Analgesis and Wound Healing Effect of Chitosan and Carboxymethyl Chitosan on Scalded Rats
2014-04-20HUANGShuyaHANBaoqinSHAOKaiYUMiaoandLIUWanshun
HUANG Shuya, HAN Baoqin, SHAO Kai, YU Miao, and LIU Wanshun
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
Analgesis and Wound Healing Effect of Chitosan and Carboxymethyl Chitosan on Scalded Rats
HUANG Shuya, HAN Baoqin, SHAO Kai, YU Miao, and LIU Wanshun*
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
Analgesis and wound healing effect of chitosan and carboxymethyl chitosan on scalded rats were investigated. A II degree scald model was established in rats, which was subsequently treated with chitosan and carboxymethyl chitosan solution, respectively. The concentration of bradykinin and 5-hydroxytryptophan was detected by assaying enzyme-linked immunosorbent. Healing condition was observed and pathological sections were made to determine the healing effect of chitosan and carboxymethyl chitosan. Results showed that the concentration of bradykinin and 5-hydroxytryptophan peaked at the third hour post-wound in all groups, while the concentration of hydroxyproline peaked at the seventh day post-wound in both chitosan and carboxymethyl chitosan group. The concentration of bradykinin and 5-hydroxytryptophan of carboxymethyl chitosan group was significantly lower than that of control (P < 0.05), while that of chitosan group was similar to that of control (P > 0.05). These findings indicated that carboxymethyl chitosan reduced the concentration of algogenic substances, resulting in analgesia. During the whole recovery process, the hydroxyproline concentration in chitosan and carboxymethyl chitosan group on day 3 and 7 was significantly higher than that of control (P < 0.01); however the significance of such a highness decreased on day 14 (P < 0.05). These findings indicated that chitosan and carboxymethyl chitosan accelerated tissue repair. Meanwhile, chitosan performed better in healing than carboxymethyl chitosan in both decrustation and healing time. In conclusion, carboxymethyl chitosan showed significant analgesis and wound-healing promotion effect, but chitosan only showed wound-healing promotion effect.
bradykinin; 5-hydroxytryptophan; hydroxyproline; carboxymethyl chitosan
1 Introduction
Inflammation is at the root of most serious complications occurring after infection and injury. With the aim of establishing an immune barrier against invading microorganisms, inflammation leads to a reduced threshold of nociceptors, resulting in pain (Julius and Basbaum, 2001). Algesthesia, which is triggered by tissue injury, may be evoked by some endogenous chemical substances, such as histamine, 5-hydroxytryptophan (5-HT) and prostaglandin and bradykinin (BK). These substances are algogenic, which have profound impact on capillary vasomotor state, vessel permeability and the production of algesthesia (Dray, 1995).
Bradykinin, a nonapeptide, is known as the first inflammatory mediator which has a potent algogenic property. Bradykinin shows proinflammatory effect, causing the release of prostanoids, cytokines and free raicals from a variety of cells (Dray, 1995). Certainly, bradykinin is not the only inflammatory mediator, 5-HT is another typical mediator, which is an aromatic amino acid derived from essential L-tryptophan (Birdsall, 1998). As a neurotransmitter, 5-HT mainly distributes in pineal gland and hypothalamus on capillary vasomotor state, vessel permeability and the production of algesthesia.
Collagen is a major part of animal tissues. It provides support, repair and protection for animal tissues. After the tissue injury, collagen can repair the defect and restore the organizational structure and function of tissues, which laying the foundation for the formation of wound cell matrix. But an increased collagen deposition in the wound may lead to the fibrosis. Hydroxyproline is a collagen specific amino acid, which is traditionally used to quantify this protein (Hofman et al., 2011). The concentration of hydroxyproline in different tissues can be considered as an important index of metabolism.
Chitosan, the deacetylated chitin, is the only alkaline polysaccharide in nature (Khor and Lim, 2003). Because of its biocompatibility, biodegradability and bioactivity (Chung et al., 1994), chitosan has been widely used as medicine (Mistra et al., 2001) and biomedical (Muzzarelli and Muzzarelli, 2005) and tissue engineering materials (Yilgor et al., 2009; Abarrategi et al., 2010). However, the insolubility of chitosan has greatly limited its application. Carboxymethyl chitosan is a derivative of chitosan with better water solubility and biological degradability(Lu et al., 2007), thus is more widely used than chitosan. A myriad of studies have documented that chitosan and carboxymethyl-chitosan effectively accelerate wound healing and reduce scar formation (Biagini et al., 1991). They effectively promote wound healing by accelerating collagen synthesis and the propagation of fibroblasts (Kojima et al., 2004; Peng et al., 2011). However, the analgesis of chitosan and carboxymethyl-chitosan has not been investigated.
