Histological Observation of Germ Cell Development and Discovery of Spermatophores in Ovoviviparous Black Rockfish (Sebastes schlegeli Hilgendorf) in Reproductive Season
2014-04-20FENGJunrongLIULimingJIANGHaibinWANGMaojianandDURongbin
FENG Junrong, LIU Liming, JIANG Haibin, WANG Maojian, and DU Rongbin,
1) Ocean College, Yantai University, Yantai 264005, P. R. China
2) Shandong Marine Fisheries Research Institute, Yantai 264006, P. R. China
Histological Observation of Germ Cell Development and Discovery of Spermatophores in Ovoviviparous Black Rockfish (Sebastes schlegeli Hilgendorf) in Reproductive Season
FENG Junrong1), LIU Liming1), JIANG Haibin2), WANG Maojian2), and DU Rongbin1),*
1) Ocean College, Yantai University, Yantai 264005, P. R. China
2) Shandong Marine Fisheries Research Institute, Yantai 264006, P. R. China
Black rockfish (Sebastes schlegeli) is an important species for culture; however, its reproductive characteristics have not been fully documented. In this study, we investigated the morphology and developmental process of germ cells in this ovoviviparous rockfish in reproductive season (October 2011–November 2012) with histological methods. We found that the gonad of mature fish showed notable seasonal changes in developmental characteristics and morphological structure. The sperm cells matured during a period lasting from October to December, significantly earlier than the oocytes did. A large number of spermatozoa and other cells occurred in testis at different developmental stages. Vitellogenesis in oocytes began in October, and gestation appeared in April next year. Spermatophores were discovered for the first time in Sebastes, which assembled in testis, main sperm duct, oviduct and genital tract, as well as ovarian cavity in October and April. These organs may serve either as production or hiding places for spermatophores and spermatozoa which were stored and transported in form of spermatophores. Testicular degeneration started from the distal part of testis in April, with spermatophores assembled in degenerating testis and waiting for transportation. The copulation probably lasted for a long period, during which the spermatozoa were discharged in batches as spermatophores. These spermatophores were coated with sticky materials secreted from the interstitial areas of testis and the main sperm duct, then transported into ovary.
Sebastes schlegeli; germ cell development; spermatophore; copulation period
1 Introduction
Black rockfish (Sebastes schlegeli Hilgendorf) is of economical importance for artificial reef enhancement and net-cage culture in coastal areas, Northern China. Being an ovoviviparous fish, the reproductive performance of S. schlegeli is of great interest to researchers. Previously, Eldridge et al. (1991) studied the sex ratio, age and size of S. flavidus in Cordell Bank throughout an annual cycle and examined its associating seasonal changes in gonad and fecundity. Takano et al. (1991) described the annual reproductive and spawning cycle of female Sebastiscus marmoratus. In recent decades, Beak et al. (2000) investigated the gonadosomatic and hepatosomatic index, and histological feature of the gonad and the plasma level of sex steroid hormone of S. schlegeli. Mori et al. (2003) researched the gonadal development and serum profile in reared S. schlegeli for a full year. Tsang et al. (2007) examined the reproductive performance of captured near shore rockfish. More recently, Yang et al. (2010) reported the morphology and developmental histology of S. schlegeli testis, and Shi et al. (2011) studied the annual change in S. schlegeli during ovarian development.
It was commonly found that the development of ovary and testis of S. schlegeli is asynchronic; the maturation of its sperm cells is significantly earlier than oocytes do. However, the copulation performance of S. schlegeli has rarely been studied to date (Helvey, 1982), and the transportation and storage of spermatozoa in rockfish remain unclear. In the present study, the histological structure of gonad as well as oviduct, genital tract and main sperm duct in black rockfish during reproductive season was studied in order to clarify the reproductive biology and provide reference data for its artificial reproduction.
