Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
2014-04-20WUNianCHENShiguoYEXingqianLIGuoyunYINLiangandXUEChanghu
WU Nian, CHEN Shiguo,, YE Xingqian, LI Guoyun, YIN Li’ang, and XUE Changhu
1) College of Biosystem Engineering and Food Science, Zhejiang University, Hangzhou 310058, P. R. China
2) College of Food Science and Engineering, Ocean University of China, Qingdao 266061, P. R. China
Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
WU Nian1), CHEN Shiguo1),*, YE Xingqian1), LI Guoyun2), YIN Li’ang2), and XUE Changhu2)
1) College of Biosystem Engineering and Food Science, Zhejiang University, Hangzhou 310058, P. R. China
2) College of Food Science and Engineering, Ocean University of China, Qingdao 266061, P. R. China
Acidic polysaccharide, which has various biological activities, is one of the most important components of sea cucumber. In the present study, crude polysaccharide was extracted from four species of sea cucumber from three different geographical zones, Pearsonothuria graeffei (Pg) from Indo-Pacific, Holothuria vagabunda (Hv) from Norwegian Coast, Stichopus tremulu (St) from Western Indian Ocean, and Isostichopus badionotu (Ib) from Western Atlantic. The polysaccharide extract was separated and purified with a cellulose DEAE anion-exchange column to obtain corresponding sea cucumber fucans (SC-Fucs). The chemical property of these SC-Fucs, including molecular weight, monosaccharide composition and sulfate content, was determined. Their structure was compared simply with fourier infrared spectrum analyzer and identified with high temperature1H nuclear magnetic resonance spectrum analyzer (NMR) and room temperature13C NMR. The results indicated that Fuc-Pg obtained from the torrid zone mainly contained 2,4-O-disulfated and non-sulfated fucose residue, whereas Fuc-Ib from the temperate zone contained non-, 2-O- and 2,4-O-disulfated fucose residue; Fuc-St from the frigid zone and Fuc-Hv from the torrid zone contained mainly non-sulfated fucose residue. The proton of SC-Fucs was better resolved via high temperature1H NMR than via room temperature1H NMR. The fingerprint of sea cucumber in different sea regions was established based on the index of anomer hydrogen signal in SC-Fucs. Further work will help to understand whether there exists a close relationship between the geographical area of sea cucumber and the sulfation pattern of SC-Fucs.
sea cucumber; sulfated fucan; composition analysis;1H NMR; identification
1 Introduction
Sea cucumbers in class Holothuroidea are marine invertebrates, which are habitually found in benthic areas and deep seas across the world (Bordbar et al., 2011). Sea cucumbers have been recognized as a traditional tonic food in China and other Asian countries for thousands of years, mainly due to their effectiveness against hypertension, asthma, rheumatism, cuts and burns, impotence, and constipation (Fu et al., 2005; Wen et al., 2010). It has been proven that chemical compounds extracted from different sea cucumber species exhibit several unique biological and pharmacological activities, such as antiangiogenic (Tian et al., 2005), anticancer (Roginsky et al., 2004), anticoagulant (Chen et al., 2011), anti-hypertension (Hamaguchi et al., 2010), anti-inflammatory (Collin, 1999), antimicrobial (Hing et al., 2007), antioxidant (Althunibat et al., 2009), antithrombotic (Pacheco et al., 2000), antitumor (Tong et al., 2005) and wound healing (San Miguel-Ruiz and García-Arrarás, 2007). There are hundreds of varieties of sea cucumbers in ocean. Their commercial value varies geographically between a few and thousands of US dollars per kilogram. However, no detailed distinction of sea cucumber species has been documented in terms of chemical components and nutritional value.
The major edible part of sea cucumbers is their body wall, which contains mainly collagen, acidic polysaccharides (Mourão et al., 1996; Trotter et al., 1995; Vieira et al., 1988) and some minor components such as triterpene glycosides, gangliosides and various lipids (Avilov et al., 2000; Kitagawa et al., 1982; Matsuno and Tsushima, 1995). Of these, acidic polysaccharide has various biological activities and is believed to be the most important component of sea cucumbers. It has been reported that polysaccharide isolated from sea cucumbers has anticoagulant and antithrombotic activity (Fonseca and Mourão, 2006), can modulate angiogenesis (Tapon-Bretaudiere et al., 2002) and inhibit tumor metastasis (Borsig et al., 2007). Two types of polysaccharide isolated from the body wall of sea cucumbers mainly include sea cucumber fucosylated chondroitin sulfate (SC-fCS), an acidic polysaccharide composed of D-N-acetyl galactosamine, D-glucuronic acid and L-fucose (Vieira et al., 1988), and sea cucumber fucan (SC-Fuc), a linear polysaccharide ofL-fucose (Mourão et al., 1996). There have been several studies on the structure of SC-fCSs isolated from sea cucumbers, such as Stichopus japonicus (Casu et al., 1996) and Ludwigothurea grisea (Mourão et al., 1996), via mild acid hydrolysis, methylation analysis and1H and13C nuclear magnetic resonance (NMR) spectroscopy. SC-fCSs from these sea cucumbers have a sulfated fucose branch linked to the 3-O position of the glucuronic acid (GlcA) residue, with different sulfation patterns of the fucose branches. However, characteristics of sea cucumber polysaccharide, such as the sulfation inconsistency, the inhomogeneity of microstructure and molecular weight, and the polytrope of residue quantity, cause the complexity of their purification and structural analysis. So far, the majority of studies on SC-Fuc have been focused on the analysis of sugar chain structure and influence of substitutional positions and quantities of sulfate on its activity (Mourão et al., 1996; Vieira et al., 1988).