In this study, the rats scald model was established to explore the analgesis of chitosan and carboxymethylchitosan and their effect on wound healing.
2 Materials and Methods
2.1 Materials
72 adult Sprague-Dawley rats, 200 g ± 20 g in body weight, Male and female, were purchased from Qingdao Institute for Drug Control, China. Chitosan (CTS, deacetylation degree 95%) and carboxymethyl-chitosan (CMCTS, substitution degree 98.6%, deacetylation degree 91.3%) were purchased from Biotemed Co., Ltd. of China. Rats bradykinin and 5-HT ELISA kit were obtained from R&D Systems, USA. Hydroxyproline kit was manufactured by Nanjing JianCheng Bioengineering Institute, China. Thermo Fisher 1510 Microplate Reader was the product of Thermo Fisher Scientific, USA.
2.2 Scald Models and Analgesis Experiments
Rats were raised for 5 d by feeding standard laboratory food and water. Before experiment, the normal concentration of BK and 5-HT in tail venous blood for 10 rats were determined by bradykinin and 5-HT ELISA kits respectively, in order to determine the normal concentration of BK and 5-HT. After the wounded tails recovered, 72 rats were randomly divided into 3 experimental groups, control (n=24), CTS (n=24), CMC (n=24), half female and half male each group.
In order to establish II degree scald model, all the rats of 3 groups were shaved on the back with an electric razor, and the shaved skin was touched 100 mL of hot water (95℃ ± 1℃) in a glass tube (2.8cm in diameter) for 15 s as was described early (Vorauer-Uhl et al., 2001). Animals were raised independently in cages. At 3 h, 6 h, 12 h after scalding, 8 rats were randomly selected from each group for taking venous blood from tails in order to determine the concentration of BK and 5-HT.
2.3 Healing Promotion Effect Analysis
After being scalded, the rats were exposure to either chitosan or carboxymethyl chitosan for 40 d in order to therapy their wounds. Sterile saline, 3% CTS in acetic acid (about pH 6) and 3% CM-CTS in saline were applied to the wounds of scalded rats, respectively, twice a day. The wound healing state was observed with the wound areas and the scabs off time were evaluated every day.
2.4 Pathological Observation of Scalded Skin and Determination of Wound Organization Hydroxyproline Content
Rats were executed on days 3, 7, 14 and 28 after being wounded, and their skin biopsies were taken from their wounds. A part of biopsy was fixed in formalin and analyzed with paraffin slice HE dying. Colorimetry was used to assay hydroxyproline level of the remaining biopsy. At the same time, normal rats were as the comparison.
3 Results
At 3 h after being scalded, the concentration of 5-HT and BK in venous blood reached the highest, and then descended to the normal at 12 h. The concentration of 5-HT and BK of CTS group was similar to that of the control; however, the concentration of 5-HT and BK of CMC group was significantly lower than that of control and CTS group, and more close to the normal level (Fig.1).
The hydroxyproline concentration in wound skin tissue of CTS and CMC group peaked at day 7 after being scalded (Fig.2). During the whole process, hydroxyproline concentration of CTS and CMC group was significantly higher than that of control.