2 Materials and Methods
Individuals of S. schlegeli (body weight > 400 g) were obtained from a commercial fish culturing farm in Yantai, China. The fish were taken from pond in October and December, 2011 and April and November, 2012 in order to investigate histological changes in the reproductive system of S. schlegeli during development and to elucidate the reproductive mechanism of ovoviviparous fish. All fish individuals were temporarily cultured for twodays before being weighed. The fish were sacrificed for internal examination of the gonad followed by histological examination. Altogether the gonad of 14 rockfish, 6 males and 8 females, were collected. The gonads were examined macroscopically and then fixed in Bouin’s fluid, dehydrated in an ethyl alcohol series, embedded in paraffin, sectioned to 5–7 μm, and stained with Delafield’s haematoxylin or Hansen’s haematoxylin followed by eosin. The prepared specimens were examined and photographed with an Olympus BX51-DP72 microscope system. The developmental stages of gonad specimens were determined according to the reference standard commented early (Lin and You, 2000; Lin et al., 2000).
3 Results
3.1 Development of Ovary
In October, the ovary of rockfish was small in size, pinkish-white and sac-like. The ovarian wall was thick and opaque. Thus, the eggs were invisible. In November and December, the ovary enlarged and turned yellow. The ovarian wall became thinner and more translucent; showing the eggs tightly adhered each other. It was notable that dark spot-like substances were observable underneath the ovarian wall in early December (Fig.1a). In April next year, the ovary was relatively large and orange-colored while the ovarian wall was even thinner and more transparent, turgid with free eggs and abundant ovarian fluid. The blood vessels gradually thickened and their abundance increased in the ovary during development.
Light microscopic observation showed that during studying period, the ovarian development involved 3 stages while the development of oocytes involved 4 phases in terms of morphological characteristics. The ovary in different developmental stages contained various phases of oocytes.
At stage III (October), the ovary consisted of the 2ndand 3rdphase oocytes. In the early 2ndphase, the oocytes were round-shaped and 38– 46 μm in average diameter. The cytoplasm was highly basophilic, and no follicular envelope surrounded the cells. In the late 2ndphase, the oocytes were round, oval, or irregular-shaped, 77–100 μm in length and 45–73 μm in width. A layer of loose follicle cell surrounded the oocytes. The cytoplasm was basophilic while the nucleus was acidophilic. The 3rdphase oocytes had 1–2 layers of follicle cell that were spindle-shaped, 133–177 μm in length and 77–127 μm in width. The cytoplasm was mild basophilic, and 1–2 layers of vacuole formed (Fig.1b).
At stage IV (December), the ovary mainly contained 4thphase oocytes with a number of 3rdphase cells and a few 2ndphase cells. The 4thphase oocytes were 292–365 μm in length and 195–234 μm in width, and 2 layers of follicle cells located outside and nucleus located the central of the cell. Yolk granules began to deposit around the edge of the cytoplasm with a detectable zona radiata. At the late 4thphase, the oocytes were 410–539 μm in length and 359– 411 μm in width, and the cytoplasm was mostly filled with round yolk granules (Figs.1c, d). In the ovaries at stage IV (early December), there were masses of brown and black substances between oocytes. Results of biopsy showed that capsule-like materials were released from the masses (Fig.1a), and microscopic observation of the sections indicated that the masses tended to scatter in the connective tissue near the 4thphase oocytes (Fig.1c).
At stage VI (April), the ovary was filled with embryos at different developmental stages. The diameter of the eggs was 443–820 μm (Fig.1e).
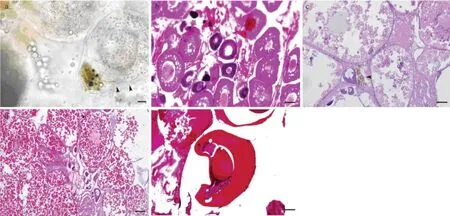
Fig.1 Histological changes in the ovary of female Sebastes schlegeli during development. a, Biopsied ovary collected in early December. Arrows indicate the spermatophores just released from the mass. Scale bar = 20 μm; b, Section of stage-III ovary collected in October. Scale bar = 50 μm; c, Section of stage-IV ovary collected in December with oocytes in an early vitellogenic period. Arrow indicates spermatophores between oocytes. Scale bar = 50 μm; d Section of stage-IV ovary collected in December with oocytes in a late vitellogenic period. Scale bar = 100 μm; e, Embryo in ovary collected in April. Scale bar = 100 μm.
3.2 Development of Testis
Results of anatomical study showed that spermatogenesis occurred in the testis of mature rockfish during a period lasting from October to December. The small, grayish white testis of mature fish gradually enlarged and turned white. When crosscut and gently squeezed, the testis released white, dense seminal fluid. In December, the testis of subadult fish showed a thin, translucent, elongated pinkish structure. In April, testicular degeneration was observed. The testis, which remained pinkish, gray, and lumpy near the urogenital sinus, had degenerated to a black ribbon in distal part.