Recently, the development of modern spectroscopic techniques, for example, the NMR spectroscopy, makes the structural characterization of complex carbohydrates possible. Both1H and13C NMR spectroscopy have been used for the sequence analysis of heparin oligosaccharides and the characterization of subtle structural differences among different preparations of heparin (Casu et al., 1996). It is possible to identify sulfated sugar contaminants associated with adverse clinical events via direct analysis of heparin polysaccharide materials (Guerrini et al., 2008).
In the present study, we isolated SC-Fucs from four species of sea cucumbers with different commercial value from three geographical zones, Pearsonothuria graeffei from Indo-Pacific, Holothuria vagabunda from Western Indian Ocean, Stichopus tremulus from Norwegian Coast and Isostichopus badionotus from Western Atlantic. The composition and structure of SC-Fucs obtained from these four species of sea cucumbers were determined for the first time in this study. Comparative analysis was carried out on anomer proton signals by high temperature1H NMR, and the sulfation pattern of fucose branches was determined. The index of anomer hydrogen signals in SC-Fucs was thus established for identifying species of sea cucumbers from different sea regions. The results will provide reference data for further studies on the structure activity relationship of SC-Fucs.
2 Materials and Methods
2.1 Sea Cucumber
Four species of sea cucumbers (50 g each), Pearsonothuria graeffei (from Indo-Pacific), Holothuria vagabunda (from Norwegian Coast), Stichopus tremulus (from Western Indian Ocean), and Isostichopus badionotus (from Western Atlantic) were purchased from a local market in Qingdao, Shandong, China.
2.2 Isolation of Crude Polysaccharide
Crude sea cucumber polysaccharide was prepared following the method reported previously (Matsuno and Tsushima, 1995). Briefly, sea cucumber body wall (about 1 g) was dried, minced and homogenized. The homogenate was treated with CH3Cl3/MeOH (4:1) to remove lipids before autoclaving at 50℃ for 4 h. The resulting residue was digested with 100 mg papain in 30 mL of 0.1 mol L-1sodium acetate buffer solution (pH 6.0) (5 mmol L-1EDTA and 5 mmol L-1cysteine) at 60℃ for 10 h. The digested mixture was centrifuged (2000 r min-1, 15 min, 10℃) and the polysaccharide in the clear supernatant was precipitated with 1.6 mL of 10% aqueous cetylpyridinium chloride solution. After standing at room temperature for 24 h, the mixture was centrifuged (2000 r min-1, 15 min) and the precipitated polysaccharide was collected and redissolved in 1000 mL of 2 mol L-1NaCl:ethanol (100:15) before further precipitation with 2000 mL of 95% ethanol. After standing at 4℃ for 24 h, the precipitate formed was collected by centrifugation (2000 r min-1, 15 min). The precipitate was dissolved in water and dialyzed against distilled water (with two exchanges). The polysaccharide solution was lyophilized before analysis.
2.3 Isolation of SC-Fucs by Anion Separation Column
The obtained sea cucumber crude polysaccharides (400 mg each) were dissolved in 10 mL of 25 mmol L-1NaH2PO4-NaOH (pH 6.3). The crude polysaccharide solution was fractionated by anion-exchange chromatography on a DEAE-cellulose column (1.6 cm × 17 cm, Whatman, Brentford, England) with elution by a linear gradient of 0–1.4 mol L-1NaCl in 1000 min at a flow rate of 0.5 mL min-1. Carbohydrate fractions were collected every 10 min with a test tube. Protein content was then determined at 280 nm with an ultraviolet detector (Agilient, California, USA). Polysaccharide content was determined by improved phenol/sulfuric assay (Chen et al., 2011). The single components in test tubes were further determined by HPLC (high performance liquid chromatography) with a TSK gel PWXL-4000 column (Tosoh Bioscience, Tokyo, Japan). The polysaccharide solution collected and dialyzed against distilled water was lyophilized.
