Development of phosphonate-terminated magnetic mesoporous silica nanoparticles for pH-controlled release of doxorubicin and improved tumor accumulation
2014-04-20
Department of Pharmaceutics,Shenyang Pharmaceutical University,Shenyang 110016,China
Original Research Paper
Development of phosphonate-terminated magnetic mesoporous silica nanoparticles for pH-controlled release of doxorubicin and improved tumor accumulation
Erxi Che,Long Wan,Ying Zhang,Qinfu Zhao,Xiling Han,Jia Li,Jia Liu, Siling Wang*
Department of Pharmaceutics,Shenyang Pharmaceutical University,Shenyang 110016,China
A R T I C L E I N F O
Article history:
Received 22 April 2014
Received in revised form
3 July 2014
Accepted 12 July 2014
Available online 27 August 2014
Magnetic mesoporous silica nanoparticles
In this study,phosphonate-terminated magnetic mesoporous nanoparticles(pMMSNs)was designed by incorporation of MNPs in the center of mesoporous silica nanoparticles(MSNs) and followed by grafting phosphonate group on to the surface of MMSNs.The carrier exhibited a typical superparamagnetic feature and the saturation magnetization was 4.89 emu/g measured by vibrating sample magnetometer(VSM).pMMSNs had a spherical morphologyandaporesizeof2.2nm.FromN2adsorption-desorptionanalysis,pMMSNshad a surface area of 613.4 m2/g and a pore volume of 0.78 cm3/g.Phosphonate modif i cation improved the colloidal stability of MMSNs,and the hydrodynamic diameter of pMMSNs was around 175 nm.The hydrophilic phosphonate group signif i cantly enhanced the negative surface charge of MMSNs from-19.3 mV to-28.8 mV pMMSNs with more negative surface charge had a 2.3-fold higher drug loading capacity than that of MMSNs.In addition,the rate and amount of release of doxorubicin(DOX)from DOX/pMMSNs was pH-dependent and increased with the decrease of pH.At pH 7.4,the release amount was quite low and only approximately17wt%ofDOXwasreleasedin48h.AtpH5.0and3.0,thereleaserateincreased signif i cantly and the release amount achieved 31 wt%and 60 wt%in 48 h,respectively.To evaluatethemagnetictargetingperformanceofpMMSNs,FITClabeledpMMSNswasinjected intomicebearingS180solidtumor.FITClabeledpMMSNscontrolledbyanexternalmagnetic f i eld showed higher tumor accumulation and lower normal tissue distribution.
© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
Magnetic targeting drug delivery systems(MTDDS)based on magnetic nanoparticles and external magnetic f i eld have been considered as a promising approach for localized accumulation of chemotherapeutical agent at the tumor sites[1,2]. Compared with traditional nanotechnology,MTDDS possess theadvantagesof easepreparation,facile response to external magnetic f i eld and low cost.Moreover,many drug delivery systems depended on the blood circulation can hardly be transported to the tumor sites due to the formation of neovessels,which are often distorted and irregular[3].Development of MTDDS requires high magnetic susceptibility for optimum magnetic enrichment and loss of magnetization after removal of magnetic f i eld[4].Concerning the vascular administration of MNPs,good colloidal stability is required. Great efforts have been made to encapsulate MNPs with various shells,such as,polymeric stabilizers and surfactants, e.g.,dextran,poly(vinyl alcohol)(PVA),poly(ethylene glycol) (PEG)or oxide surfaces(e.g.,SiO2)to ensure biocompatibility, water dispersibility,as well as appropriated functionalization for further conjugation with bioactive molecules or targeting ligands[5].In the past decade,mesoporous silica coated MNPs have received much attention due to their advantages:(1) MSNs protect MNPs from being eroded by acidic body f l uid;(2) very high surface area and pore volume for drug loading;(3) versatile silanol groups render further modif i cation;(4)good biocompatibility[6,7].
Considering the acidic environment at the tumor sites and more acidic pH of internal cell organelles(pH 4.0-5.0 in the lysosomesandpH6.0 nearthecancercell membrane)itwaswise todesignMTDDSpossessingpH-stimulireleasefeature[8,9].Qu et al.prepared Eudragit-S100 coated MMSNs,which showed high release rate and amount of ibuprofen in simulated proximalintestinef l uid,butthe polymerwasinsolubleinsimulated gastric f l uid resulting in low release accumulation[10].Yang et al.designed MMSNs coated with a pH sensitive polymer poly methacrylic acid(PMAA).The release rate of doxorubicin from thecarrierwasfasterbelowitspKathanthatofaboveitspKa[11].
