Pharmacokinetics of oxiracetam and its degraded substance(HOPAA)after oral and intravenous administration in rats
2014-04-20XiohongLiuPnqinFeitongZhngXingrongZhng
,Xiohong Liu,Pnqin M, Feitong Zhng,Xingrong Zhng,**
aSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang,China
bNingxia Kangya Pharmaceutical Co.,Ltd,Yinchuang,China
cSchool of Traditional Chinese Medicine,Shenyang Pharmaceutical University,Shenyang,China
dGLP Center,School of Life Science and Biopharmacy,Shenyang Pharmaceutical University,Shenyang,China
Pharmacokinetics of oxiracetam and its degraded substance(HOPAA)after oral and intravenous administration in rats
Xinhuan Wana,Xiaohong Liua,Zheng Liud,Panqin Mab, Feitong Zhanga,Xiangrong Zhangc,*,Jin Suna,**
aSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang,China
bNingxia Kangya Pharmaceutical Co.,Ltd,Yinchuang,China
cSchool of Traditional Chinese Medicine,Shenyang Pharmaceutical University,Shenyang,China
dGLP Center,School of Life Science and Biopharmacy,Shenyang Pharmaceutical University,Shenyang,China
A R T I C L E I N F O
Article history:
Received 21 April 2014
Received in revised form
12 July 2014
Accepted 7 August 2014
Available online 27 August 2014
Oxiracetam
4-Hydroxy-2-oxo-1-pyrrolidine ace
tic acid(HOPAA)
High performance liquid
chromatography-tandem mass
spectrometry(HPLC-MS/MS)
Pharmacokinetics
Toxicity
The pharmacokinetics of oxiracetam and its degraded substance(4-hydroxy-2-oxo-1-pyrrolidine acetic acid,HOPAA)after oral and intravenous administration in rats were studied using an established UPLC-MS/MS method.Three groups of rats after an overnight fasted received 10 g/kg(n=6)oxiracetam suspensions orally,and 2 g/kg(n=6)normal or degraded oxiracetam injections intravenously via a caudal tail vein,respectively.Before the pharmacokineticexperiment,asimplesafetyevaluationtestwasconductedonthedegradedoxiracetam injections containing 16.16%HOPAA in mice.There was no mortality by a single intravenous doseof2g/kgofdegradedoxiracetaminjectionswithintwoweeks,demonstratingthatHOPAA was non-toxic in mice.Following intravenous administration of the normal injections,the plasmaconcentration-timecurvesofoxiracetamand HOPAAbothshoweda rapid elimination phase.Thevaluesoft1/2were3.1 ± 1.5hforoxiracetamand0.8 ± 0.2hforHOPAA,andthemean residencetimes(MRT)were1.2 ± 0.1hand0.8 ± 0.1h,respectively.OxiracetamandHOPAAafter intravenous administration of the degraded oxiracetam injections presented elimination patterns similar to thoseobserved in the normal injections.Oral pharmacokinetic results showed that the Tmaxwas less than 1.5 h for the two analytes,and both had a longer t1/2and MRT than thoseofintravenousadministration.ContentsofHOPAAinthreegroupswerecalculatedbased on AUC0-tvalues of the two analytes.The quantitative change of HOPAA in vivo was also evaluatedbycomparingtheplasmaconcentrationsofHOPAAandoxiracetamatthesametime for every group.Additionally,the values of absolute bioavailability of oxiracetam were about 8.0%and7.4%calculatedby thenormal or degradedoxiracetaminjections,whichwerefar less than the value of 75%reported in literature,indicating the necessity of further study.
© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
Oxiracetam,4-hydroxy-2-oxo-1-pyrrolidine acetamide,is a cyclic hydroxy-amino butyric acid derivative,and is widely applied in improving central nervous system disorders as a cognition enhancer[1,2].It has been found to improve learning,memory and acquisition processes[3,4].Recently, there are also some domestic researches on oxiracetam. Huang RY verif i ed its effect on vascular dementia combined with nimodipine,and Li JW et al.then conf i rmed its protective effect on traumatic brain injury in rats[5,6].
