hspA9基因5′侧翼区SNPs对矮脚黄羽肉鸡耐热性的影响
2014-07-02张文武甘建伉张细权张德祥罗庆斌
张文武,甘建伉,张细权,张德祥,罗庆斌*
(1.华南农业大学动物科学学院,广东 广州 510642;2.农业部鸡遗传育种与繁殖重点实验室(广州),广东 广州510642)
hspA9基因5′侧翼区SNPs对矮脚黄羽肉鸡耐热性的影响
张文武1,2,甘建伉1,2,张细权1,2,张德祥1,2,罗庆斌1,2*
(1.华南农业大学动物科学学院,广东 广州 510642;2.农业部鸡遗传育种与繁殖重点实验室(广州),广东 广州510642)
对矮脚黄羽肉鸡纯系hspA9基因5′侧翼区进行PCR测序和PCR–RFLP检测,将检测到的突变位点与矮脚黄羽肉鸡纯系5个耐热性状(T3、皮质酮、异嗜性粒细胞与淋巴细胞比率(H/L)、CD3+T细胞和CD4+T细胞)进行关联分析。结果表明:矮脚黄羽肉鸡纯系hspA9基因5′侧翼区有3个单核苷酸多态性(SNP)位点(C.–85C>A、C.–485G>A和C.–568G>A),根据C.–85C>A的酶切位点,这个基因可分为AA、AC、CC型;根据C.–485G>A的酶切位点,这个基因可分为GG、GA、AA型;根据C.–568G>A的酶切位点,这个基因可分为GG、GA、AA型;热应激状态下,C.–485G>A位点与皮质酮极显著相关(P<0.01),GG基因型个体的皮质酮值极显著高于GA基因型个体;常温状态下,C.–485G>A位点与T3显著相关(P<0.05),GA基因型个体的T3值显著高于AA 基因型个体,C.–568G>A 位点与 T3显著相关(P<0.05),GG基因型个体T3值显著高于GA和AA基因型个体,C.–568G>A位点与CD4+T细胞极显著相关(P<0.01),GG基因型个体CD4+T细胞值显著高于AA基因型个体。C.–485G>A位点和C.–568G>A位点对鸡热耐受性有较大的影响,可望用于鸡抗逆性的分子标记辅助选择。
矮脚黄羽肉鸡;hspA9基因;热应激;耐热性
热应激蛋白(heat shock protein, HSP)是机体受到应激源(如高温等)刺激后合成的一组蛋白质[1]。这些热应激蛋白在细胞生长、发育及分化,基因转录,蛋白质合成、折叠、运输及分解,维持细胞骨架功能,膜功能等方面具有重要作用,且能保护机体免受外界刺激的损害[2]。HSP70是热应激蛋白家族中的重要成员,作为主要的分子伴侣参与机体耐受性的形成[3]。
hspA9基因属于hsp70基因家族。研究表明,hspA9在患皮肤癌[4]和食管鳞癌[5]的动物中表达上调,可能与抗癌有关。hspA9基因的过表达还能抑制apoptin诱导的HepG2细胞的凋亡[6]。另外,hspA9基因可能与老鼠上颚发育有关[7],因其mRNA在老鼠上颚中高表达。而hspA9基因敲除的老鼠,其造血细胞减少,表明hspA9基因还与造血有关[8]。迄今为止,关于鸡hspA9基因的研究较少。本研究选择矮脚黄鸡纯系为试验材料,对其hspA9基因进行PCR–RFLP检测,以分析hspA9基因的位点多态性与热应激指标的相关性,旨在为利用分子标记辅助选择来改良家鸡的热适应性提供参考依据。
1 材料与方法
1.1 供试鸡
333只矮脚黄羽肉鸡纯系(N301系)母鸡选自广东温氏南方家禽育种有限公司。
1.2 外周血采集
在35 ℃(20周龄)、15 ℃(32周龄)条件下采集母鸡的外周血1.5 mL,分装到1.5 mL抗凝管和1.5 mL非抗凝管中,备用。
1.3 鸡基因组DNA的提取
取每只鸡的抗凝血30 μL,采用苯酚–氯仿抽提法[9]分别提取基因组DNA。DNA样品分别溶于300 μL ddH2O中,–20 ℃冰箱保存。
1.4 耐热性状相关指标的测定