The purposes of this study were to incorporate MNPs into the center of MSNs and modify the surface with the hydrophilic phosphonategroup(pMMSNs).The phosphonatetermination improved the drug loading capacity of MMSNs by increasing the negative surface charge of MMSNs resulting in a stronger electrostatic interaction between the positively charged DOX and more negatively charged pMMSNs.The DOX release prof i les at pH 3.0,5.0 and 7.4 were signif i cantly different due to the electronic repulsion between DOX and the carriers.FITC labeled pMMSNs were prepared to evaluate the magnetic targeting eff i ciency of this carrier in vivo under the control of an external magnetic f i eld.
2. Materials and methods
2.1. Materials
Doxorubicin(DOX)in the form of hydrochloride salt was obtained from Haizheng Drug Company(Zhejiang,China). FeCl3·6H2O,FeSO4·7H2O,oleic acid,and Tetraethyl orthosilicate(TEOS)were purchased from Bodi Reagent Company (Tianjin,China).Fluorescein isothiocyanate(FITC)and 3-aminopropyl triethoxysilane(APTES)were purchased from Aladdin(Shanghai,China).Trihydroxyl Silyl Proply Methyl Phosphonate(THPMP)was obtained from Sigma-Aldrich(St. Louis,MO,USA).Other chemicals were of reagent grade and were used without further purif i cation.
2.2. Animals
The male Kunming mice weighing 20-22 g were purchased from the Experimental Animal Center of Shenyang Pharmaceutical University(Shenyang,China).The animal care and experiments were performed in accordance with the guidelines of the local Animal Welfare Committee.
2.3. Preparation of pMMSNs
2.3.1. Preparation of MNPs
Oleic acid stabilized magnetic nanoparticles(MNPs)were prepared via a modif i ed coprecipitation method reported previously[12].The oleic acid stabilized magnetic nanocrystals were placed in chloroform and the concentration of the magnetic solid was adjusted to 7.5 mg/ml.
2.3.2. Preparation of MMSNs and pMMSNs
pMMSNs were produced by modif i ed sol-gel method[13]. MNPs dispersed in chloroform(1 ml)were added to an aqueoussolutionofcetyltrimethylammoniumbromide (CTAB)(20 ml,12.5 mg/ml)under vigorous ultrasonic to produce a homogeneous oil-in-water microemulsion.Then, chloroform was evaporated by heating at 85°C to form CTAB stabilized MNPs.And then the system was diluted by distilled water and the pH was adjusted to 12.When temperature was stabilized in 80°C,1 ml ethanol was mixedwith 1 ml TEOS and then the mixture was added slowly to the aqueous solution containing the CTAB stabilized MNPs.30 min later,THPMP was added.The solution was stirred for another 3 h followed by collecting the colloidal nanoparticles through centrifugation.CTAB was removed by dispersing the washed sample in 100 ml ethanol solution containing 12 mg/ml ammonium nitrate and heating the mixture at 70°C for 6 h.The product was then washed with waterand ethanolto obtained phosphonate-terminated MMSNs(pMMSNs).MMSNs without phosphonate modif i cation were also prepared but in the absence of THPMP.
2.3.3. Preparation of FITC labeled pMMSNs
1 mg of f l uorescein isothiocyanate(FITC)was dissolved in 1 ml absolute ethanol and mixed with 20 μl of 3-aminopropyl triethoxysilane(APTES)for 10 h.100 mg of pMMSNs was redispersed in 100 ml absolute ethanol and heated to 80°C. After the temperature was stabilized,0.5 ml of the ethanolic FITC-APTES solution was added slowly to the ethanolic solution containing pMMSNs.The mixture was stirred for 6 h.The synthesized product was re-dispersed in 100 ml of ethanol solution containing 12 mg/ml ammonium nitrate and heating the mixture at 70°C for 6 h.The product was then washed with water and ethanol to obtain FITC-pMMSNs.
