Pharmacokinetic performance of the nitrendipine intravenous submicron emulsion in rats
2014-04-20
Shenyang Pharmaceutical University,School of Pharmacy,No.103,Wenhua Road,Shenyang 110016,China
Pharmacokinetic performance of the nitrendipine intravenous submicron emulsion in rats
Jibin Guan,Nana Zhao,Yinglei Zhai,Chunxia Chu,Hongming Chen, Tianhong Zhang*
Shenyang Pharmaceutical University,School of Pharmacy,No.103,Wenhua Road,Shenyang 110016,China
A R T I C L E I N F O
Article history:
Received 18 March 2014
Received in revised form
11 July 2014
Accepted 14 July 2014
Available online 6 September 2014
Nitrendipine
To compare pharmacokinetic behaviors of nitrendipine submicron emulsion with nitrendipine solution following intravenous administration in rats.The plasma concentrations were analyzed by ultra-performance liquid chromatography coupled with tandem mass spectrometry detection(UPLC-MS/MS)through a new validated method.The pharmacokinetic parameters of the nitrendipine submicron emulsion and nitrendipine solution were as follows:AUC0-t900.76 ± 186.59 versus 687.08 ± 66.24 ng h/ml,Cmax854.54 ± 159.48 versus 610.59 ± 235.99 ng/ml,t1/22.37 ± 1.99 versus 2.80 ± 2.69 h.The relative bioavailability of nitrendipine submicron emulsion to nitrendipine solution was 131.4 ± 11.3%.The developed methods could meet the requirements of bioanalysis.Compared to the solution injection,intravenous submicron emulsion presents higher systematic exposure which can help to improve the therapeutic eff i cacy.
© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
Nitrendipine(NTD)is a widely used calcium channel blocker belonging to the dihydropyridine class compounds that is being developed for the treatment of hypertension[1,2].With itsmild andpersistentantihypertensiveeffect,itwas preferred drug for elderly hypertensive patients.The molecular structure of NTD is showed in Fig.1.
Due to its low solubility in water(about 2 μg/ml)[3],slow dissolution rate,and high f i rst-pass effect[4,5],NTD exhibits low and irregular bioavailability following oral administration.To improve the solubility,various techniques have been applied,such as self-microemulsifying drug-delivery system [6],solid dispersion[7],solid lipid nanoparticles[8,9],nanosuspensions[10]and inclusion complexation[11].However, the commercial dosage forms of NTD are still usual tablets and capsules only which all display low oral bioavailability.
In recent years,submicron emulsion was applied to improve the solubility and bioavailability of NTD as a new drug-delivery system.Composed of well-tolerated excipients, this drug-delivery system has many advantages including:(1)enhancing the solubility of many hydrophobic drugs and meanwhile avoiding the use of conventional co-solvent systems;(2)preventing drug hydrolysis and enhancing drug stability by incorporating it into the interior oil phase;(3) providing the possibility of sustained-release and targeted delivery of drugs[12].

Fig.1-The structure of nitrendipine.
The preparation of NTD submicron emulsion has been described in details by Nana Zhao et al.[13]in her research. But there is still no study on the intravenous pharmacokinetic behaviors of nitrendipine submicron emulsion.This paper established the analysis method of NTD in vitro and got validations.Then a pharmacokinetic study on rats was preformed to evaluate whether the bioavailability of NTD was signif icantly improved following intravenous injection of nitrendipine submicron emulsion.
2. Materials and methods
2.1. Materials
Nitrendipine was purchased from Shanxi Sciphar Biotechnology Co.,Ltd.(Shanxi,China).Egg lecithin was purchased from Shanghai advanced vehicle technology pharmaceutical Ltd.(Shanghai,China).Soybean oil and medium-chain triglyceride was supplied by Tieling Beiya Pharmaceutical Co., Ltd.(Tieling,China).Oleic acid was purchased from Lipoid GmbH Pharmaceutical Co.,Ltd.(Germany).Nimodipine was purchased from Jinan Dongfeng Pharmaceutical Co.,Ltd. (Jinan,China).Acetonitrile and methanol(HPLC-grade)were purchased from Fisher Scientif i c(Pittsburgh,PA,USA).Diethyl ether was purchased from Tianjin Fuyu Commerce&Trade Co.,Ltd(Tianjin,China),Hexane was purchased from Tianjin Bodi Commerce&Trade Co.,Ltd(Tianjin,China).