将非抗凝血直接离心吸取血清,送中山大学广州达瑞抗体中心检测三碘甲状腺氨酸(triiodothyronine,T3)和皮质酮;用瑞氏和吉姆萨复合染色法[10]对抗凝血的血涂板染色、镜检,按Alfred等[11]的白细胞分类方法进行细胞分类,并按Campo和Davila方法[12]进行计数和计算,之后送解放军458医院免疫细胞实验室检测 H/L;将抗凝血直接送解放军 458医院免疫缺陷病研究与特殊实验中心检测 CD3+T细胞和CD4+T细胞。
1.5 hspA9基因的PCR–RFLP分析
1) 引物设计与PCR扩增。根据GenBank中收录的鸡HspA9基因的cDNA序列,通过Primier5.0设计扩增hspA95′侧翼区的引物F1(5′–GCACCACG CTGTTCCATCA–3′)和R1(5′–AGGGCGATTGCAG CGAGCT–3′)。引物由上海生工生物工程技术服务有限公司合成。
随机选取10份DNA进行PCR扩增。用PCR试剂盒(Gen Star公司)配置PCR反应体系(20 μL):Mix 10 μL、20 μmol/L上、下游引物各0.2 μL、10 U/μL Taq酶0.15 μL、鸡基因组DNA 1.5 μL (35 ℃时提取的血液DNA),ddH2O 7.95 μL。PCR反应程序:94 ℃预变性3 min,32个循环(94 ℃变性30 s,59.7 ℃退火30 s,72 ℃延伸30 s), 72 ℃ 终延伸5 min, 16 ℃保存。PCR产物用1.2%的琼脂糖凝胶电泳检测后送华大公司测序。
2) PCR–RFLP分析。对PCR产物测序结果进行分析,获得多态性位点及特异性限制性内切酶识别位点,设计引物,对提取的666份DNA样品进行PCR及RFLP检测。PCR反应体系为10 μL:Mix 5 μL、20 μmol/L上、下游引物各0.1 μL、10 U/μL Taq酶 0.1 μL、鸡基因组DNA 1 μL,ddH2O 3.7 μL。PCR反应程序同上。根据引物改变退火温度。对PCR产物进行酶切,酶切体系为10 μL:ddH2O 2.1 μL、内切酶(TaKaRa公司) 0.3 μL、Buffer缓冲液 0.6 μL、PCR产物7 μL。37 ℃过夜。用2%的琼脂糖凝胶电泳检测酶切产物,根据电泳条带判定个体的基因型。
1.6 关联分析
采用 SAS 9.0软件的广义线性模型(General Linear Model,GLM)程序进行计算,对矮脚黄羽肉鸡的热耐受指标与hspA9基因多态位点进行关联分析。所用模型为 yij=μ+gi+eij。其中,yij为性状表型值,μ为该性状的总体均值,gi为基因型效应值,eij为残差效应,i表示线性方程的行,j表示线性方程的列。
2 结果与分析
2.1 hspA9基因5′侧翼区的PCR结果
对PCR产物的测序结果进行分析,发现矮脚黄羽肉鸡纯系的hspA9基因5′侧翼区有3个SNP位点:C.–85C>A、C.–485G>A和C.–568G>A。C.–85C>A 为5′侧翼区第85位出现碱基C→A的突变,可用Hae II酶(识别序列为 GGCGCC)进行区分;C.–485G>A为5′侧翼区第485位出现碱基G→A的突变,可用Sml I酶(识别序列为CTTGAG)进行区分;C.–568G>A为5′侧翼区第568位出现碱基G→A的突变,可用Ban II酶(识别序列为GAGCTC)进行区分。
2.2 hspA9基因3个SNP的分型