The testis of rockfish was of lobular type (Yang et al., 2010), with the long independent main sperm duct slightly depressed along the ventral side of testis in which the efferent duct and blood vessel distributed. Microscopic observation demonstrated that the testis was a large network of units called testicular lobule. There were lobule lumens at the center of testicular lobules and spindle-shaped fibroblast cells as well as interstitial cells between lobules. The testicular lobule was composed of a number of spermatogenic cysts showing developmental asynochronization. Within a spermatogenic cyst, the germ cells were at the same developmental stage. The interstitial cells dispersed between testicular lobules were round or oval-shaped, small in size, and usually purple red-dyed.
At the stage of spermatogenic proliferation, the sperm cells were mainly round or oval-shaped primary spermatogonia lined along lobular wall. The cells were 5.18–8.93 μm in length and 3.56–5.18 μm in width, containing deep-stained nucleus and light-stained cytoplasm (Fig.2a).

Fig.2 Histological changes in the testis of male Sebastes schlegeli during development. a, Testis at stage of spermatogonic proliferation, collected in December. Scale bar = 20 μm; b, Testis at stage of early spermatogenesis, collected in October. scale bar = μm; c, Testis at stage of medium spermatogenesis, collected in October. Scale bar = 50 μm; d, Testis at stage of late spermatogenesis, collected in December. Scale bar = 10 μm; e, Testis at stage of late spermatogenesis, collected in December and containing spermatophores. Scale bar = 20 μm; f, Degenerating testis collected in April, which contained spermatophores. Scale bar = 50 μm. sgI, primary spermatogonia; sgII, secondary spermatogonia; scI, primary spermatocyte; scII, secondary spermatocyte; st, spermatid; sz, spermatozoa; and spp, spermatophore.
At the stage of early spermatogenesis, the sperm cells became diversified. The testicular lobule was composed of a number of spermatogenic cysts, and the development of germ cells in the same spermatogenic cyst was in synchronization. In the spermatogenic cyst, there existed primary and secondary spermatogonia and spermatocytes as well as spermatids. A limited number of primary spermatogonia occurred in the wall of testicular lobules and a few secondary spermatogonia, about 4.5 µm in diameter and usually purple-dyed, assembled in a small spermatogenic cyst near the wall of testicular lobules. The primary spermatocytes were round and 2.48–3.71 μm in size, dispersed in a cyst much bigger than that of spermatogonia. Their nucleus was basophilic and blue purple-dyed while the cytoplasm was difficult to be distinguished. The secondary spermatocytes were round and 1.33–2.49 μm in size, with chromophobe cytoplasm and even more basophilic nucleus. Most spermatids were crescent (1.96–2.27 μm in length) and highly basophilic (Fig.2b).
During testicular development, the primary spermatogonia disappeared while the lobule lumen was filled with spermatozoa, the smallest cells in the testis. A large number of crescent blue spermatozoa assembled in a whirlpool pattern in the lobule lumen, whose tail was difficult to be distinguished (Fig.2c).
At the late spermatogenic stage, spermatocytes, spermatids and spermatozoa occurred in the testicular lobule. In addition, there existed a large number of empty sper-matogenic cysts that had discharged the spermatozoa. The lobule lumen substantially enlarged and was merged into the network (Fig.2d).
During the discharge of spermatozoa, the wall of testicular lobule thickened and the quantity of primary spermatogonia increased significantly (Fig.2e).
When spermatozoa were discharged completely, the testis degenerated into the stage of spermatogenic proliferation. In April, the distal part of the testis had degenerated into a black ribbon while the proximal part near the urogenital sinus remained large and pinkish gray. These observations assured that testicular degeneration started from the distal part of testis. It was notable that a large number of oval black substances existed in the proximal part of the degenerating testis (Fig.2f).
At each developmental stage, there were unknown deep-brown structures scattered in interstitial area of testis, especially in spermatogenic testis. The deep-brown structures tended to scatter near blood vessels, with higher density near sperm-duct than in other part of testis (Figs.2e, 3a–c) and a few tiny round granules scattered nearby. The quantity of interstitial cells decreased with the maturation of testis, and it was difficult to find these cells when the majority of sperm cells matured.
