QSAR for Photodegradation Activity of Polycyclic Aromatic Hydrocarbons in Aqueous Systems
2014-05-02XUXiangandLIXianguo
XU Xiang, and LI Xianguo,
1) College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao 266109, P. R. China
2) Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
QSAR for Photodegradation Activity of Polycyclic Aromatic Hydrocarbons in Aqueous Systems
XU Xiang1),2), and LI Xianguo2),*
1) College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University, Qingdao 266109, P. R. China
2) Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
The relationship between chemical structures and photodegradation activity of 12 PAHs is studied using DFT and HF methods, and stepwise multiple linear regression analysis method. The equilibrium geometries and vibration frequency have been investigated by considering Solvent effects using a selfconsistent reaction field based on the polarizable continuum model. With DFT and HF methods, different quantum chemical structural descriptors are obtained by quantum chemical calculation and the results with DFT method are better for QSAR model. It is concluded that the photodegradation activity is closely related to its molecular structure. In the regression analysis, the main factors affecting photodegradation rate include the energy of the highest occupied orbital EHOMOand the number of six-carbon benzene ring N1, and the QSAR model successfully established is logkb=6.046 + 54.830EHOMO+ 0.272N1. Statistical evaluation of the developed QSAR shows that the relationships are statistically significant and the model has good predictive ability. EHOMOis the most important factor influcing the photodegradation of PAHs, because the higher EHOMOis, the more easily electron will be excited and the more easily molecular will be degraded. Comparison of the photodegradation of PAHs with their biodegradation shows that the committed step of biodegradation is that the effects of microorganisms make the chemical bond break, while in the committed step of photodegradation PAHs eject electrons.
quantitative structure-activity relationship (QSAR); polycyclic aromatic hydrocarbons (PAHs); density functional theory (DFT); photodegradation
1 Introduction
PAHs are aromatic hydrocarbons with two or more fused benzene rings. They are mostly produced by the incomplete combustion or the thermal decomposition under reducing atmosphere of fossil fuels like oil and coal, and materials containing hydrocarbon like wood, natural gas, gasoline, heavy oil, organic polymer compounds, paper, crop straw, tobacco,etc. Now, they widely exist in natural water (Grimmer and Misfeld, 1983; Grimmer and Pott, 1983; Mekenyanet al., 1994; Sunet al., 2011; Wanget al., 2012) and the direct photodegradation is one important transformation for PAHs.
Because of the limits of various conditions, the experimental study about the direct photodegradation of PAHs is relatively less than the biodegradation. Two classical sets of data were reported by Smith (Smithet al., 2001) and Zepp (Zepp and Schlotzhauer, 1979), which listed the photodegradation half-life of 13 PAHs at noon of midsummer days. By using PM3 semi-empirical method, Chenet al. (2001) calculated the quantum chemical parameters of 13 PAHs and established the QSAR model with three structural parameters (ELUMO−EHOMO, (ELUMO−EHOMO)2, ELUMO+EHOMO). Based on the same data sets as Chen, Luet al. (2005) calculated the quantum chemical parameters by using HF and DFT method and also established the QSAR model with only one structural parameter (EHOMO).
Through the studies above we can raise the following questions: 1) The experimental data comes from different researchers and the experiments are performed under different conditions, so the two sets of data inevitably contained certain inconsistencies. 2) All the structural parameters to establish QSAR models which are calculated by quantum chemistry are just the gas state without considering solvent effect, while the photodegradation reactions proceed in aqueous system. 3) The research results of Chen and Lu have some different points. For the reasons mentioned above, using the experimental data of Fasnacht and Blough (Fasnachtet al., 2002) for 12 PAHs in aqueous system, we studied the relationship between chemical structures and photodegradation activity of 12PAHs with stepwise multiple linear regression analysis method and successfully established the QSAR model.
2 Materials and Methods
2.1 Experimental Data
The experimental data on photodegradation activity of 12 PAHs were obtained from literature (Fasnachtet al., 2002). The photodegradation rate constantskbof 12 PAHs and the photodegradation activity expressed by logkbare listed in Table 1.