2.2. Bioavailability and pharmacokinetic studies of injection
2.2.1. Drug preparation and administration
The NTD submicron emulsion was prepared using egg lecithin,soybean oil,medium-chain triglyceride and oleic acid. They were mixed evenly and processed by homogenization. NTD solution:the drug was dissolved by water for injection with sodium metabisulf i te,niacinamide,propanediol and ethanol which was mentioned by Nana Zhao et al.[13].
The animal experiments were approved by the Animal Experiment Ethics Committee of Shenyang Pharmaceutical University.The in vivo studies were conducted in male Sprague-Dawley rats with body weights ranging from 190 g to 210 g which were group-housed(6 animals per cage)with free access to food and water for at least one week before the pharmacokinetic study and then the rats were fasted for approximately 12 h prior to dosing.Each animal was weighed to ensure that an accurate dose was given.Nitrendipine submicron emulsion injections were used as tests and nitrendipine solution injections as references.According to clinical dosage of NTD regimen for human,the drug was injected into the tail vein at a dose of 3.15 mg/kg which was translated from human to rat by the method of BSA.Blood samples(300 μl) were collected from the orbital plexus and put into heparinized plastic tubes at the following time after dosing:5,15,30, 45 min,1,2,4,6,8,12 and 24 h.Thenthe blood was centrifuged at 3500 rpm for 10 min and stored at-20°C until assay.
2.2.2. UPLC-MS/MS conditions
Liquid-liquid extraction technique was used to extract the drug from plasma samples and nimodipine was used as internal standard.Generally,50 μl of the plasma sample was mixed with 50 μl of internal standard solution(100 ng/ml)and 50 μl of acetonitrile-water(70:30,v/v),then 1 ml of hexanediethyl ether was added and vortexed for 3 min followed by centrifugation at 3500 rpm for 5 min.Then,the upper layer was collected and dried at 37°C under a stream of nitrogen. The residue was reconstituted in 100 μl mobile phase by vortex mixing(1 min)followed by centrifugation at 15,000 rpm for 10 min.Then the upper layer was used for analysis and 5 μl was injected into UPLC-MS/MS system.
The analyses were performed on a triple-quadrupole mass spectrometer equipped with an ESI source in positive mode. The capillary voltage was 3.3 kV,and the cone voltage was 26 V for nitrendipine.The source temperature was 120°C and the desolvation temperature was 380°C.The cone gas f l ow was 50 l/h and the desolvation gas f l ow was 550 l/h.Quantif i cation was carried out in the multiple reaction monitoring mode.The fragmentation transitions were m/z 361 → 315 and 419 → 343 for nitrendipine and nimodipine,respectively.The separation was performed on an ACQUITY UPLC™ BEH C18column(50 mm × 2.1 mm,1.7 μm,Milford,MA,USA)with acetonitrile-water(70:30,v/v)as the mobile phase at a f l ow rate of 0.2 ml/min.The column temperature was set at 40°C. All data were acquired and processed using MassLynx 4.1 software with the QuanLynx program(Waters Corp.,Milford, MA,USA).