2.4 Molecular Weight Measurement
GPC measurement was performed by Aglient 1100 HPLC equipped with a refractive index detector. For polysaccharide measurement, a TSK gel PWXL-4000 column (Tosoh Bioscience, Tokyo, Japan) was used. A 0.2 mol L-1NaNO3solution was used as solvent at a flow rate 0.5 mL min-1.
2.5 Chemical Composition Analysis
Monosaccharide composition was determined by HPLC as described elsewhere (Strydom, 1994). In brief, sea cucumber polysaccharide (2 mg each) was hydrolyzed with 1 mL of 2 mol L-1TFA at 110℃ for 8 h. The hydrolysate was vacuum dried and conjugated to PMP (Sangon Biotech, Shanghai, China). The derivatization was carried out with 450 μL of PMP solution (0.5 mol L-1, in methanol)and 450 μL of 0.3 mol L-1NaOH at 70℃ for 30 min. The reaction was stopped by neutralization with 450 μL of 0.3 mol L-1HCl and extraction three times with chloroform (1mL).
HPLC analysis was performed on an Agilent ZORBAX Eclipse XDB-C18 column (5 μm, 4.6 mm×150 mm, Agilient, California, USA) at 25℃ with UV detection at 250 nm. The mobile phase was aqueous 0.05 mol L-1KH2PO4 (pH 6.9) with 15% (solvent A) and 40% acetonitrile (solvent B), respectively. A gradient of B from 8% to 20% in 30 min was used.
Sulfate content was determined by ion chromatography as described previously (Ohira and Toda, 2006). Briefly, sea cucumber polysaccharide (about 2 mg each) was hydrolyzed with 1 mL of 2 mol L-1TFA at 110℃ under nitrogen for 8 h. The hydrolysate was dried under vacuum and then dissolved in water prior to ion chromatography.
2.6 NMR Assay
For NMR (JEOL, Japan) analysis, polysaccharide (50 mg each) was evaporated with 500 μL of D2O (99.8%, Sigma-Aldrich, Missouri, USA) twice by lyophilization before final dissolution in 500 μL of high quality D2O (99.96%) containing 0.1 μL of acetone.1H NMR experiment was carried out at 600 MHz and13C NMR at 150 MHz. Spectrum was recorded at 25℃ for13C NMR and 60℃ for1H NMR. The observed1H and13C chemical shift were reported relative to an internal acetone standard (2.03 and 33.1 ppm, respectively).
2.7 IR Spectroscopy
IR spectrum of the polysaccharide (KBr pellets, 0.5 mg sample with 150 mg KBr) was taken on a Perkin-Elmer instrument (Bruke, Genman) .
2.8 Statistical Analysis
All the data were statistically evaluated with SPSS/ 13.00 software. P < 0.05 and P < 0.01 were considered to indicate statistical significance. All the results were expressed as mean ± SE.
3 Results and Discussions
3.1 SC-Fucs Isolation and Purification
The yield of polysaccharide isolated from four species of sea cucumbers, Pearsonothuria graeffei (Pg), Holothuria vagabunda (Hv), Stichopus tremulus (St), and Isostichopus badionotus (Ib), was 11.0%, 6.3%, 7.0% and 9.9% by weight, respectively. Ion exchange chromatography of the extracted polysaccharides on the DEAE-cellulose column showed two peaks each sea cucumber (Fig.1), which were respectively identified as SC-fCSs (fractions I) and SC-Fucs (fractions II) based on their chemical composition (Table 1, see 3.2 for discussion on Chemical Compositions). The relative content of two polysaccharide fractions was different in each species of sea cucumbers, as SC-fCSs were abundant in St, Pg and Hv (about 63%, 65%, 64%, respectively) (Figs.1a–c), whereas SC-Fucs was abundant in Ib (65%) (Fig.1d). According to the tube quantity and the eluent concentration in the region of rising peaks, the salinity of the two peaks ranged from 0.63 to 0.99 mol L-1and from 0.88 to 1.28 mol L-1, respectively. The polysaccharide had different salinity of elution peaks, varying in sulfate content. SC-Fucs from these four species of sea cucumbers were called Fuc-Pg, Fuc-Hv, Fuc-St and Fuc-Ib. Fuc-Ib had relatively higher (41.1%) sulfate content than others (29.1%–34.8%) (Fig.1).
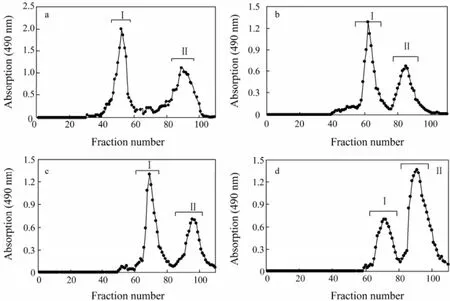
Fig.1 Isolation of sea cucumber fucosylated chondroitin sulfates (SC-fCSs) and sea cucumber fucans (SC-Fucs) from crude polysaccharides extracted from sea cucumbers by anion-exchange chromatography. (a) Pearsonothuria graeffei, (b) Holothuria vagabunda, (c) Stichopus tremulus, and (d) Isostichopus badionotus.