HOPAA,4-hydroxy-2-oxo-1-pyrrolidine acetic acid,is a degraded substance of oxiracetam.Determined by high performance liquid chromatography(HPLC)in our lab,the contentsofHOPAA were0.5% and 0.04% forlab-made oxiracetam injections and oxiracetam tablets,and 1.0%and 0.07%for the original commercial injections and tablets (Korean Drug Co.,Ltd),respectively.HOPAA was proved to be the main degraded substance comparing with other related substances,whose content had exceeded the impurity limit (0.3%)according to our own product quality standard.So far, there is no further research on it besides an examination in formulations and a sensitive HPLC-MS/MS method for its determination in rat plasma[7,8].In order to verify whether contents of HOPAA would increase and thus bring any underlying danger to patients in vivo,a simple toxicity test in mice was conducted to ensure the safety of HOPAA and the degradedinjectionscontaining16.16%HOPAAobtained based on a violent hydrolysis reaction and the normal oxiracetam injections were used as the test and the reference preparations,respectively.Then,the pharmacokinetic experiment was developed to investigate the pharmacokinetics behaviors ofoxiracetam and HOPAA in rats simultaneously.
2. Materials and methods
2.1. Materials
Oxiracetam(99.7%pure,Fig.1A)and piracetam(100.0%pure, Fig.1C)were both supplied by the National Institutes for Food and Drug Control(Beijing,China).HOPAA(97.0%pure,Fig.1B) was purchased from Shenzhen Yasuo Sci-Tech Co.,Ltd (Shenzhen,China).Methanol and acetonitrile(HPLC grade) were bought from Fisher Scientif i c(Pittsburgh,USA).Formic acid was acquired from Dikma Reagent Company(Ohio,USA). Oxiracetam injections(1 g/5 ml,purity of 99.50%)and oxiracetam tablets(800 mg)were both lab-made.
2.2. Toxicity test of oxiracetam injection after hydrolysis reaction
HOPAA is the degradation product of oxiracetam in which amino was instead by hydroxyl.16.16%HOPAA solution in the toxicity study was prepared by a destructive test of heating the solution of oxiracetam injection in the water bath for 120 h under pH adjusted from 4.35 to 7.2 with 0.1 M sodium hydroxide solution.Then the oxiracetam injection after heating was regulated to the original pH 4.35 by 0.1 M phosphoric acid solution,sterilized under 121°C for 15 min after packaging in ampoules,and stored at-4°C before use.
40 Kunming mice male and female(20 ± 2 g),provided by Shenyang Pharmaceutical University Animal Care and Use Committee,were housed at the room temperature(25 ± 2°C) and a relative humidity of 65 ± 5%under the natural light/dark conditions for 1 week.Then,mice were fasted for 12 h prior to the experiment.All mice were divided into two groups for the experimental group and the control,and mice in each group were divided evenly between male and female.Then the experimental group was administered the degraded oxiracetaminjections containing 16.16%HOPAA intravenously via a caudal tail vein at a dose of 2 g/kg body weight calculating by oxiracetam.Similarly,the control was administered in the same way at the same dose level after conversion.Then all animals were observed for any adverse clinical signs immediately and restore their normal diet for the 2 weeks'observation period.
2.3. Pharmacokinetics study design
18 Sprague Dawley male rats weighed of 250 ± 10 g in this pharmacokinetics experiment,were approved by animal centerof Shenyang Pharmaceutical University(Shenyang,P.R. China).All animals were housed in a temperature-and humidity-controlled environment,which was in accordance with institutional guidelines and approved by Shenyang Pharmaceutical University Animal Care and Use Committee. The rats were fasted overnight with ad libitum access to water prior to the experiment and divided into three groups randomly(n=6).The oral suspensions of oxiracetam was prepared by suspending the drug powder in 0.5%CMC-Na with the concentration of 1 g/ml and orally administered at a single dose of 10 g/kg.The normal oxiracetam injections and degraded injections were intravenously administered at a dose of 2 g/kg calculating by oxiracetam after conversion by the tail vein.No any adverse effect was observed during the whole experimental period.
Blood samples(about 300 μl)were collected into heparinized tubes and were centrifuged at 3000 rpm for 10 min to obtain the plasma.The blood samples were collected at 0(predose),0.17,0.33,0.50,0.75,1,1.5,2,3,5,8,10,12,and 24 h after oral administration and at 0(pre-dose),0.08,0.17,0.33,0.50, 0.75,1,1.5,3,5,8,12,24 h after intravenous administration viatail vein.All blood samples were immediately processed by centrifugationat 3000rpmfor 10 minto obtain the plasmaand stored at-20°C before analysis.

Fig.1-Chemical structures of the:(A)oxiracetam,(B) HOPAA and(C)piracetam.