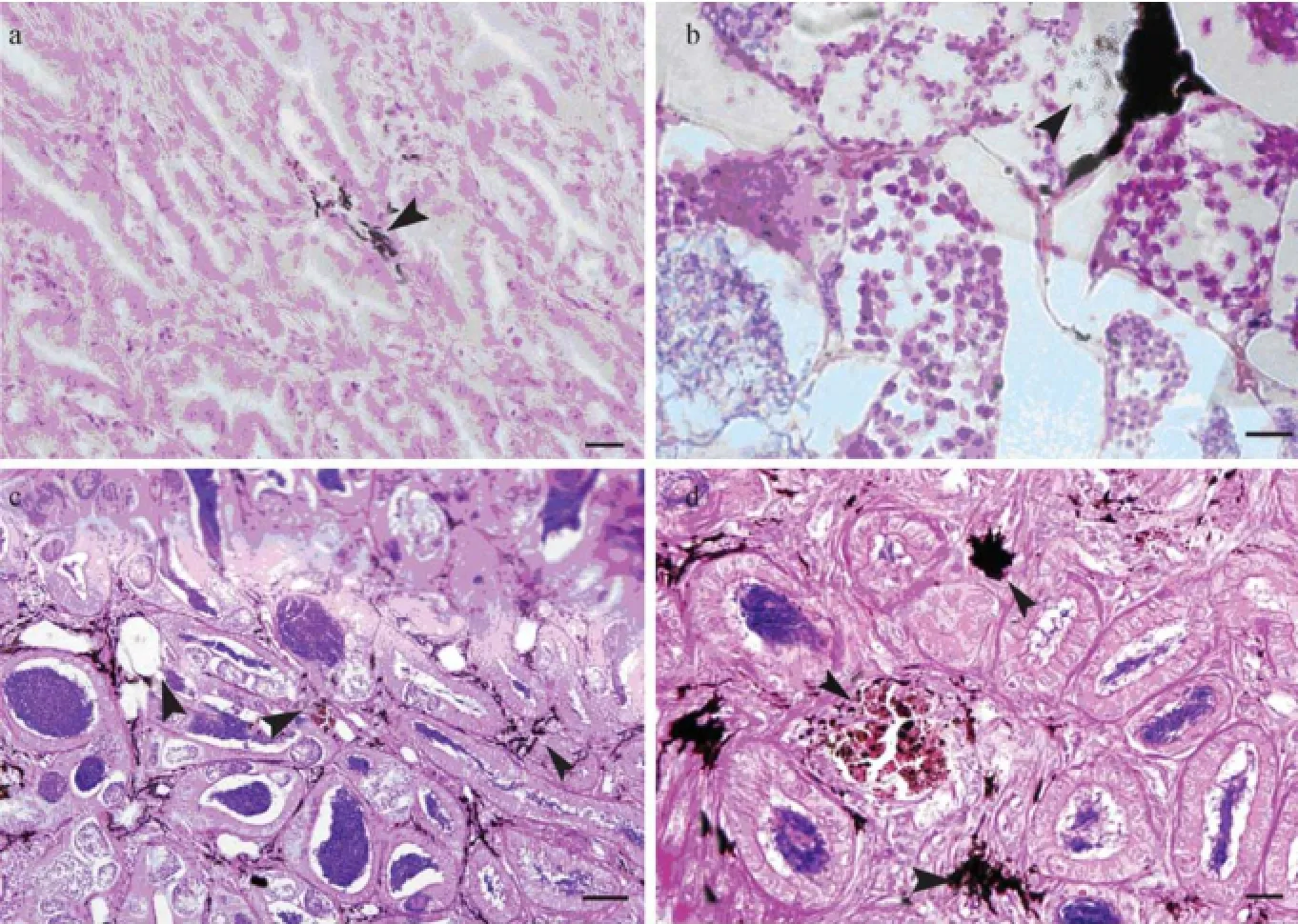
Fig.3 Brown substances (arrows) in testis and main sperm duct of Sebastes schlegeli during development. a, Brown substance in testis at stage of spermatogenic proliferation. Scale bar = 20 μm; b, Brown substance in testis at stage of late spermatogenesis. Scale bar = 10 μm; c, Brown substance in testis at stage of late spermatogenesis. Triangle indicates coated spermatophores in testis. Scale bar = 50 μm; d, Brown substance and masses in main sperm duct. Triangle indicates spermatophore mass. Scale bar = 20 μm.