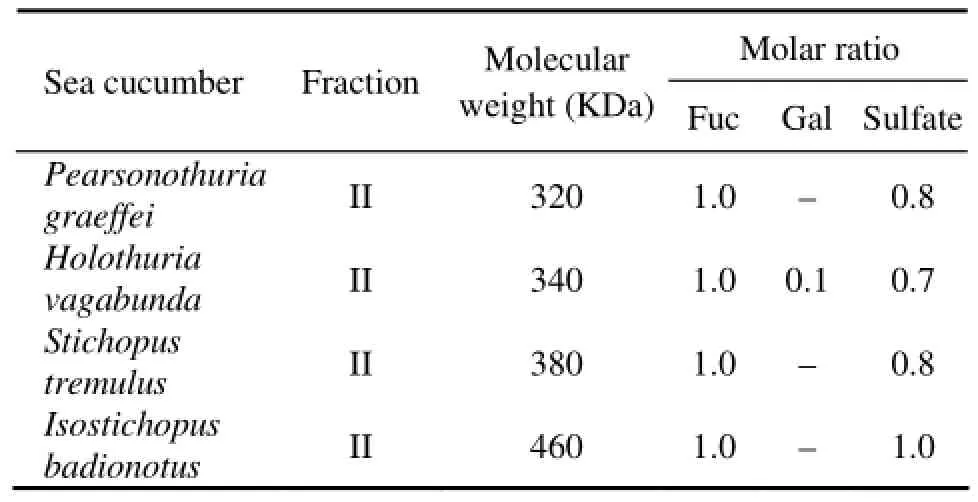
Table 1 Molecular weight (MW) and the molar ratio of monosaccharide SC-Fuc
3.2 Chemical Composition
Three monosaccharides, glucuronic acid (GlcA), N-acetylgalactosamine (GalNAc), and fucose constituted the polysaccharide fraction I (Fig.1). The fraction I was then assigned to be chondroitin sulfate, as GlcA and GalNAc were in an approximately 1:1 ratio, consistent with the polysaccharide backbone structure, -4GlcAβ1-3GalNAcβ1-. On the contrary, only one monosaccharide, fucose, was found to be the major component of polysaccharide fraction II (Fig.1). The fucose and sulfate content in SC-fCSs obtained from different sea cucumber species varied as well. The molecular weight of Fuc-Ib was lager than that of other three SC-Fucs and its sulfate content was the highest (Table 1), consistent with the results regarding the salinity of elution peaks.
3.3 IR
In the IR spectrum of SC-Fucs, characteristic signals were assigned to sulfate groups, including S=O asymmetric stretching vibration at 1261–1220 cm-1and symmetric C-O-S stretching vibration at 860–820 cm-1(Matsuhiro, 1996; Matsuhiro et al., 2012) (Fig.2). The absorption at 848 cm-1, 837 cm-1, and 820 cm-1indicated the presence of 4-O-sulfated, 2,4-O-sulfated and 2-O-sulfated Fucs, respectively. Fuc-Pg and Fuc-Hv showed strong absorption at 820 cm-1(Figs.2a, b), indicating the presence of 2-O-sulfated Fuc according to previous reports on sea weed fucoidans (Bernardi and Springer, 1962). Conversely, Fuc-St and Fuc-Ib respectively exhibited strong absorption at 837 cm-1and 848 cm-1, indicating the presence of 2,4-O-sulfated and 4-O-sulfated Fucs (Bernardi and Springer, 1962).