2.4. DOX loading procedure
Positively charged DOX was selected as a model drug.In order to enhance the drug loading capacity,two-steps drug loading process was adopted involving adsorption equilibrium and solvent evaporation.Typically,the adsorption of DOX into mesopores of pMMSNs was carried out by adding pMMSNs to 4 ml of DOX solution(2 mg/ml).The drug/carrier ratio in the loading solution was fasten to 1:10(w/w)and then the hybrid was mixed under ultrasonic for 1 h and stirred at room temperature to achieve maximum loading for another 24 h.The process was in a closed container to prevent ethanol evaporation.Finally,the container was opened and ethanol as the solvent was evaporated at 37°C.The DOX loaded carrier was continually dried at 40°C under vacuum to remove solvent residue.The drug loaded samples were labeled with DOX/ pMMSNs.
2.5. Characterization techniques
The morphology of the samples plated with gold was characterized using TEM(Tecnai G2 20,FEI,USA).Nitrogen adsorption isotherms at-196°C were measured using a nitrogen adsorption analyzer(V-Sorb 2800P,China).The carriers were degassed to remove physically adsorbed water before analysis.The magnetic curves were analyzed by vibrating magnetometer(Lake Shore 7410,USA)at 300 K.The hysteresis of the magnetization was obtained by changing H between +10,000 Oe and-10,000 Oe.The hydrodynamic diameter,PDI and zeta potential of the samples were measured by photo correlation spectroscopy using a Zetasizer nano(Nano ZS, Malvern Co.,UK).TGA-50 instrument(Shimadzu,Japan)was also employed to calculate the weight percentage of phosphonate groups grafted on to the surface MMSNs.
2.6. Analysis of drug content
To determine the drug loading capacity,DOX/MMSNs and DOX/pMMSNs were resuspended in methanol and sonicated for 2 h.The concentration of DOX of the supernatant was determined by ultraviolet(UV)spectroscopy(UV-2000,Unico, USA),while the detection wavelength was 480 nm.The standard curves were linear over the concentration range of 1.0-30 μg/ml.The drug loading capacity(DLC)was calculated by the following equation:DLC=WDOx/WDOx-carrier.Where WDOxis the weight of DOX loaded and WDOx-carrieris the weight of DOX loaded carrier.
2.7. In vitro DOX release study
A typical in vitro drug release experiment was performed as follows.An aliquot of 20 mg of drug/carrier composite was immersed in 20 ml of 0.1 M phosphate buffer solution of required pH(pH 3.0,pH 5.0 and pH 7.4).The release medium was stirred at 100 rpm and at 37°C.At predetermined sampling time,4 ml of medium was extracted for measurement, and then put back to the container.The amount of DOX in the medium was determined by UV-spectroscopy at a detection wavelength of 480 nm for all pH values.The experiments were performed in triplicate.
2.8. Ex vivo tissue imaging
The in vivo imaging system(FX Pro,Kodak,USA)was applied to evaluate the magnetic targeting eff i ciency of pMMSNs controlled by an external magnet f i eld in Kunming mice bearing S180 tumor.Brief l y,when tumor sizes reached 1500 mm3,mice were received the FITC labeled pMMSNs (10 mg/ml)intravenously and the blank group receive saline. The test group was then treated with permanent magnet attached to the surface of the tumor of the mice for 1 h.Mice were scarif i ed at 2 h,and then tumor and normal tissues were imaged with appropriate wavelength(λex:480 nm, λem: 535 nm).
3. Results and discussion
3.1. Preparation of MNPs,MMSNs and pMMSNs
In this study,MNPs were synthesized according to the traditional coprecipitation method and stabilized with oleic acid [13].A simple sol-gel method was applied to prepare monodispersedMNPsembeddedin mesoporoussilicashell. Scheme 1 shows a typical synthetic process of pMMSNs in.As previouslyreported,pre-existingCTAB stabilized MNPs served as the nucleation seeds[13,14].Under basic conditions, the silica oligomers which were generated by the hydrolysis of TEOS,self-assembled with surfactant micelles to form CTAB/ silica mesostructured nano-composites,which subsequently deposited on the magnetic seeds to construct the shell framework.However,in the case of MSNs,the mesostructured nano-composites aggregated together directly and grew to the whole particle by further condensation of silica species. CTAB served not only as the organic template for the formation of mesopores but also as the secondary surfactant to transferMNPsfromorganicsolventto aqueous phase[15].The surface of MMSNs was modif i ed with hydrophilic phosphonate groups shortly after the particle formation resulted in increased stability and dispersibility of the carrier in aqueous solution even after drying process.Previous research had reported that MMSNs easily aggregated and settled to the bottom quickly after drying and re-dispersing process.The irreversible aggregation might be attributed to the interparticle hydrogen-bonding interaction between the surface silanol groups and can be prevented by grafting hydrophilic molecules on to the surfaces[16,17].