3.3 Spermatophores in Reproductive System
The pair of testes was connected by two independent long main sperm ducts which united at the posterior part, forming a short vas deferent opened almost immediately into the urogenital sinus. Light microscopic observation showed that the main sperm duct contained whirls of spermatozoa as well as numerous black seed-like substances, 5.12–8.03 μm in length and 2.56–5.04 μm in width in April (Figs.4a, b). In early December, a large quantity of similar substances were found in the longitudinal section of main sperm duct. Numerous spermatozoa gushed out and formed seed-like substances (Fig.4c). Similar substance was observed in spermatogenic and degenerating testes in April (Figs.2e, f). The structure of such substances was unclear under light microscope.
The two oviducts of rockfish joined in genital tract and then opened at genital pore. In October, December and April, the oviduct and genital tract of females contained numerous black seed-like substances being 6.15–8.69 μm long and 3.33–4.22 μm wide with similar characteristics as those in the reproductive system of males (Figs.4d–g). Similar substances were scattered in the ovarian lamellae of ovaries with developed embryos and those of fish collected in early December (Figs.4h, i).
Considering their location and quantity, we proposed that the black seed-like substances were spermatozoa assembled and covered with specific materials, the socalled spermatophore. The quantity of spermatophores in female and male rockfish was apparently different, that is, much less in the former than in the latter.
The unknown deep-brown structures similar to the substances in the testis scattered in the longitudinal section of main sperm duct, with a few deep-stained masses as well (Fig.3d). The masses appeared similar to those in spermatogenic testis and early stage-IV ovary (Figs.3c, 4i, 1c). The masses in testis, main sperm duct and ovary, together with those in biopsied ovary (Fig.1a), were coated withspermatophores. In biopsied ovary, the spermatophores just released from the coating were observed (Fig.1a).

Fig.4 Spermatophores (arrows) in the reproductive system of Sebastes schlegeli. a, Spermatophores in the main sperm duct of male fish collected in April. Scale bar = 100 μm; b, Enlarged photograph of spermatophores in the main sperm duct of male fish collected in April. Scale bar = 20 μm; c, Spermatophores and spermatozoa in the main sperm duct of male fish collected in December. Scale bar = 20 μm; d, Spermatophores in the oviduct of female in October. Scale bar = 50 μm; e, Spermatophores in the genital duct of female fish collected in December. Scale bar = 100 μm; f, Spermatophores in the oviduct of female fish collected in April. Scale bar = 50 μm; g, Spermatophores in the genital duct of female fish collected in April. Scale bar = 20 μm; h, Spermatophores in ovary of fish collected in April. Scale bar = 20 μm; i, Spermatophores in ovary of fish collected in early December. Triangle indicates the brown masses. Scale bar = 20 μm.
4 Discussion
4.1 The Copulation Period and Storage Place of Spermatozoa
The copulation of rockfish, which had rarely been studied previously, is commonly assumed to occur soon after the maturation of sperm cells and finishes in a short time (Eldridge et al., 1991; Beak et al., 2000; Mori et al., 2003). Previously, Helvey (1982) observed the courtship behavior of the blue rockfish S. mystinus, which mainly occurred during late summer to early winter (Helvey, 1982). The nearshore rockfish introduced into culturing fish farm in an advanced stage of vitellogenesis were found pregnant in the absence of males after 14–55 d (Tsang et al., 2007). Reports assured that in Sebastes sp. insemination commonly occurs up to six months prior to fertilization (Sorokin, 1961; Moser, 1967). Although copulation of black rockfish had never been observed, we infer that this process occurred significantly earlier than gestation, as observed in the present study, a large number of spermatophores were discovered in the oviduct of female in October, while the embryos were detected in April next year.
If the copulation occurred in a short period around October and December (when most of the sperm cells matured), where would the spermatozoa be stored during a long period between copulation and fertilization? How would the activity of spermatozoa be maintained? In the absence of special genitals, how the spermatozoa are transported to ovary? According to Beak et al. (2000), sperm masses are present in the ovarian cavity. Additionally, Mori et al. (2003) reported that sperms are stored freely in ovary and under ovigerous lamellae epithelium during early and late vitellogenic period, respectively. In the present study, results indicated that spermatophores are the transportation and storage form of spermatozoa. The spermatophores were found under ovigerous lamellae epithelium during early vitellogenic and embryo developmental period. In addition, spermatophores were found in main sperm duct, testis, oviduct and genital tract.