2.2 Calculation Method
The geometries of all the molecules were optimizedusing DFT and HF method at B3LYP/6-31+G (d, p) level. The stationary points were characterized by frequency calculations in order to verify that the transition states have one and only one imaginary frequency. In chemistry, solvent effect is actually the group of effects that a solvent has on chemical reactivity. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction. Reactivity and reaction mechanisms are often pictured as the behavior of isolated molecules in which the solvent is treated as a passive support. However, solvents can actually influence reaction rates and the order of a chemical reaction (Reichardt, 1990; Jones, 1984; James, 1985, Sarwaret al., 2010)). In solution, the behaviour of ions and molecules is dictated mainly by the solvent and only to a lesser extent by their intrinsic properties. Because the photodegradation reactions in this study happened in aqueous system, solvent effects have been considered as the using of a selfconsistent reaction field (SCRF) (Simkin and Sheikhet, 1995) based on the polarizable continuum model (PCM) of Tomasi’s group (Baroneet al., 1998). All calculations were carried out with Gaussian 03 program package (Frischet al., 2003).

Table 1 Compounds studied, structure and the experimental first-order biomass-normalized rate coefficients (kb) and the photodegradation activity (logkb)
In this study the data used to establish QSAR model were obtained from the calculation results, which included the energy of the highest occupied molecular orbital (EHOMO), the energy of the lowest unoccupied molecular orbital (ELUMO), the HOMO and LUMO orbital energy difference (△E= ELUMO−EHOMO), the energy of the next-HOMO (NEHOMO), the energy of the next-LUMO (NELUMO), total energy (ET), molecular dipole moment (μ), molar volume (Vm), the number of six-carbon benzenoid ring (N1), the number of all types of rings (N2), the most negative charge of carbon atomthe most positive charge of hydrogen atomand the in-plane bending vibration frequency of the conjugated ring of PAHs (Freq).
QSAR model was established using SPSS 13.0 statistical analysis software package, including dependent variable logkband independent variables mentioned above and obtained from calculated results. In the process of multivariate stepwise linear regression analysis, the independent variables having high correlation coefficients with the dependent variable were chosen and introduced into the regression equation (Xiaet al., 2011). The validity of the established model was judged by R (correlation coefficient between observed values and fitted values),R2(determination coefficient),S.E. (standard error of regression estimation),F(value of theFstatistic), Sig.F(significance of the F statistic), Std. Dev. (standard deviation),RMSE(the root mean squared error) andn(the number of analytical compounds).
3 Results
3.1 Structural Parameters and Related Relationship
To establish QSAR model, the relationship between logkband each of molecular descriptors should be firstobtained. In this paper, DFT and HF methods are adopted in all calculations, and the calculation results are compared. The 14 molecular descriptors of 12 PAHs are listed in Table 2, and the correlation coefficients of each molecular descriptor with logkbare shown in Table 3. By comparing the values of correlation coefficients, the molecular descriptors which have a significant relationship with logkbare chosen to establish QSAR model. As is shown in Table 3, the results with DFT and HF method are consistent. EHOMOhas the highest correlation coefficient with logkband the value is 0.843 by DFT method and it is 0.800 by HF method, which is different from the calculation results of Lu (Luet al., 2005) by using DFT and HF methods. It can be found that except for the correlation coefficient between (ELUMO+EHOMO) and logkb, the results calculated by the two methods are similar. Because the correlation coefficient between (ELUMO+EHOMO) and logkbis not relatively high, (ELUMO+EHOMO) will be eliminated before establishing QSAR model without affecting the final results. So in this paper, the calculation results of DFT method are chosen to study the relationship between the structure and photodegradation activity of PAHs.