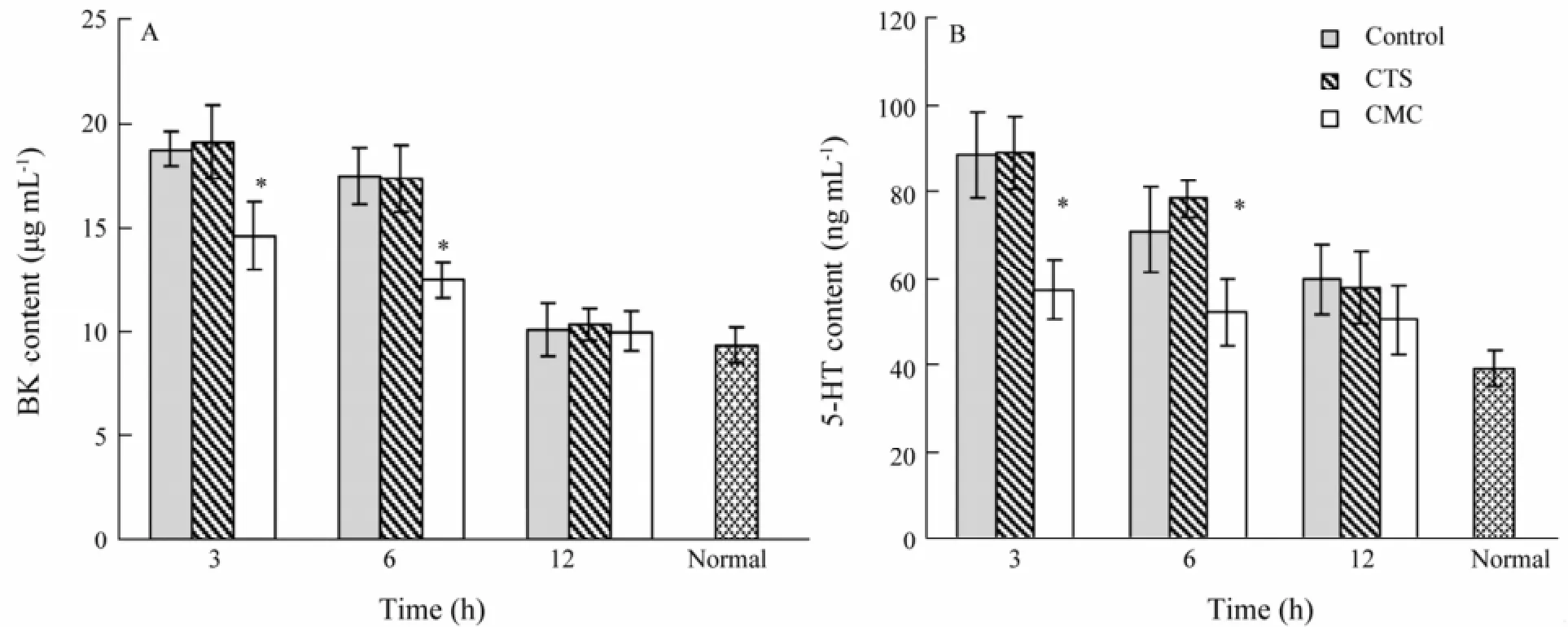
Fig.1 Effect on the synthesis of bradykinin (BK) and 5-hydroxytryptophan (5-HT). *, P < 0.05 vs. control, CTS.
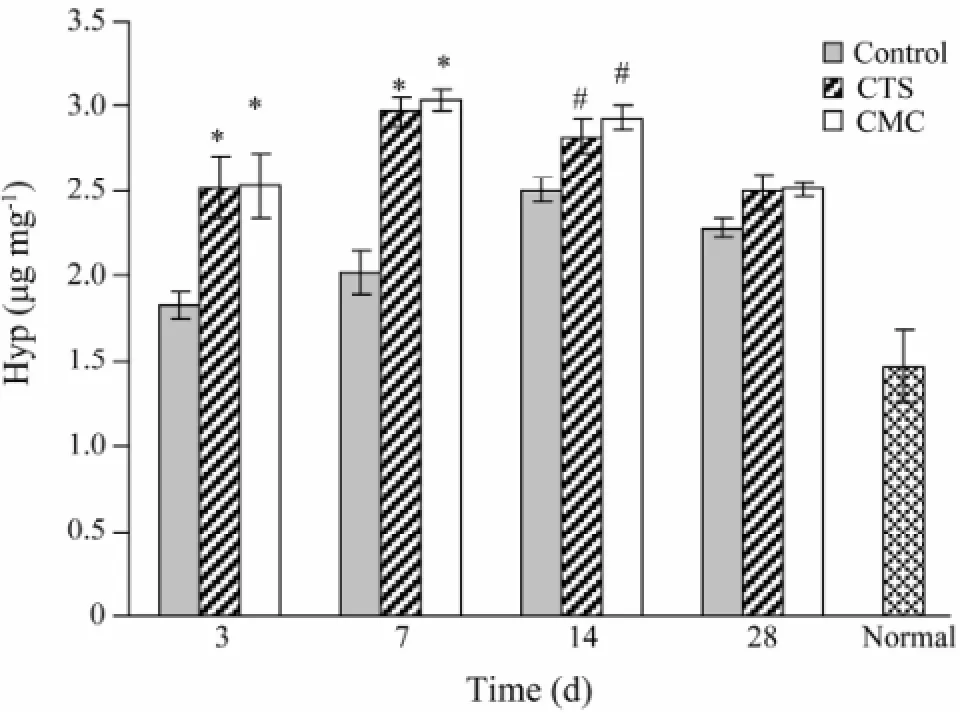
Fig.2 Hydroxyproline content after being scalded. *, P <0.01, #, P < 0.05 vs. control.