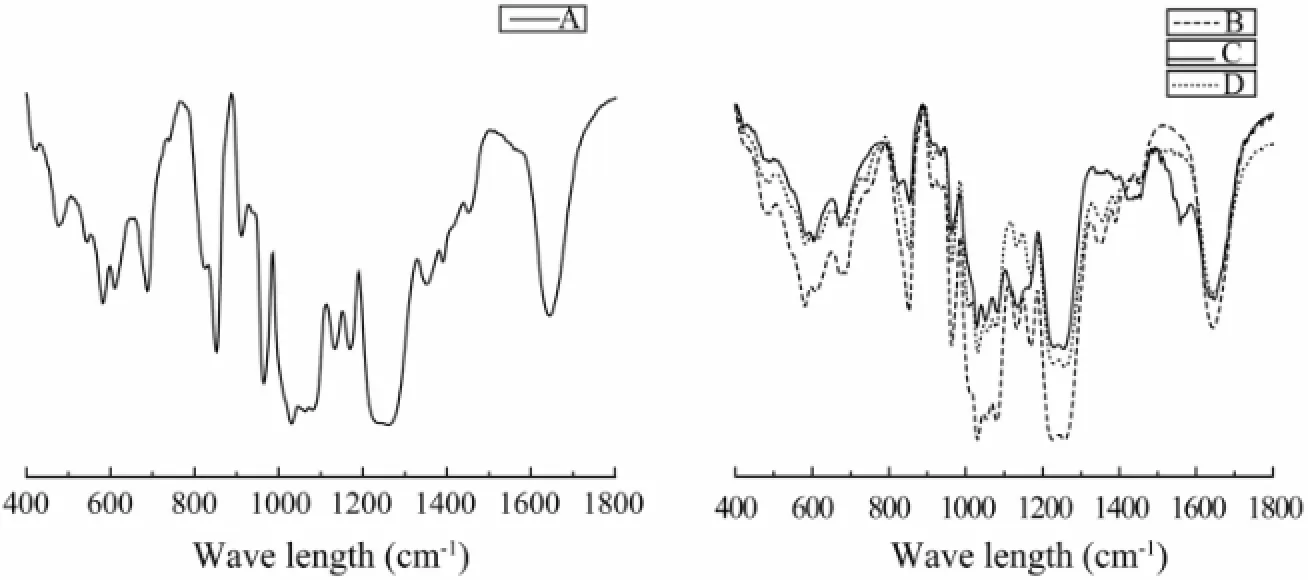
Fig.2 IR spectra of fucans isolated from sea cucumbers. (A) sea cucumber Pearsonothuria graeffei fucan (FUC-Pg), (B) sea cucumber Holothuria vagabunda fucan (FUC-Hv), (C) sea cucumber Stichopus tremulus fucan (FUC-St), and (D) sea cucumber Isostichopus badionotus fucan (FUC-Ib).
3.41H NMR
At first,1H NMR spectrum of Fuc-Ib was obtained at room temperature (Fig.3). In the spectrum, the methyl protons of fucose (CH3) showed a clear signal around 1.2 ppm, but it was difficult to differentiate signals of anomeric protons of sulfated fucose residues between 5.1 and 5.4 ppm. Conversely, high temperature1H NMR spectrum of Fuc-Ib (60℃) provided clearly identifiable signals that were undetectable at room temperature (Fig.4d).
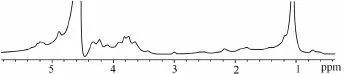
Fig.3 Room temperature1H NMR spectra of sea cucumber Isostichopus badionotus fucan (FUC-Ib).
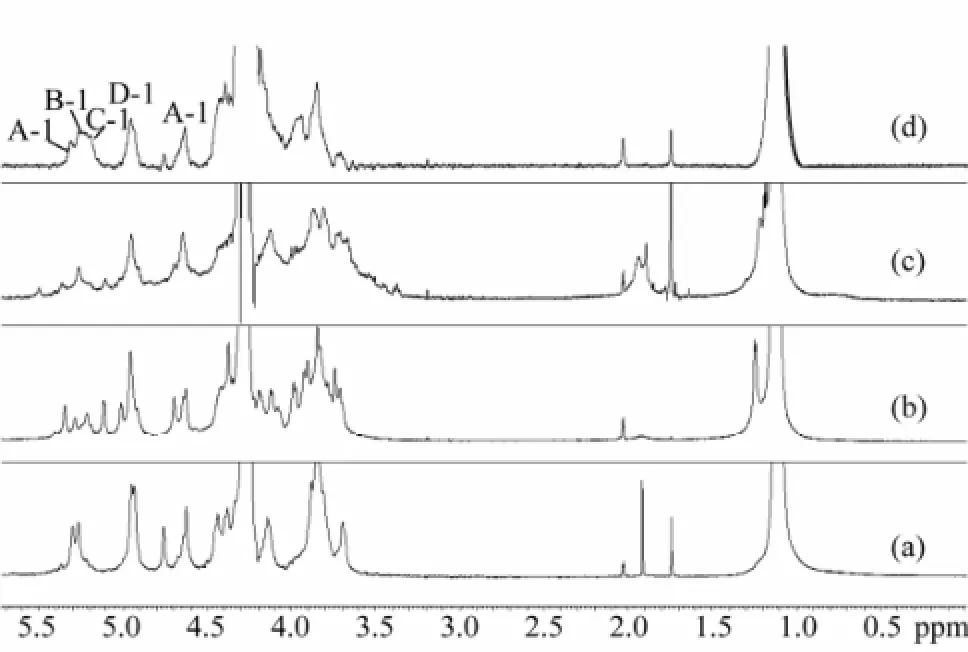
Fig.4 High temperature1H NMR spectra of four sea cucumber fucans (SC-Fucs). (a) sea cucumber Pearsonothuria graeffei fucan (FUC-Pg), (b) sea cucumber Holothuria vagabunda fucan (FUC-Hv), (c) sea cucumber Stichopus tremulus fucan (FUC-St), and (d) sea cucumber Isostichopus badionotus fucan (FUC-Ib).