2.4. Analysis of oxiracetam and HOPAA concentration in the plasma
The oxiracetam and its related substance HOPAA in rat plasma were analyzed by HPLC-MS/MS method[8].
A non-compartmental method was used in the pharmacokinetic analysis.Pharmacokinetic parameters,including maximum plasma concentration(Cmax),time to reach Cmax(Tmax),area under the curve(AUC),elimination half-life(t1/2), Mean residence time(MRT),plasma clearance(CL)and the apparent volume of distribution(Vd)were calculated using DAS 2.1.1 pharmacokinetic software(Chinese Pharmacology Society).The contents of HOPAA in three groups in vivo and the absolute bioavailability value of oxiracetam were calculated by the following two equations:

AUCHOPAAand AUCoxiracetamin Equation A were the values of AUC0~tof HOPAA and oxiracetam at the same time and in the same group.AUCoraland AUCivin Equation B were the values of AUC0-tof oxiracetam after oral and intravenous dosing,respectively.All of the pharmacokinetic parameters were expressed as mean ± SD.
3. Results and discussion
3.1. Toxicity test of oxiracetam injection after hydrolysis reaction
Generalpharmacologyshowedthatoxiracetamhasno adverse effect on the nerves system,respiratory system and cardiovascular system.Acute toxicity study revealed that the LD50values of oxiracetam in mice and rat were both not less than 10 g/kg[1].The fundamental purpose of this study was to verify the safety of HOPAA in oxiracetam injection,so an appropriate dose of 2 g/kg was chosen for administration in mice.The degraded oxiracetam injections containing 16.16% HOPAA were prepared by heating the lab-made injections under the condition of weak alkaline and tests proved that pH of 7.2 was the best hydrolysis condition.
The immediate observation within 3 h after single intravenous administration showed that there were no signif i cant difference between two group mice in dieting,action and mental state.All the mice survived to the 14 d of observation period.
3.2. Pharmacokinetics study
In order to detect HOPAA accurately in plasma samples,a higher dose of 10 g/kg for oral administration and 2 g/kg for intravenous were used.The pharmacokinetic parameters ofoxiracetam and HOPAA after oral administration of oxiracetamsuspensionsandintravenousadministrationof normal or degraded oxiracetam injections are presented in Table 1 and Table 2,respectively.The mean plasma concentration versus time prof i les of oxiracetam and HOPAA in three groups are shown in Figs.2-4,respectively.

Table 1-Major pharmacokinetic parameters of oxiracetam after oral administration of 10 g/kg and intravenous administration of 2 g/kg normal or degraded injections to rats(n=6).
For intravenous administration of the normal oxiracetam injections,the plasma concentrations of oxiracetam and HOPAA both decreased rapidly.Mean values of t1/2were 3.1 ± 1.5 h for oxiracetam and 0.8 ± 0.2 h for HOPAA,and the mean residence times(MRT)of them were 1.2 ± 0.1 h and 0.8 ± 0.1 h,respectively.Parameters including t1/2and MRT for oxiracetam and HOPAA in two intravenous groups proved no signif i cant difference(P > 0.05),indicating that the two analytes after intravenous administration of the degraded injections exhibited similar elimination patterns to those observed in the normal injections.For oral administration, oxiracetam and HOPAA reached the peak plasma concentration simultaneously at about1.4 h,demonstratingthatHOPAA was absorbed as rapidly as oxiracetam.Overall,for bothHOPAA and oxiracetam,the values t1/2and MRT in oral administration were longer than those of intravenous groups clearly,which may due to that the drug absorption did not occur and complete immediately because of the high dose. However,the t1/2values of 7.0 ± 3.4 h for oxiracetam in oral groups was similar to that of 8.5 ± 1.7 h in human[9]and a little longer than the results reported by some domestic researches in rats[10,11].It may be explained by the relative shorter sampling schedule of 12 h in their investigations, which would lead to the inaccurate evaluation on t1/2.