根据C.–85C>A位点的特征,设计引物F2(5′–ATGGGGGTGCTCGGAAAAC–3′)和R2(5′–AGGG CGATTGCAGCGAGCT–3′)对鸡的DNA进行PCR扩增,退火温度为59.7 ℃,产物大小为267 bp。用Hae II对PCR产物分别进行酶切,结果(图1)证实矮脚黄鸡hspA9可以分为CC、AC和AA型:CC 型hspA9片段可被Hae II酶切成2条带,长度分别为175、92 bp;AC型hspA9片段可被酶切成3条带,长度分别为267、175、92 bp;AA型hspA9片段不可以被酶切,为1条带,长度为267 bp。根据C.–485G>A、C.–568G>A位点的特征,用F1和R1引物对鸡的DNA进行PCR扩增,产物分别用Sml I和Ban II进行酶切分型。根据C.–485G>A位点的酶切结果(图2),矮脚黄鸡hspA9可以分为GG、GA 和AA型:GG型hspA9片段可被Sml I酶切成2条带,长度分别为493、229 bp;GA型hspA9片段可被酶切成3条带,长度分别为722、493、229 bp;AA 型hspA9片段不可以被酶切,为1条带,长度为722 bp。根据C.–568G>A位点的酶切结果(图3),矮脚黄鸡hspA9可以分为GG、GA和AA型:GG 型hspA9片段可被Ban II酶切成2条带,长度分别为561、161 bp;GA型hspA9片段可被酶切成3条带,长度分别为722、561、161 bp;AA 型hspA9片段不可以被酶切,为1条带,长度为722 bp。
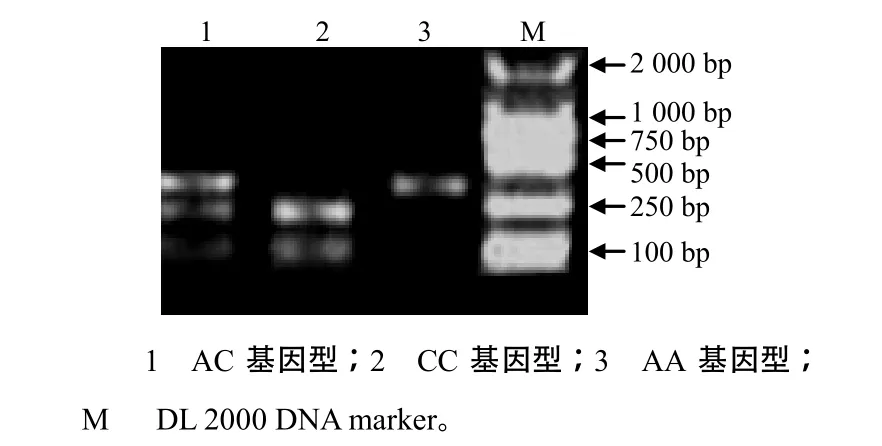
图1 hspA9基因C.–85C>A位点特异酶切结果Fig.1 Digestion of hspA9 gene by Hae II specific for C.–85C>A site
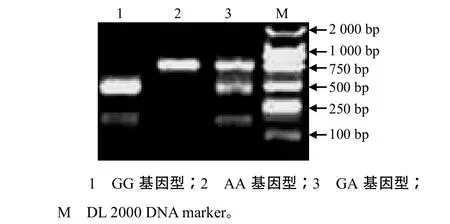
图2 hspA9基因C.–485G>A位点特异酶切结果Fig.2 Digestion of hspA9 gene by Sml I specific for C.–485G>A site

图3 hspA9基因C.–568G>A位点特异酶切结果Fig.3 Digestion of hspA9 gene by Ban II specific for C.–568G>A site
2.3 hspA9基因多态位点与耐热性状的相关性
热耐受指标与hspA9基因3个多态位点的关联分析表明,在35 ℃热应激状态下,C.–485G>A与皮质酮浓度极显著相关(P<0.01),GG基因型个体的皮质酮浓度极显著高于 GA基因型个体(P<0.01),但GG与AA,GA与AA基因型之间无显著差异(P>0.05);C.–85C>A和C.–568G>A和耐热性状的关联均不显著(表1)。

表1 热应激状态下3个SNP与耐热性状的关联分析Table 1 Association analysis between 3 SNPs and thermal tolerance traits at high temperature
在 15 ℃的常温状态下,矮脚黄羽肉鸡 hspA9基因C.–485G>A 与T3的浓度显著相关(P<0.05),GA基因型个体的T3的浓度显著高于AA基因型个体(P<0.05),但GG与AA,GG与GA基因型之间无显著差异(P>0.05);C.–568G>A 与T3浓度显著相关(P<0.05),GG基因型个体的T3浓度显著高于AA和GA基因型个体(P<0.05),但GA与AA基因型之间无显著差异(P>0.05);C.–568G>A与CD4+T细胞含量极显著相关(P<0.01),GG基因型个体的CD4+T细胞含量极显著高于AA基因型个体(P <0.01),但GG与GA、GA与AA基因型之间无显著差异(P>0.05);C.–85C>A位点在常温状态下和耐热性状关联不显著(表2)。
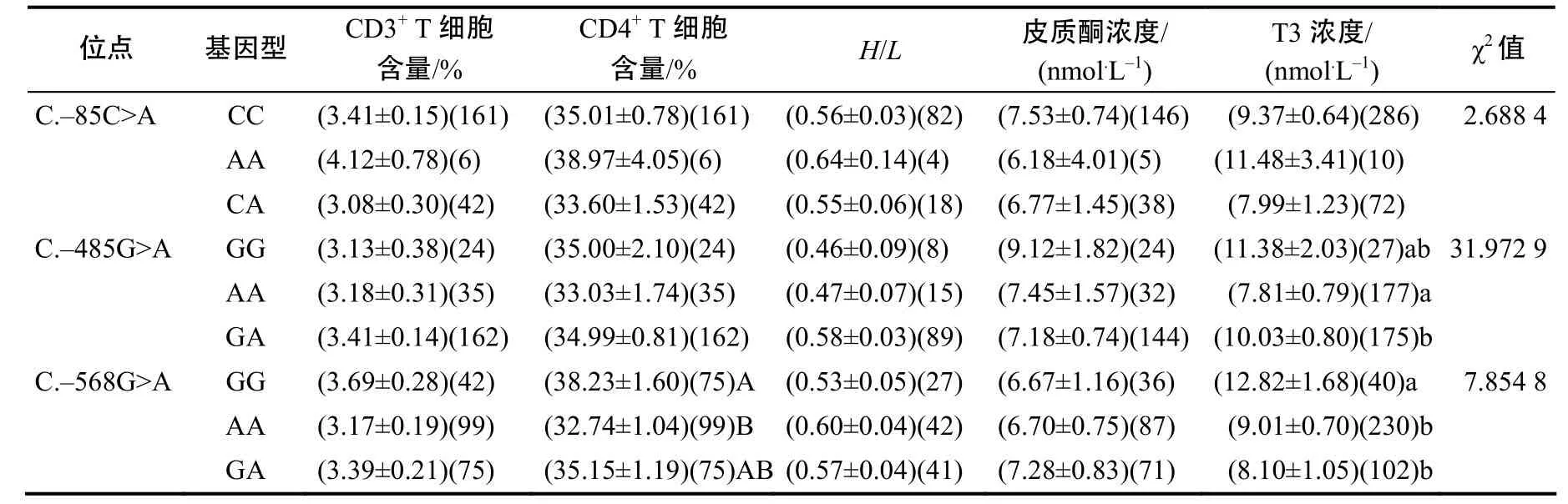