Therefore, high temperature1H NMR spectrum (Fig.4 and Table 2) of the four SC-Fucs was acquired and used for comparing their structures. Compared to the spectrum of SC-fCS (Chen et al., 2011), the signals between 1.1 and 1.3 ppm were the methyl protons of fucose (CH3) but without the methyl protons of GalNAc (CH3CO). The results further indicated that the monosaccharide composition of SC-Fucs was only fucose. The protons signals of C-2 to C-6 were between 3.2 and 4.8 ppm. The signals between 5.1 and 5.4 ppm were anomeric protons of sulfated fucose residues (Mourão et al., 1996; Wu et al., 2012) and apparent difference was observed in this region.

Table 21H chemical shift of the fucose residue in the SC-Fucs
Fuc-Pg showed major signals at 5.06 and 5.32 ppm, which were from the non-sulfated fucose and 2,4-O-disulfated fucose, respectively. These two signals were similar to those of Fuc isolated from the sea cucumber L. Grisea (Mourão et al., 1996). Fuc-Ib exhibited signals at 5.06, 5.22, 5.25 and 5.32 ppm (Fig.4d), indicating the presence of non-sulfated, 2-O-sulfated, 2-O-sulfated, and 2,4-O-disulfated residues, respectively. However, anomeric signals of Fuc-St and Fuc-Hv were more complex and difficult to be assigned, while their anomeric protons were found to be mainly non-sulfated fucose.
3.513C NMR
13C NMR spectra of SC-Fucs (Fig.5) did not show any apparent difference in the sulfation patterns. However, the major signals exhibited were useful to assign the fucose backbone sequence, as these carbon signals were similar to those present in the spectrum of a standard fucose.
In Fig.5, the signals at 16–18 ppm could be readily assigned to the methyl carbon. Differences between anomeric protons in the overlapping signal region of 60–82 ppm were slightly evident. Although the anomeric signals between 90 ppm and 100 ppm in the other three species of sea cucumbers were unclear, Fuc-Ib (Fig.5d) showed clear signals at 99.8, 98.5, 95.2 and 93.8 ppm, assigned to non-sulfated, 2-O-sulfated, 2-O-sulfated, 2,4-O-disulfated residues, respectively. Therefore,13C NMR was as efficient as1H NMR for distinguishing SC-Fucs.
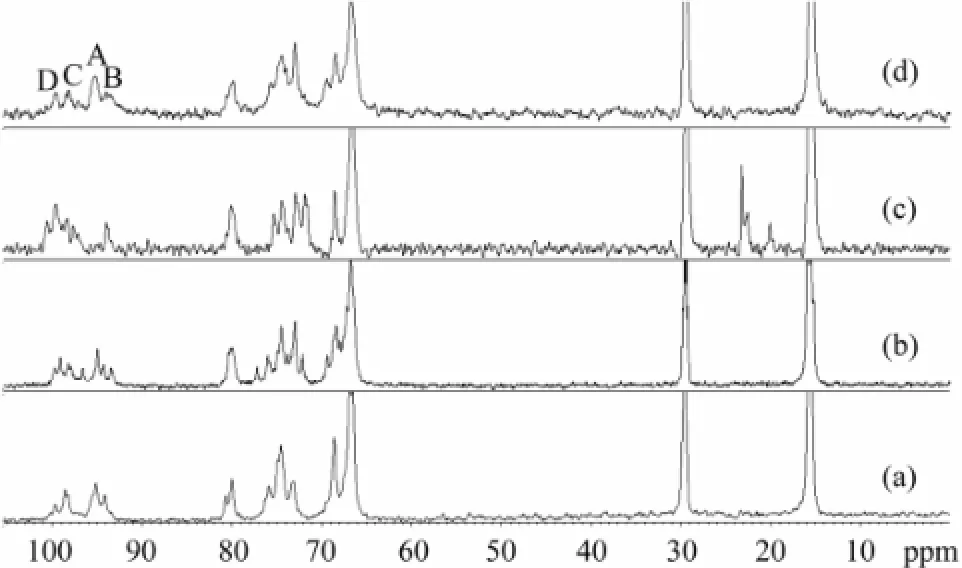
Fig.513C NMR spectra of four sea cucumber fucans (SC-Fucs). (a) sea cucumber Pearsonothuria graeffei fucan (FUC-Pg), (b) sea cucumber Holothuria vagabunda fucan (FUC-Hv), (c) sea cucumber Stichopus tremulus fucan (FUC-St), and (d) sea cucumber Isostichopus badionotus fucan (FUC-Ib).