3.2. Characterization of MNPs,MMSNs and pMMSNs
Fig.1A presents the TEM images of oleic acid modif i ed MNPs with a diameter of around 10 nm.However,the nanoparticles showed a slight aggregation.MNPs with a diameter of about 10 nm behaved superparamagnetism which meant that they were magnetic only under the controlled of external magnetic f i eld and became inactive once the external magnetic f i eld is removed.Fig.1B shows that pMMSNs were discrete spheres with a diameter of around 100 nm.The appearance of the pores was distorted and ran radically to the surface.MNPs were individually imbedded in the center of silica shell due to vigorous ultrasonic stirring in the stage of transforming MNPsfrom organic solvent to aqueous solvent.The pore space supplied to further DOX laden.
The pore structure of MMSNs and pMMSNs were measured through N2adsorption-desorption analysis.Fig.2A shows the N2adsorption isotherms of MMSNs and pMMSNs,which were typical type IV isotherms with two steps conf i rming their mesoporous structure according to the IUPAC classif i cation. Compared with MMSNs,the adsorbed nitrogen amount of pMMSNs was slightly reduced,but the shape of the hysteresis remained unchanged.The BET surface area,pore volume and pore diameter of MMSNs were 923.6 m2/g,1.48 cm3/g and 2.8 nm,respectively.The parameters of pMMSNs were reduced to 613.4 m2/g,0.78 cm3/g,and 2.2 nm,respectively (Table 1).These results suggested thatphosphonatetermination did not destruct the pore structure.However, phosphonate-group distributed on the interior pore walls and exterior surface of MMSNs occupied some pore space which could be used to storage drugs molecules.
Fig.3 illustrates the f i eld-dependent magnetization curves of MNPs,MMSNs and pMMSNs measured at 300 K.They exhibited a typical superparamagnetism and no hysteresis was observed in low f i elds.The saturated magnetization value of MNPs was 37.5 emu/g.But it dramatically decreased to 6.74 emu/g and 4.89 emu/g for MMSNs and pMMSNs.As shown in Fig.3(inserted),pMMSNs with superparamagnetic characteristic and high magnetization value could quickly respond to external magnetic f i eld and redisperse in aqueous solution once the external magnetic f i eld was removed and with gentle shaking or sonication.
Fig.4 shows the thermogravimetric analysis(TGA)of MMSNs and pMMSNs.The decline in the curves started from a low temperature of about50°C.This mightbe attributed to the evaporation of absorbed water tightly bound to the carriers. TGA curves demonstrated that the weight loss of MMSNs was 6 wt%,while that of pMMSNs was 14.9 wt%.Thus,the content of phosphonate-termination was about 8.9 wt%.
The particle sizes and zeta potential measurements of MMSNs and pMMSNs were performed using dynamic light scatter(DLS)technique to evaluate the dispersibility,stability and surface charge potential after each preparation steps (Table 2).BareMMSNshadanegativesurfacechargeofaround -20 mV in PBS solution at pH 7.4,mean hydrodynamic diameter of 166.4 nm and a narrow PDI of 0.201.The particle size measured by DLS was higher than the particle size observed from TEM image of that sample.This could be attributed to the formation of hydrodynamic shell of MMSNs and slight aggregates between nanoparticles.The hydrodynamic diameter and PDI of pMMSNs were 175 nm and 0.218,respectively.Once,the material was dried and re-dispersed in aqueous solution,MMSNs without phosphonate modif i cation easily aggregated and settled down to the bottom.On the other hand,pMMSNs were stable when re-dispersed in aqueous solution,and their hydrodynamic size and PDI showed a slight increasement.Moreover,pMMSNs leaded to more negative surface charge value of-28.8 mV in PBS solution at pH 7.4.This could be explained that the phosphonate groups have a higher pKavalue than that of the silanol groups.