Numerous spermatophores clustered in main sperm duct in male rockfish collected in April, waiting for transportation through urogenital sinus for accomplishing the designated mission. Are the spermatozoa matured so late or are these only one of the multiple batches of spermatozoa? Histological observation showed that there remained spermatophores in the testis of the same fish. Thus, the later hypothesis explained the phenomenon.
The discovery of similar spermatophores in genital tract and oviduct of rockfish collected in October, December and April proved that the period between copula-tion and fertilization in cultivated rockfish population lasted for at least 5–6 months. Did these results indicated that the hiding place of spermatozoa in rockfish includes the main sperm duct and oviduct as well as genital tract rather than the ovary, where the spermatozoa were stored for months? Or did the copulation occur at all times during the maturation of spermatozoa in batches that were temporarily stored only? In the rockfish collected in April, there remained thousands of spermatophores in main sperm duct and a large quantity of spermatophores assembled in degenerating testis. Thus, we believed that the spermatozoa were discharged in batches with the main sperm duct as one of the hiding places for spermatophores, allowing the copulation to last for a relatively prolonged period. The development of sperm cells in testicular lobules showed asynchronism, further proving that the spermatozoa were discharged in batches. Different from most other osteichthyes, the main sperm duct of black rockfish is long enough for storage of spermatophores. In addition, spermatophores were discovered in stage-IV ovaries in October and the embryos were observed in as late as April. These observations indicated that oviduct, genital tract and the ovary itself are places of storing spermatophores.
4.2 The Function and Formation of Spermatophore
Despite lacking in many ovoviviparous fish species (Follesa et al., 2011), spermatophores have been found in ovoviviparous Gambusia affinis (Kuntz, 1914), Cymatogaster aggregate (Gardiner, 1978), Horaichthys setnai (Grier, 1984), Ophidioidea (Nielsen et al., 1968) and Poecilia reticulata (Tanaka and Oka, 2005). Compared to spermatozoon, spermatophores are commonly bigger. It has been reported that the spermatophores in G. affinis are round or spherical, 0.1–0.2 mm in diameter (Kuntz, 1914), whereas the spermatophores in Ophidioidea are more or less oval, with considerable variations in size, i.e., 40–100 μm in length (Nielsen et al., 1968). By comparison, the size of spermatophores is relatively small in other species. In the testes of brotulid fish Ogilbia cayorum and guppy Poecilia reticulata, spermatophores are less than 10 µm in length (Suarez, 1975; Wester and Canton, 1992). In black rockfish, the spermatophores are 5.12–8.69 μm in length and 2.56–5.04 μm in width. Future study will be conducted on the structure of spermatophores in black rockfish in order to clarify the reason for dramatic variations in the size of spermatophores.
Despite the difference in size, the spermatophores in black rockfish probably have the same function as those in other fishes. In agreement with Nielsen et al. (1968), we considered that the spermatophores are transferred into oviduct and ovary, where they are stored for a certain period before the spermatozoa are released. It seems that the spermatophores primarily serve as a transport device. The spermatophore-capsules will eventually dissolve while the spermatozoa are released till fertilization. The development of spermatophores may thus be a mechanism that ensures the spermatozoa to be kept alive until the eggs matured (Nielsen et al., 1968). Unlike the phenomenon observed by Nelsen et al. (1968), we found spermatophores in ovary in addition to oviduct. Further investigation is needed to elucidate the mechanism of spermatozoa release and activation by the spermatophores in black rockfish.