4 Conclusions
Instead of conventional analysis via acid preparation that was easy to cause desulfation and may affect structure determination, high temperature1H NMR and room temperature13C NMR were used for the first time to identify the four SC-Fucs by comparing their structures, especially the substitution positions of sulfate. Fuc-Pg from the torrid zone (the tropics) mostly contained 2,4-O-disulfated and non-sulfated fucose residues; Fuc-Ib from the temperate zone mainly contained non-sulfated, 2-O-sulfated, and 2,4-O-disulfated fucose residues; Fuc-St from the frigid zone and Fuc-Hv from the torrid zone (the tropics) had mainly non-sulfated fucose residues. In comparison with room temperature1H NMR, high temperature1H NMR can better resolve the protons of SCFucs, thus is useful for identifying different species of sea cucumbers.
Acknowledgements
This work was supported by the Public Service Project of Zhejiang Province (2011C22026), the Special Award Funding for Postdoc in China (16000-X91009 and 316000 -X91005), the National Natural Science Foundation of China (30972282), the National Natural Science Foundation of China (31301417), and the Zhejiang Province Public Service Project (2011C11016).
Althunibat, O. Y., Ridzwan, B. H., Taher, M., Jamaludin, M. D., Ikeda, M. A., and Zali, B. I., 2009. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. European Journal of Scientific Research, 37: 376-387.
Avilov, S., Antonov, A., Drozdova, O., Kalinin, V., Kalinovsky, A., Riguera, R., Lenis, L., and Jimenez, C., 2000. Triterpene glycosides from the far eastern sea cucumber Pentamera calcigera II: disulfated glycosides. Journal of Natural Products,63: 1349-1355.
Bernardi, G., and Springer, G., 1962. Properties of highly purified fucan. Journal of Biological Chemistry,237: 75-80.
Bordbar, S., Anwar, F., and Saari, N., 2011. High-value components and bioactives from sea cucumbers for functional foods: A review. Marine Drugs,9: 1761-1805, DOI: 10.3390/md9101761.
Borsig, L., Wang, L., Cavalcante, M. C., Cardilo-Reis, L., Ferreira, P. L., Mourão, P. A., Esko, J. D., and Pavao, M. S., 2007. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. Journal of Biological Chemistry, 282 (20): 14984-14991.
Casu, B., Guerrini, M., Naggi, A., Torri, G., De-Ambrosi, L., Boveri, G., Gonella, S., Cedro, A., Ferro, L., and Lanzarotti, E., 1996. Characterization of sulfation patterns of beef and pig mucosal heparins by nuclear magnetic resonance spectroscopy. Arzneimittel-Forschung, 46: 472.
Chen, S., Xue, C., Yin, L., Tang, Q., Yu, G., and Chai, W., 2011. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydrate Polymers, 83: 688-696.
Colliec, S., Fischer, A., Tapon-Bretaudiere, J., Boisson, C., Durand, P., and Jozefonvicz, J., 1991. Anticoagulant properties of a fucoidan fraction. Thrombosis Research, 64 (2): 143-154. Collin, P. D., 1999. Process for obtaining medically active fractions from sea cucumber. United State Patent 5,876,762, March 2.
Fonseca, R. J., and Mourão, P. A., 2006. Fucosylated chondroitin sulfate as a new oral antithrombotic agent. Thrombosis and haemostasis, 96: 822-829.
Fu, X. Y., Xue, C. H., Miao, B. C., Li, Z. J., Gao, X., and Yang, W. G., 2005. Characterization of proteases from the digestive tract of sea cucumber (Stichopus japonicus): High alkaline protease activity. Aquaculture, 246 (1-4): 321-329, DOI: 10. 1016/j.aquaculture.2005.01.012.
Guerrini, M., Beccati, D., Shriver, Z., Naggi, A., Viswanathan, K., Bisio, A., Capila, I., Lansing, J. C., Guglieri, S., Fraser, B., Al-Hakim, A., Gunay, N. S., Zhang, Z., Robinson, L., Buhse, L., Nasr, M., Woodcock, J., Langer, R., Venkataraman, G., Linhardt, R. J., Casu, B., Torri, G., and Sasisekharan, R., 2008. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nature Biotechnology, 26: 669-675, DOI: 10.1038/nbt1407.
Hamaguchi, P., Geirsdottir, M., Vrac, A., Kristinsson, H. G., Sveinsdottir, H., Fridjonsson, O. H., and Hreggvidsson, G. O., 2010. In vitro antioxidant and antihypertensive properties of Icelandic sea cucumber (Cucumaria frondosa). Presented at IFT 10 Annual Meeting & Food Expo, Chicago, IL, USA, July 17–20, 2010; presentation no. 282-04.
Hing, H. L., Kaswandi, M. A., Azraul-Mumt azah, R., Hamidah, S. A., Sahalan, A. Z., Normalawati, S., Samsudin, M. W., and Ridzwan, B. H., 2007. Effect of methanol extracts from sea cucumbers Holothuria edulis and Stichopus chloronotus on Candida albicans. Microscopy and Microanalysis, 13: 270-271, DOI: http://dx.doi.org/10.1017/S1431927607071553.