表2 常温状态下3个SNP与耐热性状的关联分析Table 2 Association analysis between 3 SNPs and thermal tolerance traits at room temperature
3 讨 论
T3是动物体维持基础代谢的重要激素,是敏感的热应激指标。Koluman等[13]报道羊在热应激时,T3含量的变化差异显著,暗示T3可以作为热应激指标。杨琳等[14]报道鸡的血浆中T3浓度会随环境温度的升高而下降,表明T3能作为鸡耐热性的指标。Maak等[15]研究长时间中等强度热刺激会使蛋鸡血浆的T3浓度显著下降,也暗示鸡血浆中T3能作为热应激指标。本试验对T3与hspA9基因多态性位点的关联分析发现,在常温状态下,hspA9基因C.–485G>A为GA基因型的矮脚黄羽肉鸡个体耐热性较好,hspA9基因C.–568G>A为GG基因型的个体耐热性也较好。
皮质酮由禽类肾上腺分泌,是应激时刺激垂体–下丘脑–肾上腺轴分泌的最终产物[12]。许多研究[16–17]表明,应激会引起皮质酮的大量分泌,皮质酮能作为准确评价家禽急性应激的生理指标。Zulkifli等[18]报道,高温应激使鸡血浆中皮质酮浓度明显提高,暗示皮质酮可以作为鸡耐热性的指标。本研究对皮质酮与hspA9基因多态性位点的关联分析表明,热应激状态下hspA9基因C.–485G>A的GG基因型的矮小型黄羽肉鸡个体耐热性较好。
在抗原识别过程中,CD4+T细胞具有辅助细胞免疫和体液免疫应答的作用。研究显示,热应激可使鸡外周血CD4+T细胞显著减少[19–20]。高温对鸡各免疫器官中CD4+T细胞具有明显抑制作用,导致机体细胞免疫能力降低[21]。CD4+T细胞含量可以作为耐热性的免疫指标[22]。本研究对CD4+T细胞与hspA9基因多态性位点的关联分析表明,常温状态下,C.–568G>A的GG基因型的矮脚黄羽肉鸡个体耐热性较好。
综上所述,鸡 hspA9基因的 C.–485G>A和C.–568G>A两个SNP位点对鸡耐热性有影响,是潜在的鸡耐热性分子标记。
[1] Gray C C,Amrani M,Yacoub M H.Heat stress proteins and myocardial protection:Experimental model or potential clinical tool[J].The International Journal of Biochemistry and Cell Biology,1999,31(5):559–573.
[2] Kaldis A,Atkinson B G,Heikkila J J.Molecular chaperone function of the Rana catesbeiana small heat shock protein,hsp30[J].Comparative Biochemistry and Physiology,2004,139(2):175–182.
[3] Hartl F U.Molecular chaperones in cellular protein folding[J].Nature,1996,381(6583):571–580.
[4] Schlieben P,Meyer A,Weise C,et al.Differences in the proteome of high–grade versus low-grade canine cutaneous mast cell tumours[J].Veterinary Journal,2012,194(2):210–214.