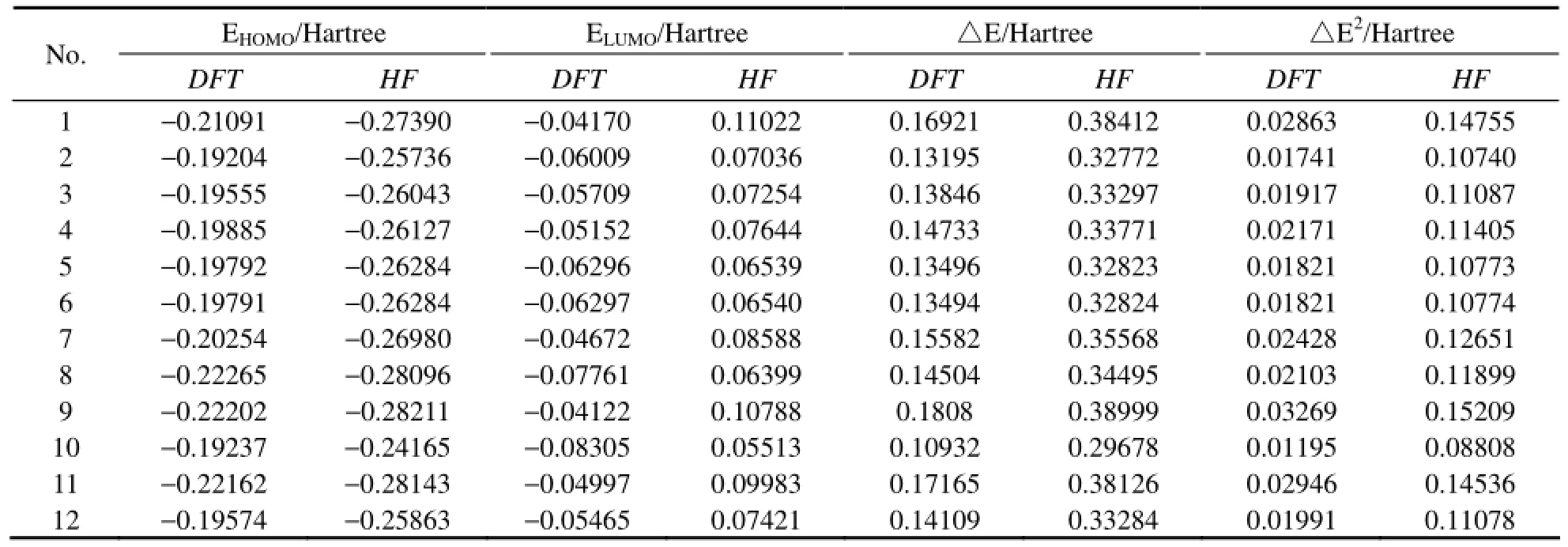
Table 2a Descriptors of PAHs used in QSAR study
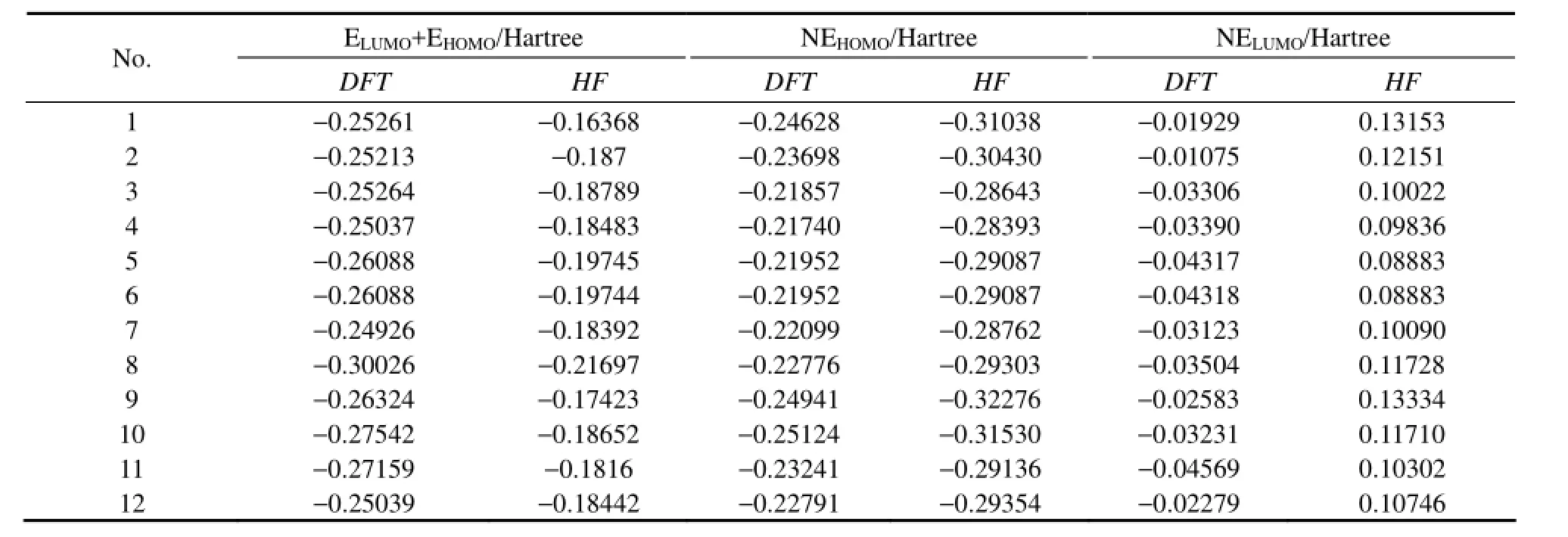
Table 2b Descriptors of PAHs used in QSAR study

Table 2c Descriptors of PAHs used in QSAR study
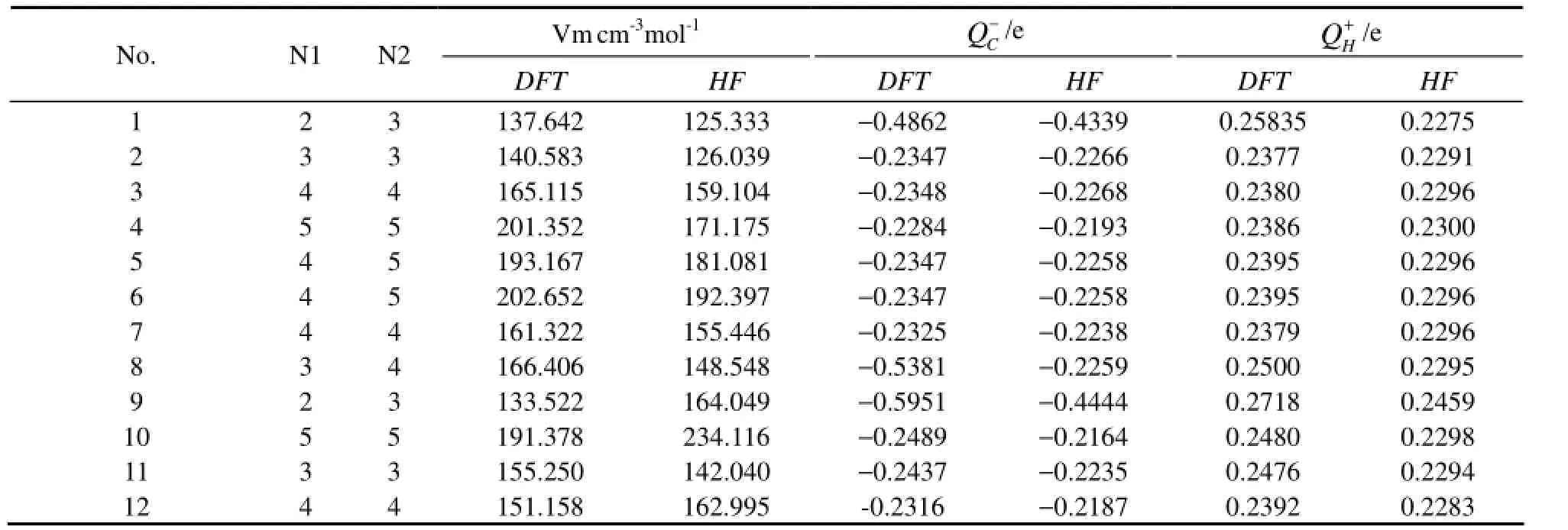
Table 2d Descriptors of PAHs used in QSAR study
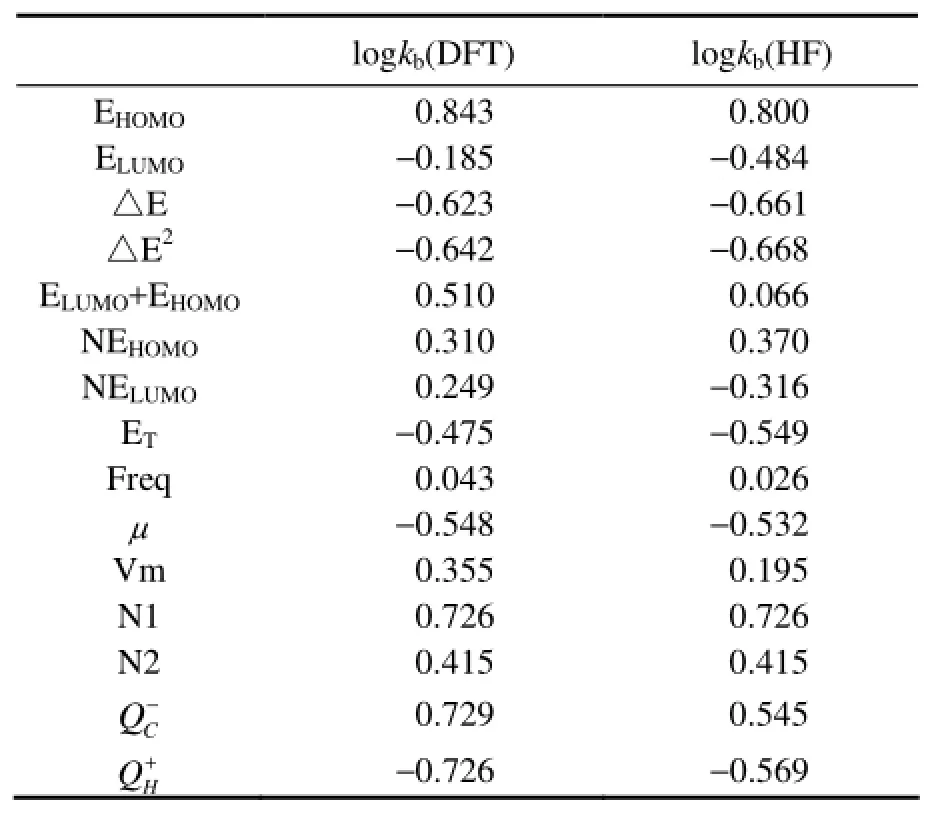
Table 3 Correlation coefficients of descriptors used in this study
The eight molecular descriptors EHOMO, N1,△E, △E2, ELUMO+EHOMOandμhave higher correlation coefficient with logkband are selected to establish QSAR model. To ensure the stability of the built model, the eight descriptors are taken pairwise in collinearity diagnostics. If the correlation of two parameters is significant, the parameter with smaller correlation coefficient with logkbwill be eliminated before