Inflammation and healing of wound skin tissue were shown in Fig.3. Epidermis of all 3 groups partially disappeared on day 3. The cutaneous appendages shrunk. Migration of macrophages was accelerated by chitosan and carboxymethyl chitosan treatment. Large areas of inflammatory cell infiltrates were observed in wound skin tissue of all three groups at day 7, while inflammatory cell was more obvious in control. At day 14, inflammation response disappeared in all groups and tissue granulation was accelerated by chitosan and carboxymethylchitosan treatment. At day 28, re-epithelization was totally completed in CTS and CMC group, while only partial re-epithelization was found in control. As showed in Table 1, CMC group had better healing result than CTS group, as its decrustation (19.0 ± 1.2 d vs. 17.0 ± 1.3 d, P < 0.05) and healing time (34.0 ± 1.6 d vs. 30.0 ± 1.1 d, P < 0.05) were short.
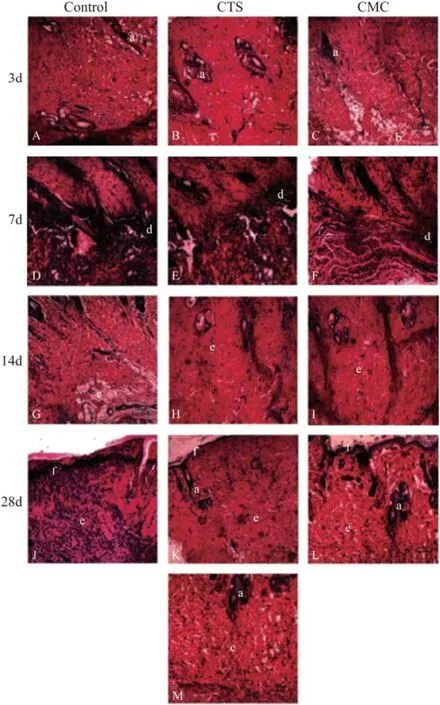
Fig.3 Pathological images of the wounded skin by H&E staining (A–M), HE sections from day 3 to day 28 (A–L). Image of the normal skin without injury (M); Large zones of inflammatory cell infiltrates observed in all three groups on day 7 (D, E and F). Inflammation response disappeared in all three groups on day 14 (G, H and I). Images on day 28 showed abundant fibroblasts in the dermis and good keratinocyte maturation in the epidermis (L), compared with control and CTS group on the same day, in which the re-epithelization had not been completed. a, hair follicle; b, muscle; c, adipose tissue; d, inflammatory infiltrate; e, dermis; f, epidermis.

Table 1 Decrustation and Healing time of wounds
4 Discussion
Chitosan and its derivatives have been extensively investigated for various biomedical and pharmaceutical applications because of their low toxicity, excellent biocompatibility and biodegradability (Mourya and Inamdar, 2008) (Aranaz et al., 2009). Meanwhile, they have numerous beneficial functions including stopping bleeding, promoting healing and functioning as good bacteriostat (Chang et al., 2008; Muzzarelli, 2009), and have been applied in different biomedical areas (Dash et al., 2011). However, their analgesis has not been documented. This experiment explored the algesthesia of scald rats, aiming to find whether chitosan and its derivatives have analgesis. Both 5-hydroxytryptophan and bradykinin belong to algogenic substances. They are released upon damages to the cells and impact the production of algesthesia. It has been reported that BK is a physiologic mediator of pain (Regoli and Barabe, 1980), and BK antagonists have analgesic activity in both acute and chronic pain model (Steranka et al., 1988), while 5-HT is involved in spontaneous pain from inflamed peripheral tissues through excitation or sensitization of fine afferent units by 5-HT3receptor (Babenko et al., 1999). Thus the concentration of 5-HT and BK can reflect the degree of pain. Moreover, during wound repairing process, the collagen synthesis and secretion are decisive factors. Hydroxyproline of animal tissues is mainly found in collagen, so the concentration of hydroxyproline can reflect wound healing situation (Nemethy and Scheraga, 1986).