The mechanism of spermatophore formation in a variety of invertebrates and vertebrates has previously been studied (Fanciulli et al., 2012; Sperone et al., 2009; Ye et al., 2011). It is speculated that the spermatophores in Ophidioidea are formed within the ducts of testis rather than within the spermatogenic tissues (Neilsen et al., 1968). However, a light microscopic study demonstrated that spermatophores actually are formed by lobule boundary cells within testis, whereas the spermatophore capsule in Horaichthys setnai is produced by sertoli cells secreted into the germinal cyst after germ maturation is completed (Grier, 1984). Gardiner (1978) proposed that in teleost Cymatogaster aggregate, the sperms are packaged into high-density aggregations and then introduced into female genital tract at insemination. Germ cell differentiation occurs within cysts formed by non-germinal sertoli cells. In late spermiogenesis, spermatozoa within the cysts come to lie parallel to each other and become more densely packed. These sperm packets (spermatophores), which contain about 600 spermatozoa, are then released into the efferent sperm ducts (Gardiner, 1978). In the present study, spermatophores were found in testis to associate with spermiogenesis as well as degenerating testis. Additionally, there are a large amount of deepstained masses that seem to secret granule-like substances in the interstitial areas of testis and likely play a role in the formation of spermatophores. The spermatophores may be formed in main sperm duct and testis, coated with substances secreted from testis and sperm duct, and further transferred into ovary.
The spermatophores in black rockfish are apparently acellular capsules. The exact nature of the spermatophore-binding material(s) needs to be investigated, but clearly, glandular cells are not involved in this phenomenon. The deep-brown sticky masses scattered in the interstitial areas of testis and the main sperm duct are very likely related to the formation of spermatophores and their surface sticky coating.
5 Conclusions
The development of germ cells in black rockfish in reproductive season was studied and the developmental characteristics of ovary and testis were described. For the first time, a large number of spermatophores were discovered in testis, main sperm and genital duct, oviduct and ovary during a period lasting from October to April. Based on our findings, we inferred that after being discharged from testicular lumen, the spermatozoa assembled in testis and main sperm duct, forming spermatophores there. The spermatophores were covered with protective materials secreted from brown substance and then transported to urogenital sinus where they waited fora certain period before copulation. The coated spermatophores were transported to oviduct. When vitellogenesis began in oocytes, the spermatophores were transferred into ovary and further released during the absorption of coating substances. The spermatophores were discharged in batches and the copulation lasted for a relatively long period. To our knowledge, this study presented the first direct evidence regarding the discovery of spermatophores in the reproductive system of black rockfish. These findings may aid to clarifying the reproductive behaviors of the ovoviviparous fish in culture as well as in nature.
Acknowledgements
We thank Dr. Daode Ji for providing microscope system. We also thank undergraduate students Jianwei Qiu and Haizhou Li for preparing samples. This study was supported financially by Grand Innovating Program of Agriculture Applying Technique in Shandong Province (No. 2008-109), Modern Agricultural Industry Technology System in Shandong Province and the Science and Technology Development Program of Yantai (No. 2013 ZH088).
Beak, J. M., Han, C. H., and Kim, D. J., 2000. Reproductive cycle of a rockfish, Sebastes schlegeli. Journal of the Korean Fisheries Society, 33 (5): 431-438.
Eldridge, M. B., Whipple, J. A., Bowers, M. J., Jarvis, B. M., and Gold, J., 1991. Reproductive performance of yellowtail rockfish, Sebastes flavidus. Environmental Biology of Fishes, 30: 91-102.
Fanciulli, P. P., Zizzari, Z. V., Frati, F., and Dallai, R., 2012. The ultrastructure of the ejaculatory duct in the springtail Orchesella villosa (Geoffroy) (Hexapoda, Collembola) and the formation of the spermatophore. Tissue and Cell, 44 (1): 32-46.
Follesa, M. C., Porcu, C., Mulas, A., Salvadori, S., and Cau, A., 2011. Reproductive characteristics of the bathyal viviparous fish Cataetyx alleni (Osteichthyes: bythitidae) from the southeastern Sardinian Sea (central-western Mediterranean). Scientia Marina, 75 (2): 391-397.
Gardiner, D. M., 1978. The origin and fate of spermatophores in the viviparous teleost Cymatogaster aggregata (Perciformes: Embiotocidae). Journal of Morphology, 155: 157-171.
Grier, H. J., 1984. Testis structure and formation of spermatophores in the atherinomorph teleost Horaichthys setnai. Copeia, 1984 (4): 833-839.
Grier, H. J., Uribe, M. C., Parenti, L. R., and Rosa-Cruz, G. D., 2005. Fecundity, the Germinal Epithelium, and Folliculogenesis in Viviparous Fishes Viviparous Fishes. New Life Publications, Homestead, Florida, 193-217.