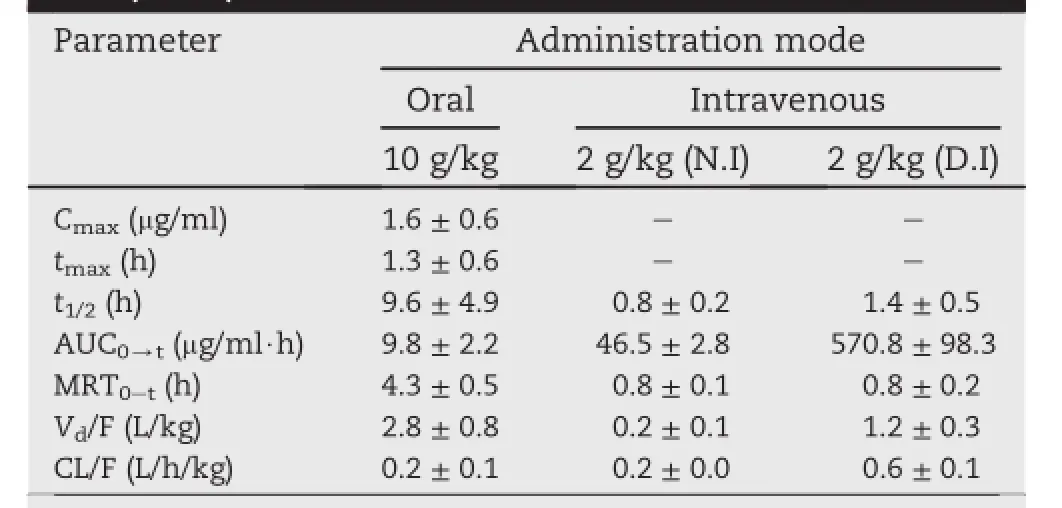
Table 2-Major pharmacokinetic parameters of HOPAA after oral administration of 10 g/kg and intravenous administration of 2 g/kg normal or degraded injections to rats(n=6).

Fig.2-Concentration-time prof i le of oxiracetam and HOPAA in rat plasma after a single oral administration oxiracetam suspensions at a dose of 10 g/kg(n=6).

Fig.3-Concentration-time prof i le of oxiracetam and HOPAA in rat plasma after a single intravenous administration normal oxiracetam injections at a dose of 2 g/kg(n=6).

Fig.4-Concentration-time prof i le of oxiracetam and HOPAA in rat plasma after a single intravenous administration degraded oxiracetam injections at a dose of 2 g/kg(n=6).

Table 3-Contents of HOPAA for three administration group in vitro and in vivo(n=6).
Based on the high dose administered,the degraded substance HOPAA was determined successfully in all groups.The contents of HOPAA for the three groups in vivo were calculated using the Equation A.Table 3 presented a comparison for the percentage of HOPAA in oxiracetam in vivo and the contents of it in formulations.Comparison results showed that the contents of HOPAA in vivo in three groups versus the data in vitro proved to be three different results completely.For the oral group,content of HOPAA in vivo exceeded that in vitro by approximately seven fold revealing that part of oxiracetam maybetransformed intoHOPAAby hydrolysisreactionduring the oral absorption process in the gastrointestinal tract.For the group administered normal oxiracetam injections,content of HOPAA in vivo was equal to that in pharmaceutical formulations.However,for the other intravenous group, content of HOPAA detected in vivo was only half of that in vitro, which may be explained by the metabolism of HOPAA in vivo. According to the structure of HOPAA with two-OH,it is liable to form phaseⅡ metabolism in vivo including glucuronidation, acetylation and so on.Thus,contents of HOPAA in vivo and in vitro have no linear correlation.
Additionally,the ratios of concentrations of HOPAA and oxiracetam at the same time for the three groups were calculated to investigate the quantitative changes of HOPAA in vivo.Fig.5 shows the mean plasma concentration ratios of HOPAA and oxiracetam versus time prof i le for three groups. For oral group,the ratio was decreased mildly at the range of 0.45%~0.29%within 10 h.For the normal oxiracetam group, the ratio was decreased faster than oral group with a minor f l uctuation.And for the group administered degraded injections,the ratio was decreased rapidly from 13.20%to 2.63% within 12 h.

Fig.5-Mean concentration ratios of HOPAA and oxiracetam at the same time for the:(A)oral oxiracetam suspensions (n=6),(B)normal oxiracetam injections(n=6)and(C)degraded oxiracetam injections(n=6).
The mean AUC0-tvalues of oxiracetam after oral dosing were 2832.3 ± 622.9 μg h/ml.Similarly,the mean AUC0-tvalues of the two intravenous groups were 7085.0 ± 773.9 μg h/ml and 7607.1 ± 1121.3 μg h/ml for the normal oxiracetam injections and degraded injections, respectively. The absolutebioavailability of oxiracetam were 8.0%and 7.4%calculating by the two intravenous groups,respectively,far less than that of 75%in healthy volunteers[9].It may be due to the species differencesfrom humanandrat,dosagediscrepancythat 10g/ kg orally administered to rat in our study and 2 g/kg used in the previous literature.