Our experiments showed that 5-HT and BK concentration of CMC group was significantly lower than that of control and CTS group, and peaked in 3 h post-wound. This indicated that carboxymethyl chitosan had better analgesis and anti-inflammatory effect than chitosan. The hydroxyproline concentration of CTS and CMC group was higher than that of control during the whole process, indicating that collagen synthesis promoted the wound healing process. The analysis of tissue slice showed that the inflammatory reaction of CTS and CMC group was lighter than that of control. Within healing time, No hyperplasia phenomenon of post-wound collagen alignment were found in CTS and CMC group compared with the normal skin.
From our study, we found that carboxymethyl chitosan had analgesis and wound healing effect, while chitosan had wound healing effect only. When tissue injury or burn occurs, pain happens the first time by forming inflammatory reaction. Hyperblastosis repairing, wound remodeling generate gradually accompanied by formation of scar tissue usually. In particular after the occurrence of burn, burn tissue repair process is often accompanied by excessive hyperplasia causing serious scar tissue. The analgesis, anti-inflammatory effect, and promoting healing of burn tissue of carboxymethyl chitosan, making it play an important role in burn wound and tissue healing, which can significantly reduce pain and inflammation and restrain the formation of burn scar in tissue healing. These findings provided some clues to the analgesic mechanism of chitosan and its derivative. The innovation of the study was that we used the concentration of inflammatory mediators to measure the degree of the pain, this method was more convincing. But there are some problems unresolved, such as whether the weak acidity of chitosan solution stimulated the wound which covered the analgesis of chitosan, and what is the analgesic mechanism of carboxymethyl chitosan, etc. From the results, we can find there must be more researches in the future, our initial idea is to carry out cell experiment, we can inject the inflammatory mediators receptors into cells and study the effect of our materials to the expression of these receptors. All in all, there is a long way, we will continue our research.
5 Conclusions
In conclusion, carboxymethyl chitosan showed analgesis while chitosan did not. Both of them showed the same effect on promoting wound healing. The carboxymethyl chitosan analgesic mechanism needs to be deciphered future.
Acknowledgement
This work was supported by National High-Tech R&D Program of China (863 Program, 2014AA093605).
Abarrategi, A., Lópiz-Morales, Y., Ramos, V., Civantos, A., López-Durán, L., Marco, F., and López-Lacomba, J. L., 2010. Chitosan scaffolds for osteochondral tissue regeneration. Journal of Biomedical Materials Research Part A, 95: 1132-1141.
Aranaz, I., Mengíbar, M., Harris, R., Paños, I., Miralles, B., Acosta, N., Galed, G., and Heras, A., 2009. Functional characterization of chitin and chitosan. Current Chemical Biology, 3: 203-230.
Babenko, V., Graven-Nielsen, T., Svensson, P., Drewes, A., Jensen, T. S., and Arendt-Nielsen, L., 1999. Experimental human muscle pain induced by intramuscular injections of bradykinin, serotonin, and substance P. European Journal of Pain, 3: 93-102.
Biagini, G., Bertani, A., Muzzarelli, R., Damadei, A., DiBenedetto, G., Belligolli, A., Ricotti, G., Zucchini, C., and Rizzoli, C., 1991. Wound management with N-carboxybutyl chitosan. Biomaterials, 12: 281-286.
Birdsall, T. C., 1998. 5-Hydroxytryptophan: A clinically-effecttive serotonin precursor. Alternative Medicine Review, 3: 271-280.
Chang, J., Liu, W. S., Han, B. Q., and Liu, B., 2008. Theevaluation on biological properties of carboxymethyl-chitosan and carboxymethyl-chitin. Journal of Ocean University of China, 7: 404-410.