establishing QSAR model. For instance, when the correlation coefficient between △Eand △E2is 0.997 and the correlation coefficients with logkbare −0.623 and −0.642, respectively, △E is eliminated. Finally,before establishing QSAR model.
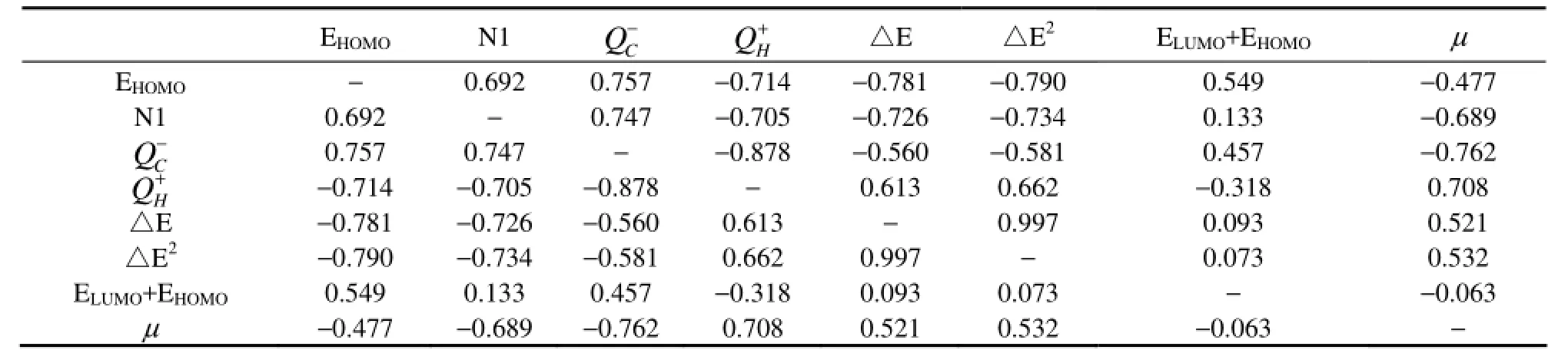
Table 4 The colinearity analysis between two parameters having close relationships with logkb
3.2 Development of QSARs
After collinearity diagnostics, the major parameters influencing photodegradation activity,i.e. EHOMO, N1, (ELUMO+EHOMO) andμwere used to establish QSAR model. In order to get the best QSAR model, we tried to select different parameter groups as independent variables and logkbas dependent variable and carried out multiple linear regression analysis. We take EHOMOas independent variable, carried out linear regression analysis, and got Eq. (1):
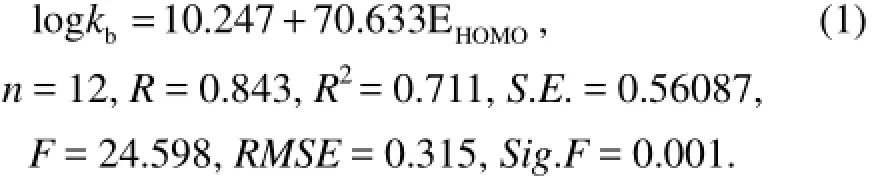
Eq.(1) has high correlation coefficient 0.843, small standard error, large Fisher check value, and the significance of the F statistic 0.001. So there are obvious linear regression relations between independent variables and dependent variable. Eq.(1) shows that the photodegradation activity of PAHs is closely related to the energy of the highest occupied molecular orbital, which is in agreement with the research result of Lu (Luet al., 2005). This result is closely related to the photodegradation mechanism of PAHs that the committed step of its photodegradation in aqueous system is ejecting one electron with absorbing one photon and ejecting electrons is absolutely crucial. The higher EHOMOis, the more easily electron will be excited and the more easily molecular will be degraded.