Kitagawa, I., Kobayashi, M., and Kyogoku, Y., 1982. Marine natural products IX structural elucidation of triterpenoidal oligoglycosides from the bahamean sea cucumber Actinopyga agassizi Selenka. Chemical and Pharmaceutical Bulletin, 30: 2045-2050.
Matsuhiro, B., 1996. Vibrational spectroscopy of seaweed galactans. Hydrobiologia, 326/327: 481-489.
Matsuhiro, B., Osorio-Román, I. O., and Torres, R., 2012. Vibrational spectroscopy characterization and anticoagulant activity of a sulfated polysaccharide from sea cucumber Athyonidium chilensis. Carbohydrate Polymers, 88: 959-965.
Matsuno, T., and Tsushima, M., 1995. Comparative biochemical studies of carotenoids in sea cucumbers. Comparative Biochemistry and Physiology–PartB: Biochemistry and Molecular Biology, 111: 597-605, DOI: 10.1016/0305-0491(95)00028-7.
Mourão, P. A., Pereira, M. S., Pavao, M. S., Mulloy, B., Tollefsen, D. M., Mowinckel, M. C., and Abildgaard, U., 1996. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. Journal of Biological Chemistry, 271: 23973-23984.
Ohira, S., and Toda, K., 2006. Ion chromatographic measurement of sulfide, methanethiolate, sulfite and sulfate in aqueous and air samples. Journal of Chromatography A, 1121: 280-284.
Pacheco, R. G., Vicente, C. P., Zancan, P., and Mourão, P. A., 2000. Different antithrombotic mechanisms among glycosaminoglycans revealed with a new fucosylated chondroitin sulfate from an echinoderm. Blood Coagul Fibrinolysi, 11: 563-573.
Roginsky, A., Singh, B., Ding, X. Z., Collin, P., Woodward, C., Talamonti, M. S., Bell, R. H., and Adrian, T. E., 2004. Frondanol(R)-A5p from the sea cucumber Cucumaria frondosa induces cell cycle arrest and apoptosis in pancreatic cancer cells. Pancreas, 29: 335.
San Miguel-Ruiz, J. E., and García-Arrarás, J. E., 2007. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Developmental Biology, 7: 1-19, DOI: 10.1186/1471-213X-7-115.
Strydom, D., 1994. Chromatographic separation of 1-phenyl-3-methyl-5-pyrazolone-derivatized neutral, acidic and basic aldoses. Journal of Chromatography A, 678: 17-23, DOI: 10. 1016/0021-9673(94)87069-1.
Tapon-Bretaudiere, J., Chabut, D., Zierer, M., Matou, S., Helley, D., Bros, A., Mourão, P. A., and Fischer, A. M., 2002. A fucosylated chondroitin sulfate from echinoderm modulates in vitro fibroblast growth factor 2-dependent angiogenesis. Molecular Cancer Research, 1: 96-102.
Tian, F., Zhang, X., Tong, Y., Yi, Y., Zhang, S., Li, L., Sun, P., Lin, L., and Ding, J., 2005. PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biology and Therapy, 4: 874-882.
Tong, Y., Zhang, X., Tian, F., Yi, Y., Xu, Q., Li, L., Tong, L., Lin, L., and Ding, J., 2005. Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. International Journal of Cancer, 114: 843-853.
Trotter, J. A., Lyons-Levy, G., Thurmond, F. A., and Koob, T. J., 1995. Covalent composition of collagen fibrils from the dermis of the sea cucumber, Cucumaria frondosa, a tissue with mutable mechanical properties. Comparative Biochemistry and Physiology–Part A: Physiology, 112: 463-478, DOI: 10.1016/0300-9629(95)02015-2.
Vieira, R., and Mourão, P., 1988. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. Journal of Biological Chemistry, 263: 18176-18183.
Wen, J., Hu, C., and Fan, S., 2010. Chemical composition and nutritional quality of sea cucumbers. Journal of the Science of Food and Agriculture, 90: 2469-2474, DOI: 10.1002/jsfa. 4108.
Wu, M., Huang, R., Wen, D., Gao, N., He, J., Li, Z., and Zhao, J., 2012. Structure and effect of sulfated Fuc on anticoagulant activity of the fucosylated chondroitin sulfate from sea cucumber Thelenata ananas. Carbohydrate Polymers, 87: 862-868.
(Edited by Qiu Yantao)
(Received March 3, 2013; revised April 18, 2013; accepted April 24, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-571-88982151
E-mail: chenshiguo210@163.com
杂志排行
Journal of Ocean University of China的其它文章
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- The Appearance of Ulva laetevirens (Ulvophyceae, Chlorophyta) in the Northeast Coast of the United States of America
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Study on Internal Waves Generated by Tidal Flow over Critical Topography
- Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
