chromatography,J.Chromatogr.A 1052(2004)217-221.
[7]Y.Xie,Y.Liang,H.W.Chen,T.Y.Zhang,Y.Ito,Preparativeisolation and purif i cation of anthraquinones from Cassia seed by high-speed countercurrent chromatography,J. Liq.Chromatogr.Relat.Technol.30(2007)1475-1488.
[8]S.Genovese,F.Tammaro,L.Menghini,G.Carlucci,F.Epifano,M.Locatelli,Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine,emodine,rheine,chrysophanol and physcione in the bark of Rhamnus alpinus L.(Rhamnaceae),Phytochem.Anal.21(2010)261-267.
[9]Y.X.Gong,S.P.Li,Y.T.Wang,P.Li,F.Q.Yang,Simultaneous determination of anthraquinonesinRhubarb by pressurized liquid extraction and capillary zone electrophoresis,Electrophoresis 26(2005)1778-1782.
[10]Z.J.Tan,F.F.Li,J.M.Xing,Separation and purif i cation of aloe anthraquinones using PEG/Salt aqueous two-phase system,Sep.Sci.Technol.46(2011)1503-1510.
[11]M.Locatelli,F.Tammaro,L.Menghini,G.Carlucci,F.Epifano,S.Genovese,Anthraquinone prof i le and chemical f i ngerprint of Rhamnus saxatilis L.from Italy,Phytochem. Lett.2(2009)223-226.
[12]M.Mojski,I.Gluch,Characteristics and applications of three-phase extraction systems,J.Anal.Chem.51(1996)329-342.
[13]H.Liu,C.Guo,J.Yu,J.Xing,Z.Chang,Y.Guan,Principle and Applications of Microemulsion Phase Extraction,Science Press,Beijing,2005.
[14]L.H.Silva,W.Loh,Polymer induced multiphase generation in water/organic solvent mixtures.Strategies towards the design of triphasic and tetraphasic liquid systems, Chem.Commun.1(1998)787-788.
[15]L.Liu,Y.Dong,Z.Xiu,Three-liquid-phase extraction of diosgenin and steroidal saponins from fermentation of Dioscorea zingibernsis CH Wright,Process Biochem.45 (2010)752-756.
[16]S.Shen,Z.Chang,J.Liu,X.Sun,X.Hu,H.Liu,Separation of glycyrrhizic acid and liquiritin from Glycyrrhiza uralensis Fisch extract by three-liquid-phase extraction systems,Sep.Purif.Technol.53(2007)216-223.
[17]J.Chen,H.Liu,B.Wang,Z.An,Q.Liu,Study on the three-phase extraction of penicillin G with a single-step method,Proceedings of the International Solvent Extraction Conference,Johannesburg,South Africa,2002.
[18]S.Shen,Z.Chang,X.Sun,X.Hu,H.Liu,Application of block copolymer in threeliquid-phase extraction system,Tsinghua Sci.Techol.11(2006)248-251.
[19]P.Yu,K.Huang,H.Liu,Two and three-phase separation of phenol and onitrophenol:correlation between phasebehavior and partitioningbehavior,Colloids Surf.A 403(2012)15-24.
[20]X.He,K.Huang,P.Yu,C.Zhang,K.Xie,P.Li,J.Wang,Z.An,H.Liu,Liquid-liquidliquid three phase extraction apparatus:operation strategy and inf l uences on mass transfer eff i ciency,Chin.J.Chem.Eng.20(2012)27-35.
[21]P.Yu,Z.Chang,Y.Ma,S.Wang,H.Cao,C.Hua,H.Liu,Separation of p-nitrophenol and o-nitrophenol with three-liquid-phase extraction system,Sep.Purif.Technol. 70(2009)199-206.
[22]S.Shen,Z.Chang,H.Liu,Three-liquid-phase extraction systems for separation of phenol and p-nitrophenol from wastewater,Sep.Purif.Technol.49(2006)217-222. [23]C.Zhang,K.Huang,P.Yu,H.Liu,Salting-out induced three-liquid-phase separation of Pt(IV),Pd(II)and Rh(III)in system of S201-acetonitrile-NaCl-water,Sep.Purif. Technol.80(2011)81-89.