4. Conclusion
Toxicity test for degraded injection containing 16.16%HOPAA conductedinthisresearchdemonstratedthatitwasnon-toxic in mice.Above all,this was the f i rst time to investigate the pharmacokinetics behaviors of oxiracetam and its degraded substance HOPAA in rats simultaneously.HOPAA and oxiracetam were both absorbed rapidly in the gastrointestinal tract in the oral group and exhibited a rapid elimination phase in intravenous groups.The contents of HOPAA in vivo in three groups appeared to be different results completely versus those in vitro suggesting that there was no linear correlation between the contents of HOPAA in vivo and vitro.Meanwhile, the absolute bioavailability of oxiracetam in rat was estimated to be about 8%,which was far less that of 75%evaluated in human indicating the necessity of further development. Finally,HOPAA,a main degraded substance of oxiracetam, has been conf i rmed to be non-toxic and the further investigation of its eff i cacy is under investigation in rats in our laboratory.
Acknowledgment
This work was f i nancially supported from the National Nature Science Foundation of China(No.81173009).
R E F E R E N C E S
[1]Nicolaus Bruno JR.Chemistry and pharmacological of nootropics.Drug Dev Res 1982;2:463-474.
[2]Malykh Andrei G,Sadaie M Reza.Piracetam and piracetamlike drugs from basic science to novel clinical applications to CNS disorders.Drugs 2010;70:287-312.
[3]Saletu B,Linzmayer L,Grunberqer L,et al.Double-blind, placebo-controlled,clinical,psychometric and neurophysiological investigations with oxiracetam in the organic brain syndrome of late life.Neuropsychobiology 1985;13:44-52.
[4]Banf iS,Dorigotti L.Experimental behavioral studies with oxiracetam on different types of chronic cerebral impairment.Clin Neuropharmacol 1986;9:S19-26.
[5]Huang Ruoyan.Evaluation of clinical effect of oxiracetam combined with nimodipine in treatment of vascular dementia in 49 cases.China Prac Med 2013;8:36-37.
[6]Li JW,Yang DJ,Chen XY,et al.Protective effect of oxiracetam on traumatic brain injury in rats.Zhongguo Ying Yong Sheng Li Xue Za Zhi 2013;29:298-300.
[7]Gagliardi L,de Orsi D,Cavazzutti G,et al.HPLC determination of oxiracetam,its impurities,and piracetam in pharmaceutical formulations.Anal Lett 1994;27:879-885.
[8]Wan Xinhuan,Wang He,Ma Panqin,et al.Simultaneous quantitative determination of oxiracetam and its degraded substance(HOPAA)in rat plasma by HPLC-MS/MS after a single high dose intravenous administration.J Chromatogr B 2014;969:95-100.
[9]Perucca E,Albrici A,Gatti G,et al.Pharmacokinetics of oxiracetam following intravenous and oral administration in healthy volunteers.Eur J Drug Metab Pharmacokinet 1984;3:267-274.
[10]Liu Changxiao,Gu Yibao,Li Quansheng.Pharmacokinetics of oxiracetam in rats and mice.Acta Pharmacol Sin 1999;34:85-89.
[11]Liu Xiuju,Zhang Zhiqing,Wang Chuanping,et al.Effects of omeprazole on pharmacokinetics of oxiracetam in rats. China Pharm 2012;23:1172-1174.
*Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel./fax:+86 24 23986522.
**Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel./fax:+86 24 23986325.
E-mail addresses:zhangxr@vip.sina.com,xrzhxr@126.com(X.Zhang),sunjin0529@aliyun.com(J.Sun).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.006
1818-0876/© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- LC-MS/MS assay for pitavastatin in human plasma and subsequent application to a clinical study in healthy Chinese volunteers
- Studies on the spray dried lactose as carrier for dry powder inhalation
- Pharmacokinetic performance of the nitrendipine intravenous submicron emulsion in rats
- Effect of the glyceryl monooleate-based lyotropic phases on skin permeation using in vitro diffusion and skin imaging
- Development of phosphonate-terminated magnetic mesoporous silica nanoparticles for pH-controlled release of doxorubicin and improved tumor accumulation
- Pharmaceutical particle technologies:An approach to improve drug solubility,dissolution and bioavailability