To investigate the influence of adding variables to QSAR model, we add N1 as an independent variable and get Eq.(2) based on Eq.(1):
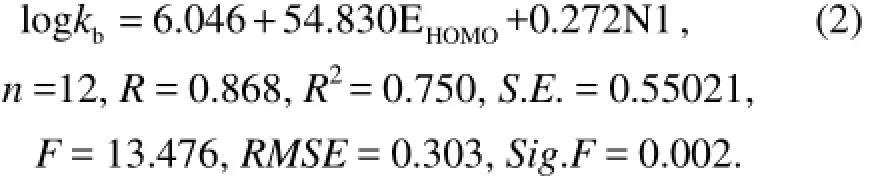
Moreover, adding (ELUMO+EHOMO) as an independent variable gives Eq.(3) based on Eq.(2):
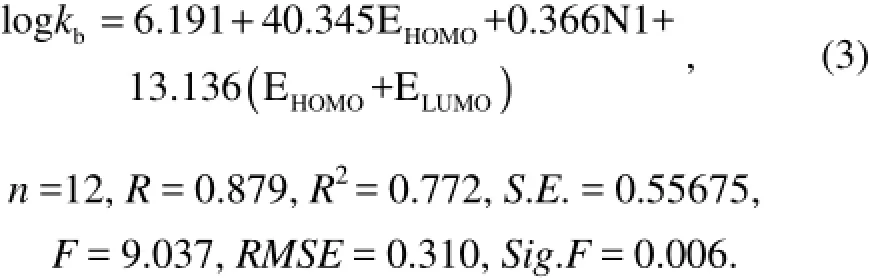
Addingμas an independent variable gives Eq.(4) based on Eq.(3):
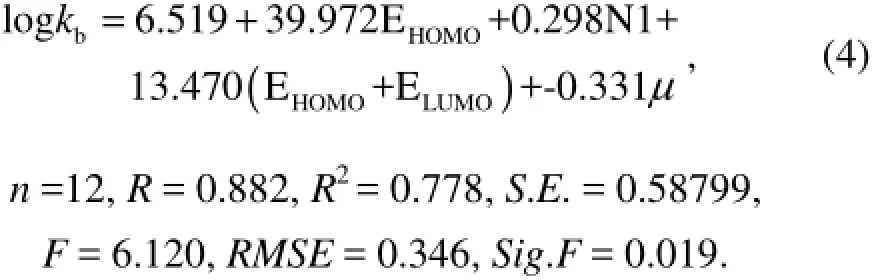
4 Discussion
By comparing the various parameters in Eqs.(1−4), it can be found that Eq.(2) is an ideal QSAR model. This is because with the increase of the variable, correlation coefficient R and determination coefficientR2become bigger, but Eq.(2) has smaller standard deviationS.E., smaller root mean squared error RMSE and relatively larger F check value. Comparing the photodegradation of PAHs with their biodegradation shows that though there is one high correlation coefficient between the vibration frequency of benzene ring and biodegradation activity (Xuet al., 2012), there is almost no correlation between the vibration frequency of benzene ring and the photodegradation activity with a correlation coefficient 0.043. This is because the reaction mechanisms of biodegradetion and photodegradation are different. The committed step of biodegradation is that microorganisms make the chemical bond break, while in the committed step of photodegradation PAHs eject electrons.