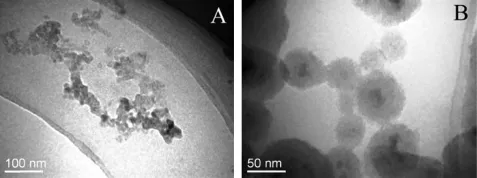
Fig.1-TEM images of(A)MNPs and(B)pMMSNs.

Fig.2-(A)N2adsorption-desorption isotherms and(B)pore size distributions of MMSNs and pMMSNs.
3.3. Drug loading capacity(DLC)and in vitro release study
In this study,positively charged DOX was selected as a model drug to investigate the drug loading capacity(DLC)of the carriers and to study the pH-dependent release of DOX/ pMMSNs.Table 1 shows that the DLC of pMMSNs(6.9 wt%) was higher than that of MMSNs(2.8 wt%).The probable reasons for the enhanced DLC of DOX in pMMSNs were as follows.The electronic attraction between negatively charged carriers and positively surface charge of DOX might be the main factor that affected DLC.Even though,pMMSNs had lower surface area and pore volume than that of MMSNs,the DLC value of DOX/pMMSNs was 2.3-fold higher than that of DOX/MMSNs.Therefore,we could speculate that the surface area and pore volume of pMMSNs were high enough for DOX incorporation.
Drug delivery systems with the pH-responsive release prof i le mean that drugs do not or hardly release in normal tissues and blood(pH ~ 7.4),but can responsively release intumor tissues,or even within cancer cells,to selectively kill cancer cells(pH 3.0-5.0)[18].The release prof i les of DOX from DOX/MMSNs and DOX/pMMSNs were investigated in PBS solution at three different pH.The pH 3.0 and pH 5.0 represented the acidic environment of tumor tissues,while pH 7.4 represented the neutral pH of normal tissues and blood.On the whole,the observed release rate and amount of DOX from DOX/MMNSs and DOX/pMMSNs increased with the decrease in the pH values.Fig.5A shows that,at pH 3.0,the release rate of DOX from DOX/MMSNs was relatively fast and 63%amount of DOX diffused to release medium at 48 h.At pH 5.0,the release rate and amount of DOX from DOX/MMSNs were decreased reached 51%at 48 h.However,at pH 7.4,the release curveofDOXfrom DOXfrom DOX/MMSNswas almostf l atand the release accumulation was 14%.The reasons for this phenomenon was that amine groups from doxorubicin are partially deprotonated at pH 7.4(pKa=8.22),while they are fully protonated at pH 3.0 and 5.0.On the other hand,MMSNs have a higher total positive charge at pH 5.0 and even higher positive charge at pH 3.0 because of the presence of surface silanol groups(pKa=6.8)[19].Therefore,the electronic repulsion between DOX and the carrier contributed to the pH-dependent release prof i le of DOX/MMSNs.
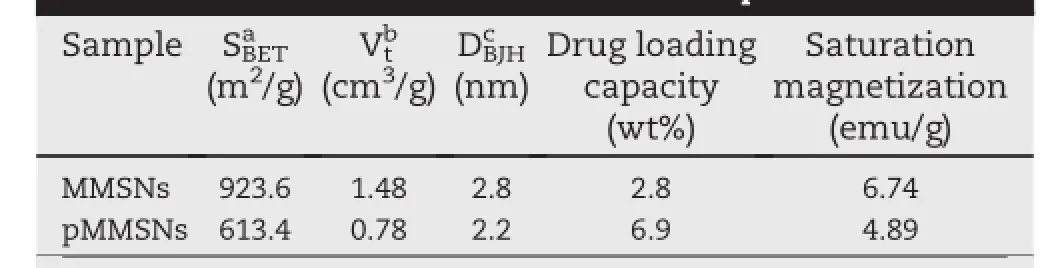
Table 1-Characteristics of MMSNs and pMMSNs.

Fig.3-Field-dependent magnetization of MNPs,MMSNs and pMMSNs and photograph demonstrating high magnetic responsiveness of pMMSNs with an external magnetic f i eld(inserted).

Fig.4-TGA curves of MMSNs and pMMSNs at 300 K.

Table 2-Hydrodynamic size,size distribution and zeta potential values of MMSNs and pMMSNs.