2.2.3. Method validation
Validation of UPLC-MS/MS method was evaluated by limit of quantif i cation,linearity,precision,accuracy and stability.The limit of quantif i cation(LLOQ)was def i ned as the concentration with a peak to peak signal-to-noise ratio of 10.A 1/x2-weighted least-squares linear regression was applied to the calibration standards using the peak area ratio of analyst to internal standard.Intra-and inter-day precision and accuracy were evaluated as the relative standard deviation(RSD)byanalysis of low,medium and high QC samples(n=6)on 3 consecutive d.The absolute recovery was determined by comparing the mean peak areas of six extracted samples at low,medium,and high QC concentrations with the mean peak areas of the corresponding standard samples.The postpreparative stability of extracted samples in the autosampler rack at room-temperature for 12 h,the room-temperature stability (22°C for 4 h) and freeze-thaw stability (-20°C-22°C)were evaluated by analyzing low,medium and high QC samples(n=3).
2.2.4. Pharmacokinetic and statistical analysis
The pharmacokinetic parameters were analyzed by using a pharmacokinetic program DAS 2.0.The mean peak concentration(Cmax)and peak time(Tmax)were obtained directly from the concentration-time curve.The elimination rate constant(ke)was obtained from the linear f i tted log-linear of the plasma concentration-time prof i le.The elimination half life(t1/2)was calculated as 0.693/ke.The area under the concentration-time curve to the last time point(AUC0-t)was estimated according to the linear trapezoidal rule.
The statistical analysis was performed using analysis of variance(ANOVA)at a signif i cance level of P < 0.05.
3. Results and discussion
3.1. Mass spectrometry
The ion scan spectra of nitrendipine are shown in Fig.2.The main mass spectrometry parameters,such as the source temperature,the capillary voltage,cone voltage and collision energy were optimized to get the sensitive response.
3.2. Extraction solvent
In order to choose a suitable solvent to extract the drug from plasma,several solvents were investigated by comparing the extraction recoveries.These solvents included diethyl ether, ethyl acetate,cyclohexane and isopropyl alcohol which were employed in other earlier methods[14-17].But these solvents showed a low extraction recovery(about 20%-60%).By using a binary solvent of hexane-diethyl ether(1:1),a high extraction recovery(above 84%)was obtained.So hexane-diethyl ether (1:1)was chosen to extract the drug from plasma.
3.3. Method validation
Fig.3 shows the typical chromatograms of blank plasma, plasma spiked with analyte and IS and a plasma sample from a rat after injection.It shows that no signif i cant interferences from the endogenous plasma components were observed at the retention times of the analyst and the IS.
The calibration curve of nitrendipine shows a good linearity overthe concentration range of5-1250 ng/ml (r=0.9975).The typical equations of the calibration curves using linear regression analysis were as follows: y=4.3x+29.19,where y is the peak area ratio of nitrendipine to the IS and x is the concentration of nitrendipine added to the plasma.The lowest limit of quantitation(LLOQ)was 5 ng/ ml.The precision at the corresponding LLOQ level was less than 15%and the accuracy was in the range of 80%-120%.
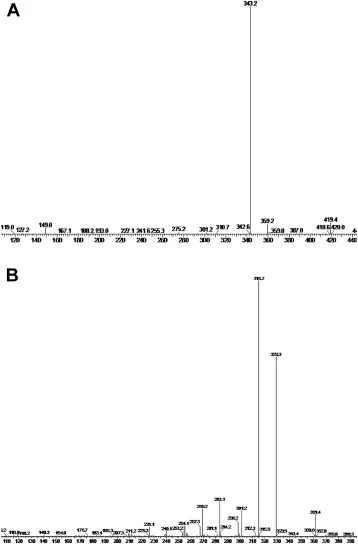
Fig.2-Massspectrumofnitrendipine(A)andnimodipine(B).
The recoveries of nitrendipine in three QC concentration levels(10,250 and 1000 ng/ml)were shown in Table 1.The mean recoveries were 84.1 ± 8.18%,97.1 ± 3.07%and 97.4 ± 2.96%,respectively.The mean absolute recovery of the internal standard was 97.6 ± 2.79%.All recoveries had a RSD less than 9.73%at three different concentrations,showing good assay consistency,precision and reproduction.