In order to validate the predictive ability of Eq.(2), the variable logkbof 12 PAHs are calculated by Eq.(2), and Fig.1 is the scatter graph of experimental and predicted values, showing that the predicted values are in good agreement with the experimental values. Fig.2 is the plots of regression standardized residuals versus the predicted values, which shows that the plots are randomly and uniformly distributed at horizontal band (−1.5, 1.5). Through the above analysis, it can be concluded that the QSAR model has a good predictive ability.
Furthermore, a leave-one-out cross-validation procedure was performed to assess the robustness of each QSAR. The procedure created perturbed training sets by removing one of the compounds from the original training sets. The perturbed training sets were used to develop partial QSARs to predict the activity of the removed compound. The squared differences between the experi-mental and predicted activities for the removed compound were used to calculate the mean cross-validated coefficient of determination (2LOOQ). For Eq.(2), the2LOOQ,FLOOandRMSELOOof validation results are 0.7383, 11.2830 and 0.3324, respectively, which change little compared with the value of the developed model (R2=0.750,F=13.476,RMSE=0.303). So the obtained QSAR model has good robustness.
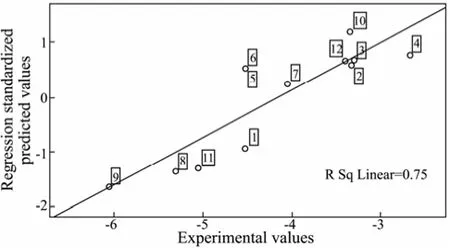
Fig.1 The scatter graph of experimental and predicted values.
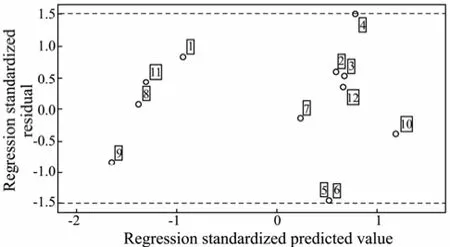
Fig.2 The plots of regression standardized residuals versus the predicted values.
5 Conclusions
With DFT and HF methods, different quantum chemical structural descriptors are obtained by quantum chemical calculation and the results with DFT method are more superior for QSAR model. By means of regression analysis, the main factors affecting photodegradation rate are picked out, and EHOMOhas the highest correlation coefficient with logkbas the committed step of PAHs’photodegradation in aqueous system is ejecting electrons. The higher EHOMOis, the more easily electron will be excited and the more easily molecular will be degraded. By multiple linear regression analysis, the QSAR model for chemical structure of PAHs and their photodegradation activity is successfully established. From the comparison between experimental and predicted values and that between the distribution of regression standardized residuals and the leave-one-out cross-validation, it can be found that the established QSAR model has good robustness and predictive ability. Comparison between the photodegradation of PAHs with their biodegradation shows that the committed step of biodegradation is that the effects of microorganisms make the chemical bond break, while in the committed step of photodegradation PAHs eject electrons.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 40976041 and 20775074). We are also grateful to the reviewers of the paper.
Barone, V., Cossi, M., and Tomasi, J., 1998. Geometry optimization of molecular structures in solution by the polarizable continuum model. Journal of Computational Chemistry, 19: 404-417.
Chen, J., Peijnenburg, W., Quan, X., Che, S., Martens, D., Schramm, K. W., and Kettrup, A., 2001. Is it possible to develop a QSPR model for direct photolysis half-lives of PAHs under irradiation of sunlight? Environmental Pollution, 114: 137-143.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J. A., Vreven, J. T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., and Pople, J. A., 2003. Gaussian 03. Revision B.05. Gaussian, Inc., Pittsburgh, PA.
Fasnacht, M. P., and Blough, N. V., 2002. Aqueous photodegradation of polycyclic aromatic hydrocarbon. Environmental Science and Technology, 36: 4364-4369.
Grimmer, G., and Misfeld, J., 1983. Environmental carcinogens: A risk for man? Concept and strategy of the identification of carcinogens in the environment. In: Environmental Carcinogens: Polycyclic Aromatic Hydrocarbons, Chemistry, Occurrence, Biochemistry, Carcinogenicity. Grimmer, G., ed., CRC Press, Boca Raton, FL, 1-26.