[5] Pawar H,Kashyap M K,Sahaspabuddhe N A,et al. Quantitative tissue proteomics of esophageal squamous cell carcinoma for novel biomarker discovery[J].Cancer Biology and Therapy,2011,12(6):510–522.
[6] Peng C,Yang P,Cui Y,et al.HspA9 overexpression inhibits apoptin–induced apoptosis in the HepG2 cell line[J].Oncology Reports,2013,29(6):2431–2437.
[7] Zhu Y,Ren C,Wan X,et al.Gene expression of Hsp70,Hsp90 and Hsp110 families in normal palate and cleft palate during mouse embryogenesis[J].Toxicology and Industrial Health,2012,10(8):1177–1180.
[8] Chen T H,Kambal A,Krysiak K,et al.Knockdown of HspA9,a del(5q31.2) gene,results in a decrease in hematopoietic progenitors in mice[J].Blood,2011,117(5):1530–1539.
[9] 奥斯伯 F,布伦特 R,金斯敦 R E,等.精编分子生物学实验指南[M].颜子颖,王海林,译.北京:科学出版社,1998.
[10] 董书魁,贾天宠.改良的 MGG染色液[J].北京军区医药,1996,8(1):77.
[11] Alfred M L,Casimir J.Atlas of Avian Hematology[M]. Washington:United States Department of Agriculture, 1961.
[12] Campo J L,Davila S G.Estimation of heritability for heterophil:Lymphocyte ratio in chickens by restricted maximum likelihood effects of age,sex,and crossing[J]. Poultry Science,2002,81(10):1448–1453.
[13] Koluman N,Daskiran I.Effects of ventilation of the sheep house on heat stress,growth and thyroid hormones of lambs[J].Tropical Animal Health and Production,2011,43(6):1123–1127.
[14] 杨琳,杜荣,张子仪.环境温度对鸡饲粮代谢能测值及血浆中甲状腺激素浓度的影响[J].畜牧兽医学报,1993,24(6):494–499.
[15] Makk S,Melesse A,Schmidt R,et al.Effect of long–term heat exposure on peripheral concentrations of heat shock protein 70 (Hsp70) and hormones in laying hens with different genotypes[J].British Poultry Science,2003,44(1):133–138.