The intra-and inter-day precision and accuracy were within the acceptable range of ±15%and these results indicated that the method is accurate and precise.Stability results showed good stability of nitrendipine in rat plasma under the indicated conditions.
When this method is used,the analysis time for a single injection is just 4 min,therefore,the current UPLC-MS/MS method was considered as an appropriate method for the simple,sensitive and rapid determination of nitrendipine in plasma.
3.4. Pharmacokinetics
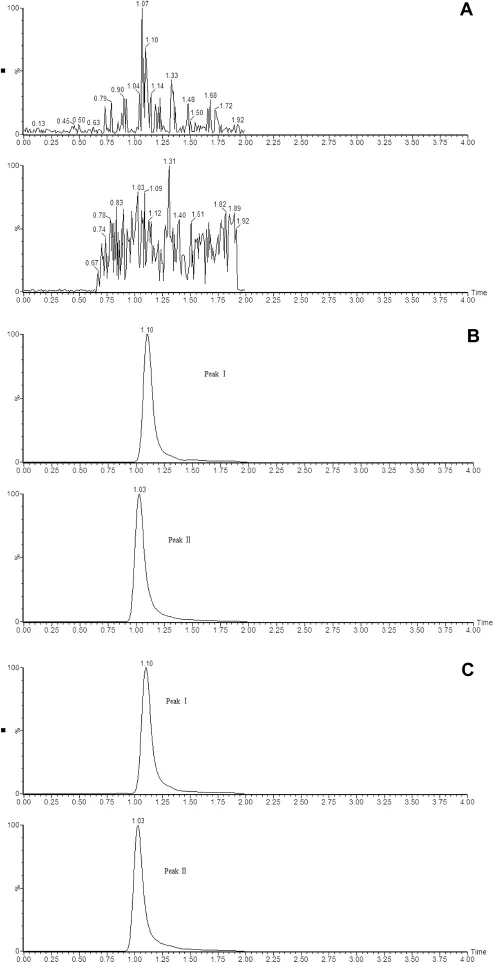
Fig.3-(A)A blank rat plasma;(B)a blank rat plasma spiked with nitrendipine at 500 ng/ml and internal standard (nimodipine,100 ng/ml);(C)rat plasma obtained from a rat 15 min after administration of nitrendipine.Peak Ⅰ,nimodipine; Peak Ⅱ,nitrendipine.
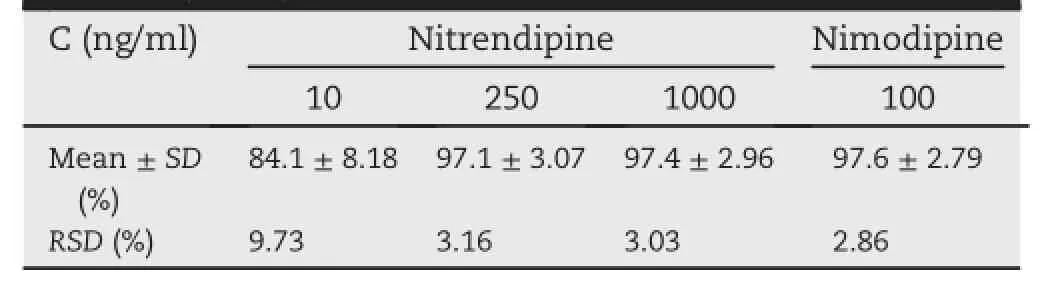
Table 1-The recovery data of nitrendipine from rat plasma(n=6).
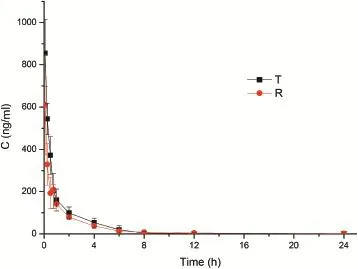
Fig.4-Mean concentration-time curves after administrating the test and reference formulations.