[24]K.Xie,K.Huang,L.Xu,P.Yu,L.Yang,H.Liu,Three-liquid-phase extraction and separation of Ti(IV),Fe(III),and Mg(II),Ind.Eng.Chem.Res.50(2011)6362-6368.
[25]C.Zhang,K.Huang,P.Yu,H.Liu,Sugaring-outthree-liquid-phaseextractionandonestep separation of Pt(IV),Pd(II)and Rh(III),Sep.Purif.Technol.87(2012)127-134.
[26]P.Yu,K.Huang,C.Zhang,K.Xie,X.He,J.Zhao,F.Deng,H.Liu,Block copolymer micellization induced microphase mass transfer:partition of Pd(II),Pt(IV)and Rh(III) in three-liquid-phase systems of S201-EOPO-Na2SO4-H2O,J.Colloid Interface Sci. 362(2011)228-234.
[27]P.Yu,K.Huang,C.Zhang,K.Xie,X.He,H.Liu,One-step separation of platinum,palladium,and rhodium:a three-liquid-phase extraction approach,Ind.Eng.Chem.Res. 50(2011)9368-9376.
[28]P.Yu,K.Huang,H.Liu,K.Xie,Three-liquid-phase partition behaviors of Pt(IV),Pd(II)and Rh(III):inf l uences of phase-forming components,Sep.Purif.Technol.88(2012)52-60.
[29]K.Xie,K.Huang,L.Yang,P.Yu,H.Liu,Three-liquid-phase extraction:a new approach for simultaneous enrichment and separation of Cr(III)and Cr(VI),Ind.Eng. Chem.Res.50(2011)12767-12773.
[30]K.Xie,K.Huang,L.Yang,H.Liu,Enhancing separation of titanium and iron by threeliquid-phase extraction with 1,10-phenanthroline as additive,J.Chem.Technol. Biotechnol.87(2012)955-960.
[31]P.Yu,K.Huang,J.Zhao,C.Zhang,K.Xie,F.Deng,H.Liu,A novel separation technique:gas-assisted three-liquid-phase extraction for treatment of the phenolic wastewater,Sep.Purif.Technol.75(2010)316-322.
[32]H.Liu,K.Wang,X.Chen,Z.Hu,Determination of rhein,baicalin and berberine intraditional Chinese medicinal preparations by capillary electrophoresis with twomarker technique,Biomed.Chromatogr.18(2004)288-292.
[33]L.Liu,L.Fan,H.Chen,X.Chen,Z.Hu,Separation and determination of four active anthraquinones in Chinese herbal preparations by f l ow injection-capillary electrophoresis,Electrophoresis 26(2005)2999-3006.
[34]P.Bi,H.Dong,J.Dong,The recentprogress of solventsublation,J.Chromatogr.A 1217 (2010)2716-2725.
[35]P.Å.Albertsson,Separation of particles and macromolecules by phase partition, Endeavour 1(1977)69-74.
[36]K.Ananthapadmanabhan,E.Goddard,Aqueous biphase formation in polyethylene oxide-inorganic salt systems,Langmuir 3(1987)25-31.
[37]P.Bi,H.Dong,Y.Yuan,Application of aqueous two-phase f l otation in the separation and concentration of puerarin from Puerariae extract,Sep.Purif.Technol.75(2010)402-406. [38]H.O.Johansson,G.Karlstroem,F.Tjerneld,Experimental and theoretical study of phase separation in aqueous solutions of clouding polymers and carboxylic acids, Macromolecules 26(1993)4478-4483.
[39]J.Zhang,C.Bao,Y.Wang,W.Kong,F.Li,Separation and enrichment of the active constituents in Radix Et Rhizoma Rhei by solvent f l otation,J.Anal.Sci.3(2009) 317-320(in Chinese).
25 March 2013
☆Supported by the National Natural Science Foundation of China(21136009, 21106162,21106152),Ministry of Human Resources and Social Security of China and State Key Laboratory of Chemical Engineering(SKL-ChE-11A04).
*Corresponding authors.
E-mail addresses:lxf@ipe.ac.cn(X.Liang),Hzliu@ipe.ac.cn(H.Liu).
http://dx.doi.org/10.1016/j.cjche.2014.06.029
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 9 June 2013
Accepted 10 July 2013
Available online 30 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