Helvey, M., 1982. First observations of courtship behavior in rockfish, Genus Sebastes. Copeia, 1982 (4): 763-770.
Kuntz, A., 1914. Notes on the habits, morphology of the reproductive organs, and embryology of the viviparous fish Gambusia affinis. Bulletin of the United States Bureau of Fisheries, 33: 177-190.
Lin, D. J., and You, Y. L., 2000. The ovarian cyclical development of ovoviviparous telelost Sebastiscus marmoratus. Zoology Research, 21 (4): 269-274 (in Chinese with English abstract).
Lin, D. J., You, Y. L., and Chen, L. Y., 2000. The testicular cycle development of ovoviviparous telelost Sebastiscus marmoratus. Zoology Research, 21 (5): 337-342 (in Chinese with English abstract).
Mori, H., Nakagawa, M., Soyano, K., and Koya, Y., 2003. Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fisheries Science, 69: 910-923.
Moser, H. G., 1967. Reproduction and development of Sebastodes paucispinnis and comparison with other rockfishes off Southern California. Copeia, 1967: 773-797.
Nielsen, J. G., Jespersen, Ǻ., and Munk, O., 1968. Spermatophores in Ophidioidea (Pisces, Percomorphi). Galathea Reports, 9: 239-254.
Shi, D., Wen, H. S., and Yang, Y. P., 2011. The annual change of ovarian development in female Sebastes schlegeli. Periodical of Ocean University of China, 41 (9): 25-30 (in Chinese with English abstract).
Sorokin, V. P., 1961. The redfish, gametogenesis and migrations of the Sebastes marinus (L.) and Sebastes mentella Travin. ICNAF Special Publication, 3: 245-250.
Sperone, E., Bonacci, A., Brunelli, E., Tripepi, S., and Jamieson, B. G. M., 2009. Male reproductive system in the Italian newt Lissotriton italicus (Peracca 1898) (Amphibia, Urodela): Ultrastructural and morphological study with description of spermiogenesis, spermatozoon and spermatophore. Zoo Morphology, 128 (2): 183-195.
Suarez, S. S., 1975. The reproductive biology of Ogilbia cayorum, a viviparous brotulid fish. Bulletin of Marine Science, 25 (2): 143-173.
Takahama, H., Kinoshita, T., Sato, M., and Sasaki, F., 1991. Fine structure of the spermatophores and their ejaculated forms, sperm reservoirs, of the Japanese common squid, Todarodes pacificus. Journal of Morphology, 207: 241-251.
Takano, K., Takemura, A., Furihata, M., Nakanishi, T., and Hara, A., 1991. Annual reproductive and spawning cycles of female Sebastiscus marmoratus. Environmental Biology of Fishes, 30: 39-48.
Tanaka, H., and Oka, Y., 2005. Chaotropic ions and multivalent ions activate sperm in the viviparous fish guppy Poecilia reticulata. Biochimica et Biophysica Acta, 1724 (2005): 173-180.
Tsang, W. N., Chaillé, P. M., and Collins, P. M., 2007. Growth and reproductive performance in cultured nearshore rockfish (Sebastes spp.). Aquaculture, 266: 236-245.
Wester, P. W., and Canton, H. H., 1992. Histopathological effects in Poecilia reticulata (guppy) exposed to methyl mercury chloride. Toxicology Pathology, 20 (1): 81-92.
Yang, Y. P., Wen, H. S., He, F., Li, J. F., Shi, D., Chen, C. F., Zhang, J. R., Jin, G. X., Chen, X. Y., and Shi, B., 2010. The morphology and developmental histology of testis in rockfish Sebastes schlegeli. Journal of Dalian Ocean University, 25 (5): 391-396 (in Chinese with English abstract).
Ye, D. F., Wu, C. W., Lv, Z. M., and Chi, C. F., 2011. Fine structure of spermatophoric organ and formation of spermatophore in Sepiella maindroni. Oceanologia et Limnologia Sinica, 42 (2): 207-212 (in Chinese with English abstract).
(Edited by Qiu Yantao)
(Received January 3, 2013; revised February 22, 2013; accepted March 6, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. E-mail: rbdu62@163.com
杂志排行
Journal of Ocean University of China的其它文章
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Study on Internal Waves Generated by Tidal Flow over Critical Topography
- Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