The mean plasma concentration-time curves and the key pharmacokinetic parameters are shown in Fig.4 and Table 2, respectively.These results showed that the Cmaxand AUC of the submicron emulsion were signif i cantly increased(P < 0.05) compared with that of usual solution.The Cmaxwas about 854.54 and 610.59 ng/ml for nitrendipine submicron emulsion and nitrendipine solution,respectively.This similar f i nding as Kun Gao et al.[18]suggested that incorporation of durg into lipid emulsion may increase its effective plasma concentration.The AUC0-tfor nitrendipine submicron emulsion was 900.76 ng h/ml,about 1.3-fold higher than that of nitrendipine solution.In summary,the submicron emulsion showed a faster and better pharmacokinetic prof i le than the solution.
The plasma concentration-time curves revealed that the submicron emulsion shows a signif i cantly higher plasma concentration after 45 min of injection than solution formulation.This phenomenon can be explained by the state of nitrendipine in the two formulations.In the solution,nitrendipine existing as the molecule state can be eliminated fromplasma rapidly.But in the submicron emulsion,nitrendipine was entrapped into the interior oil phase,so it was slowly for the drug to release from the carrier.This result attributed to the effect of sustained-release,which was in agreement with the report[19,20].And it did not have dose dumping phenomenon in vivo.This result meant that the NTD submicron emulsion also had good stability in vivo compare with the results of stability studies in vitro from Nana Zhao et al.[13].
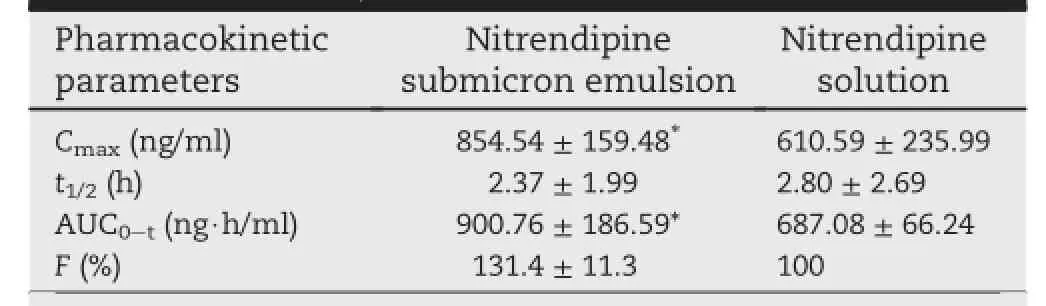
Table 2-Pharmacokinetic parameters of nitrendipine after i.v.administration(data were expressed asmean ± SD.,n=6).
4. Conclusion
Thispaperestablishedarapid,sensitiveandaccurate UPLC-MS/MS method to study the pharmacokinetics of netrendipine submicron emulsion in rats.The submicron emulsion showed a better absorption than the solution.The results suggest that it is a promising way to improve the bioavailability of poorly soluble drugs by the application of submicron emulsion.
R E F E R E N C E S
[1]Bean BP,Sturek M,Puga A,et al.Nitrendipine block of calcium channels in cardiac and vascular muscle.J Cardiovasc Pharmacol 1987;9:S17-24.
[2]Wang L,Cui FD,Hayase T,et al.Preparation and evaluation of solid dispersion for Nitrendipine-Carbopol and Nitrendipine-HPMCP systems using a Twin Screw Extruder. Chem Pharm Bull 2005;53:1240-1245.
[3]Li J,Li QX,Xie XF,et al.Differential roles of dihydropyridine calcium antagonist nifedipine,nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats.Eur J Pharmacol 2009;620:97-104.
[4]Krol GJ,Lettieri JT,Yeh SC,et al.Disposition and pharmacokinetics of 14 C-nitrendipine in healthy volunteers. J Cardiovasc Pharmacol 1987;9:s122-8.