Fig.5B presents sustained release patterns of DOX from the phosphonate modif i ed MMSNs(pMMSNs)in PBS solution at three different pH.At pH 3.0,the release pattern showed a burst release amount of 31%at the initial 4 h and then the release rate turned slow to reach a total release accumulation of 60%.At pH 5.0,the release rate and amount of DOX from DOX/pMMSNs decreased and only 43%of DOX dissolvedin the medium.At pH 7.4,the whole release amount of DOX from DOX/pMMSNs was also incomplete with a f i nal accumulation of 17%at 48 h.The reasons for the sustained release behavior of DOX from phosphonate modif i ed carrier may be that phosphonate modif i cation made the surface charge potential of MMSNs even more negative,thus the attractive interaction between DOX and pMMSNs was stronger than the attractive force between DOX and MMSNs.
3.4. In vivo distribution of pMMSNs(MF+)and pMMSNs(MF-)
To demonstrate that pMMSNs could be controlled by external applied magnetic f i eld,FITC labeled pMMSNs was injected intravenously into mice bearing S180 tumor.The test group then was f i xed with an external magnetic f i eld at the solid tumorfor1h,referredtoaspMMSNs(MF+).Thecontrastgroup also received the same formulation but without an applied magnetic f i eld(MF-).Also,mice received saline was treated as controlled group.Since the FITC f l uorescence signal had poor penetration ability in deep tissues and the background f l uorescenceintensityofmicewashigh,miceweresacrif i cedat2h afteradministrationandexvivof l uorescenceintensityimages of the major organs and tumors were obtained,as shown in Fig.6.The f l uorescence intensities in the heart and spleen of mice of the two groups were very low.Enhanced f l uorescence intensity was observed in the tumor of mice treated with pMMSNs(MF+).On the other hand,the f l uorescence intensity in liver and lung of mice treated with pMMSNs(MF-)was obviouslyhigherthanthatofmicetreatedwithpMMSNs(MF+). This phenomenon was attributed to that without external magnetic f i eld,the discontinuous gaps in the endothelium which lines the sinusoidal walls of liver allow the passive entrapment of pMMSNs[20,21].Moreover,macrophages in liver had great association with the nanoparticles than other typesofcells[22].Thehigh lungaff i nityof pMMSNs wasdueto hydrodynamic size increased in serum which leaded to transient association with capillary in lung.A high tumor accumulation of pMMSNs(MF+)demonstrated the feasibility of our designed magnetic targeting drug delivery system.
4. Conclusion

Fig.5-In vitro release prof i les of DOX from(A)DOX/MMSNs and(B)DOX/pMMSNs in PBS solution at pH 3.0,5.0 and 7.4.

Fig.6-Ex vivo optical images of tumors and other organs of S180 tumor bearing mice sacrif i ced at 2 h after intravenous injection of FITC labeled pMMSNs.(A)S180 tumor bearing mice treated with saline;(B)S180 tumor bearing mice treated with pMMSNs(MF-);(C)S180 tumor bearing mice treated with pMMSNs(MF+).
The results of this papershowed that phosphonate-terminated magnetic mesoporous silica nanoparticles(pMMSNs)with a saturation magnetization of 4.89 emu/g and a hydrodynamic diameter of around 175 nm were prepared.The negative surface charge(-28.8 mV)and surface area of 613.4 m2/g warranted successful loading of DOX using a two-steps method including adsorption equilibrium and solvent evaporation.The in vitro release prof i les of DOX/pMMSNs were pH-depended.At lower pH(3.0 and 5.0),DOX release rate and release amount of DOX was higher compared to that of at pH 7.4 and this can be attributed to the decreased electrostatic interaction between the negative surface charge of pMMSNs and the entrapped DOX from the nanoparticles.Moreover, ex vivo tissue f l uorescence intensity measurement showed that under the facilitation of external magnetic f i eld,the distribution of pMMSNs(MF+)was improved via a higher tumor retention and lower distribution in normal tissues than that of pMMSNs(MF-).We believe that these results may open the possibilities for combining magnetic targeting drug delivery systemandpH-responsivereleaseofdoxorubicintocancerous tissue.
R E F E R E N C E S
[1]Cole A,Yang V,David A.Cancer theranostics:the rise of targeted magnetic nanoparticles.Trends Biotechnol 2011;29:323-332.
[2]Arruebo M,Fern′andez-Pacheco R,Ibarra M,et al.Magnetic nanoparticles for drug delivery.Nano Today 2007;2:22-32.