[16] Post J,Rebel J M,Ter Huurne A A.Physiological effects of elevated plasma corticosterone concentrations in broiler chickens:An alternative means by which to assess the physiological effects of stress[J].Poultry Science,2003,82(8):1313–1318.
[17] Star L,Nieuwland M G,Kemp B,et al.Effect of single or combined climatic and hygienic stress on natural and specific hum oral immune competence in four layer lines[J].Poultry Science,2007,86(9):1894–1903.
[18] Zulkifli I,Al–Aqil A,Omar A R,et al.Crating and heat stress influence blood parameters and heat shock protein 70 expression in broiler chickens showing short or long tonic immobility reactions[J].Poultry Science,2009,88(3):471–476.
[19] Trout J M,Mashaly M M.The effects of adrenocorticotropic hormone and heat stress on the distribution of lymphocyte populations in immature male chickens[J]. Poultry Science,1994,73(11):1694–1698.
[20] Hammami M M,Bouchama A,Shail E,et al. Lymphocyte subsets and adhesion molecules expression in heatstroke and heat stress[J].Journal of Applied Physiology,1998,84(5):1615–1621.
[21] 李新国,王自力,朱晓宇,等.高温下清凉冲剂对鸡免疫器官中CD4+、CD8+T细胞数量的影响[J].中兽医医药杂志,2006,6(6):5–7.
[22] Chen Z Y,Gan J K,Xiao X,et al.The association of SNPs in Hsp90β gene 5′ flanking region with thermo tolerance traits and tissue mRNA expression in two chicken breeds[J].Molecular Biology Reports,2013,40(9):5295–5306.
责任编辑:罗 维
英文编辑:罗 维
Effect of SNPs of hspA9 gene 5′ flanking region with thermal tolerance traits in yellow feather dwarf broiler
ZHANG Wen-wu1,2, GAN Jian-kang1,2, ZHANG Xi-quan1,2, ZHANG De-xiang1,2, LUO Qing-bin1,2*
(1.College of Animal Science, South China Agricultural University, Guangzhou 510642, China; 2.Key Laboratory of Chicken Genetics, Breeding and Reproduction, Ministry of Agriculture(Guangzhou), Guangzhou 510642, China)
To observe the role of hspA9 of domestic chicken in tolerance of heat stress, hspA9 gene 5 'flanking region of yellow feather dwarf broiler pure line was amplified by PCR and sequenced, and then analyzed by PCR–RFLP. The relationships between the mutation sites detected in hspA9 gene and the five heat resistant traits (triiodothyronine (T3), corticosterone, heterophil/lymphocyte ratio (H/L), CD3+and CD4+T cell) were analyzed. The results indicated that three SNP sites (C.–85C>A, C.–485G>A and C.–568G>A) were found. According to the C.–85C>A specific enzyme sites, hspA9 gene is divided into AA, AC and CC genotypes; according to the C.–485G>A specific enzyme sites, this gene is divided into GG, GA and AA genotypes; according to the C.–568G>A specific enzyme sites, this gene is divided into GG, GA and AA genotypes. Under high temperature, C.–485G>A site was highly significantly associated with corticosterone (P<0.01), corticosterone value in individual of GG genotype was highly significantly higher than that of AA genotype. At room temperature, C.–485G>A site was significantly associated with T3 (P<0.05), the content of T3 in individual of GA genotype was significantly higher than that of AA genotype; C.–568G>A site was significantly associated with T3 (P <0.05), the content of T3 in individual of GG genotype was significantly higher than that of AA T3 genotype; C.–568G>A site was significantly associated with CD4+T cell (P<0.01), the percentage of CD4+T cell in individual of GG genotype was significantly higher than that of AA genotype. Both C.+258A>G and C.+276C>G sites play animportant role in thermal stress resistance, indicating their potential in marker–assisted selection of chicken.
yellow feather dwarf broiler; hspA9 gene; thermal stress; thermal stress resistance
S831.2;Q75
A
1007−1032(2014)02−0183−05
10.13331/j.cnki.jhau.2014.02.015
投稿网址:http://www.hunau.net/qks
2013–05–12
国家自然科学基金项目(30972093)
张文武(1985—),男,湖南邵阳人,硕士,主要从事动物分子遗传与育种的研究,125714992@qq.com;*通信作者,qbluo @scau.edu.cn