Chung, L. Y., Schmidt, R. J., Hamlyn, P. F., Sagar, B. F., Andrews, A. M., and Turner, T. D., 1994. Biocompatibility of potential wound management products: Fungal mycelia as a source of chitin/chitosan and their effect on the proliferation of human F1000 fibroblasts in culture. Journal of Biomedical Materials Research, 28 (4): 463-469.
Dash, M., Chiellini, F., Ottenbrite, R., and Chiellini, E., 2011. Chitosan–A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science, 36: 981-1014.
Dray, A., 1995. Inflammatory mediators of pain. British Journal of Anaesthesia, 75: 125-131.
Hofman, K., Hall, B., Cleaver, H., and Marshall, S., 2011. High-throughput quantification of hydroxyproline for determination of collagen. Analytical Biochemistry, 417: 289-291.
Julius, D., and Basbaum, A. I., 2001. Molecular mechanisms of nociception. Nature, 413: 203-210.
Khor, E., and Lim, L. Y., 2003. Implantable application of chitin and chitosan. Biomaterials, 24: 2339-2349.
Kojima, K., Okamoto, Y., Kojima, K., Miyatake, K., Fujise, H., Shigemasa, Y., and Minami, S., 2004. Effects of chitin and chitosan on collagen synthesis in wound healing. Journal of Veterinary Medical Science, 66: 1595-1698.
Lu, G. Y., Kong, L. J., Sheng, B. Y., Wang, G., Gong, Y. D., and Zhang, X. F., 2007. Degradation of covalently crosslinked carboxymethyl chitosan and its potential application for peripheral nerve regeneration. European Polymer Journal, 43: 3807-3818.
Mistra, S., Gaur, U., Ghosh, P. C., and Maitra, A. N., 2001. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. Journal of Controlled Release, 74: 317-323.
Mourya, V. K., and Inamdar, N. N., 2008. Chitosan-modifi cations and applications: Opportunities galore. Reactive and Functional Polymers, 68: 1013-1051.
Muzzarelli, R. A. A., 2009. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydrate Polymers, 76: 167-182.
Muzzarelli, R. A. A., and Muzzarelli, C., 2005. Chitosan chemistry: Relevance to the biomedical sciences. Advances in polymer Science, 186: 151-209.
Nemethy, G., and Scheraga, H. A., 1986. Stabilization of collagen fibrils by hydroxyproline. Biochemistry, 152: 267-273.
Peng, S. K., Liu, W. S., Han, B. Q., Chang, J., Li, M. Y., and Zhi, X., 2011. Effects of carboxymethyl-chitosan on wound healing in vivo and in vitro. Journal of Ocean University of China, 10: 369-378.
Regoli, D., and Barabé, J., 1980. Pharmacology of bradykinin and related kinins. Pharmacological Reviews, 32:1-46.
Steranka, L. R., Manning, D. C., Dehaas, C. J., Ferkany, J. W., Borosky, S. A., Connor, J. R., Vavrek, R. J., Stewart. J. M., and Snyder, S. H., 1988. Bradykinin as a pain mediator: Receptors are localized to sensory neurons and antagonists have analgesic actions. Proceedings of the National Academy of Sciences of the United States of America, 85: 3245-3249.
Vorauer-Uhl, K., Furnschlief, E., Wagner, A., Ferko, B., and Katinger, H., 2001. Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. European Journal of Pharmaceutical Sciences, 14: 63-67.
Yilgor, P., Tuzlakoglu, K., Reis, R. L., Hasirci, N., and Hasirci, V., 2009. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials, 30: 3551-3559.
(Edited by Qiu Yantao)
(Received January 23, 2013; revised April 15, 2013; accepted April 25, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-82032105
E-mail: WanshunLiu@hotmail.com
杂志排行
Journal of Ocean University of China的其它文章
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- The Appearance of Ulva laetevirens (Ulvophyceae, Chlorophyta) in the Northeast Coast of the United States of America
- Metabolic and Phylogenetic Profile of Bacterial Community in Guishan Coastal Water (Pearl River Estuary),South China Sea
- Distribution and Source of Main Contaminants in Surface Sediments of Tidal Flats in the Northern Shandong Province
- Histological Observation of Germ Cell Development and Discovery of Spermatophores in Ovoviviparous Black Rockfish (Sebastes schlegeli Hilgendorf) in Reproductive Season
- Identification of Five Sea Cucumber Species Through PCR-RFLP Analysis