[5]Goda KL,Sorkin EM.Nitrendipine:a review of its pharmacodynamic and pharmacokinetic properties,and therapeutic eff i cacy in the treatment of hypertension.Drugs 1987;33:123-155.
[6]Wang ZY,Sun J,Wang YJ,et al.Solid self-emulsifying nitrendipine pellets:preparation and in vitro/in vivo evaluation.Int J Pharm 2010;383:1-6.
[7]Wang L,Cui FD,Hisakazu S.Improvement of the dissolution rate of nitrendipine using a new Pulse Combustion Drying method.Chem Pharm Bull 2007;55:1119-1125.
[8]Manjunath K,Venkateswarlu V.Pharmacokinetics,tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration.J Drug Target 2006;14(9):632-645.
[9]Mohan CK,Lingam M,Reddy VP,et al.Development of nitrendipine controlled release formulations based on SLN and NLC for topical delivery:in vitro and ex vivo characterization.Drug Dev Ind Pharm 2008;34:719-725.
[10]Xia DN,Cui FD,Piao HZ,et al.Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability.Eur J Pharm Sci 2010;40:325-334.
[11]Han GC,Dae DK,Hu WJ,et al.Improvement of dissolution and bioavailability of nitrendipine by inclusion in hydroxypropyl-β-cyclodextrin.Drug Dev Ind Pharm 2003;10:1085-1094.
[12]Chansri N,Kawakami S,Yamashita F,et al.Inhibition of liver metastasis by all-trans retinoic acid incorporated into o/w emulsion in mice.Int J Pharm 2006;321(1-2):42-49.
[13]Zhao NN,Qin YM,Sun YH,et al.Formulation and preparation of a nitrendipine submicron emulsion.Asian J Pharm Sci 2011;6(3-4):151-158.
[14]Qin F,Ma YY,Wang YQ,et al.Determination of nimodipine in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and pharmacokinetic application.J Pharm Biomed Anal 2008;46:557-562.
[15]Ioannis N,Athanasios C,Daftsios S.Determination of nifedipine in human plasma by solid-phase extraction and high-performance liquid chromatography:validation and application to pharmacokinetic studies.J Pharm Biomed Anal 2003;32:1213-1218.
[16]Quan P,Shi K,Piao HZ,et al.A novel surface modif i ed nitrendipine nanocrystals with enhancement of bioavailability and stability.Int J Pharm 2012;30:366-371.
[17]Yang MS,Cui FD,Yang MH,et al.Vivo evaluation of two novel controlled-release nitrendipine formulations.Drug Dev Ind Pharm 2005;31:589-595.
[18]Gao Kun,Sun Jin,Liu Kai,et al.Preparation and characterization of a submicron lipid emulsion of docetaxel: submicron lipid emulsion of docetaxel.Drug Dev Ind Pharm 2008;34:1227-1237.
[19]Sing M,Ravin LJ.Parenteral emulsions as drug carriers systems.J Parenter Sci Technol 1986;40:34-41.
[20]Xia DN,Cui FD,Piao HZ,et al.Effect of crystal size on the in vitro dissolution and oral absorption of nitrendipine in rats.Pharm Res 2010;27:1965-1976.
*Corresponding author.
E-mail address:zhangth_student@aliyun.com(T.Zhang).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.07.004
1818-0876/© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
Submicron emulsion
Bioavailability
Method validation
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- LC-MS/MS assay for pitavastatin in human plasma and subsequent application to a clinical study in healthy Chinese volunteers
- Pharmacokinetics of oxiracetam and its degraded substance(HOPAA)after oral and intravenous administration in rats
- Studies on the spray dried lactose as carrier for dry powder inhalation
- Effect of the glyceryl monooleate-based lyotropic phases on skin permeation using in vitro diffusion and skin imaging
- Development of phosphonate-terminated magnetic mesoporous silica nanoparticles for pH-controlled release of doxorubicin and improved tumor accumulation
- Pharmaceutical particle technologies:An approach to improve drug solubility,dissolution and bioavailability