Grimmer, G., and Pott, F., 1983. Occurrence of PAH. In: Environmental Carcinogens: Polycyclic Aromatic Hydrocarbons, Chemistry, Occurrence, Biochemistry, Carcinogenicity. Grimmer, G., ed., CRC Press, Boca Raton, FL, 61-128.
Jones, R., 1984. Physical and Mechanistic Organic Chemistry. Cambridge University Press, Cambridge, 94-114.
James, T. H., 1985. Chemical reaction dynamics in solution. Annual Review of Physical Chemistry, 36: 573-597.
Lu, G. N., Dang, Z., Tao, X. Q., and Zhang, D. C., 2005. Quantum chemistry study on photolysis activity of polycyclic aromatic hydrocarbons. Environmental Chemistry, 24 (4): 459-462.
Mekenyan, O. G., Ankley, G. T., Veith, G. D., and Call, D. J., 1994. QSARs for photoinduced toxicity: 1. Acute lethality ofpolycyclic aromatic hydrocarbons to Daphnia magna. Chemosphere, 28: 567-582.
Reichardt, C., 1990. Solvent Effects in Organic Chemistry. Wiley-VCH, Marburg, 147-181.
Simkin, B. Y., and Sheikhet, I., 1995. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach. Ellis Horwood, London,1-44.
Smith, J. H., Mabey, W. R., Bahonos, N., Holt, B. R., Lee, S. S., Chou, T. W., Venberger, D. C., and Mill, T., 1979. Environmental Pathways of Selected Chemicals in Fresh Water Systems: Part II. Laboratory Studies (Interagency Energy- Environment Research Report EPA-600/7-78-074). Environmental Research Laboratory Office of Research and Development, US Environmental Protection Agency, Athens, GA.
Sun, P. Y., Gao, Z. H., Wang, X. P., Zhou, Q., Zhao, Y. H., and Li, G. M., 2011. Application of a step-by-step fingerprinting identification method on a spilled oil accident in the Bohai Sea area. Journal of Ocean University of China, 10 (1): 35-41.
Sundberg, R. J., and Carey, F. A., 2007. Advanced Organic Chemistry: Structure and Mechanisms. Springer, New York, 359-376.
Sarwar, M. G., Dragisic, B., Salsberg, L. J., Gouliaras, C., and Taylor, M. S., 2010. Journal of the American Chemical Society, 132 (5): 1646-1653.
Wang, Y., Li X. G., Peng, X. W., Tang, X. L., and Deng, X. Y., 2012. Optimization of sample pretreatment for determination of polycyclic aromatic hydrocarbons in estuarine sediments by gas chromatography. Journal of Ocean University of China, 11 (2): 159-164.
Xia, S. W., Mao Y. P., Xue, Q. Q., and Yu, L. M., 2011, QSAR and molecular design of some quinoline derivatives as antimicrobial. Chemical Journal of Chinese Universities, 32 (10): 2415-2420.
Xu, X., Li, X. G., and Sun, S. W., 2012. A QSAR study on the biodegradation activity of PAHs in aged contaminated sediments. Chemometrics and Intelligent Laboratory Systems, 114: 50-55.
Zepp, R. G., and Schlotzhauer, P. F., 1979. In: Polynuclear Aromatic Hydrocarbons. Jones, P. W., and Leber, P., eds., Ann Arbor Science Publishers, Ann Arbor, MI, 141-158.
(Edited by Ji Dechun)
* Corresponding author. Tel: 0086-532-66782215
E-mail: lixg@ouc.edu.cn
(Received May 12, 2012; revised March 4, 2013; accepted March 22, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
杂志排行
Journal of Ocean University of China的其它文章
- Properties of Klebsiella Phage P13 and Associated Exopolysaccharide Depolymerase
- Comparative Study on the Allergenicity of Different Litopenaeus vannamei Extract Solutions
- Isolation and Characterization of a Fucoidan-Degrading Bacterium from Laminaria japonica
- Toxicity of Five Phenolic Compounds to Brine Shrimp Artemia sinica (Crustacea: Artemiidae)
- Application of CFD Modeling to Hydrodynamics of CycloBio Fluidized Sand Bed in Recirculating Aquaculture Systems
- DNA Barcoding Assessment of Green Macroalgae in Coastal Zone Around Qingdao, China