[3]Mody V,Cox A,Shah S,et al.Magnetic nanoparticle drug delivery systems for targeting tumor.Appl Nanosci 2014;4:385-392.
[4]Reddy L,Arias J,Nicolas J,et al.Magnetic nanoparticles: design and characterization,toxicity and biocompatibility, pharmaceutical and biomedical applications.Chem Rev 2012;112:5818-5878.
[5]Butterworth M,Illum L,Davis S.Preparation of ultraf i ne silica-and PEG-coated magnetite particles.Colloid Surf A 2001;179:93-102.
[6]Lee J,Lee N,Kim T,et al.Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res 2011;44:893-902.
[7]Li Z,Barnes J,Bosoy A,et al.Mesoporous silica nanoparticles in biomedical applications.Chem Soc Rev 2012;41:2590-2605.
[8]Pillay C,Elliott E,Dennison C.Endolysosomal proteolysis and its regulation.Biochem J 2002;363:417-429.
[9]Griff i ths J.Are cancer cells acidic?Br J Cancer 1991;64:425-427.
[10]Xing R,Lin H,Jiang P,et al.Biofunctional mesoporous silica nanoparticles for magnetically oriented target and pH-responsive controlled release of ibuprofen.Colloid Surf A 2012;403:7-14.
[11]Wen H,Guo J,Chang B,et al.pH-responsive composite microspheres based on magnetic mesoporous silica nanoparticle for drug delivery.Eur J Pharm Biopharm 2013;84:91-98.
[12]Liu C,Guo J,Yang W,et al.Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release.J Mater Chem 2009;19:4764-4770.
[13]Kim J,Lee JE,Lee J,et al.Magnetic f l uorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals.J Am Chem Soc 2006;128:688-689.
[14]Nooney R,Thirunavukkarasu D,Chen Y,et al.Self-assembly of mesoporous nanoscale silica/gold composites.Langmuir 2003;19:7628-7637.
[15]Fan H,Yang K,Boye DM,et al.Self-assembly of ordered, robust,three-dimensional gold nanocrystal/silica arrays. Science 2004;304:567-571.
[16]Xu H,Yan F,Monson E,et al.Room-temperature preparation and characterization of poly(ethylene glycol)-coated silica nanoparticles for biomedical applications.J Biomed Mater Res A 2003;66:870-879.
[17]Bagwe R,Hilliard L,Tan W.Surface modif i cation of silica nanoparticles to reduce aggregation and nonspecif i c binding. Langmuir 2006;22:4357-4362.
[18]Gao W,Chan J,Farokhzad O.pH-responsive nanoparticles for drug delivery.Mol Pharm 2010;7:1913-1920.
[19]Kneˇzevi′c N,Trewyn B,Lin V.Light-and pH-responsive release of doxorubicin from a mesoporous Silica-Based nanocarrier.Chem Eur J 2011;17:3338-3342.
[20]Gratton S,Pohlhaus P,Lee J,et al.Nanofabricated particles for engineered drug therapies:a preliminary biodistribution study of PRINT™ nanoparticles.J Control Release 2007;121:10-18.
[21]Xie G,Sun J,Zhong G,et al.Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch Toxicol 2010;84:183-190.
[22]Yu T,Malugin A,Ghandehari H.Impact of silica nanoparticle design on cellular toxicity and hemolytic activity.Acs Nano 2011;5:5717-5728.
*Corresponding author.Tel.:+86 24 23986348;fax:+86 24 23986348.
E-mail addresses:silingwang@syphu.edu.cn,silingwang@hotmail.com(S.Wang). Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.07.003
1818-0876/© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
Doxorubicin
pH-depended release
Tumor accumulation
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- LC-MS/MS assay for pitavastatin in human plasma and subsequent application to a clinical study in healthy Chinese volunteers
- Pharmacokinetics of oxiracetam and its degraded substance(HOPAA)after oral and intravenous administration in rats
- Studies on the spray dried lactose as carrier for dry powder inhalation
- Pharmacokinetic performance of the nitrendipine intravenous submicron emulsion in rats
- Effect of the glyceryl monooleate-based lyotropic phases on skin permeation using in vitro diffusion and skin imaging
- Pharmaceutical particle technologies:An approach to improve drug solubility,dissolution and bioavailability
