Optimization of Two-species Whole-cell Immobilization System Constructed with Marine-derived Fungi and Its BiologicalDegradation Ability*
2014-03-25陈慧英王明霞沈煜斌姚善泾
(陈慧英)(王明霞)(沈煜斌)(姚善泾)**
Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Optimization of Two-species Whole-cell Immobilization System Constructed with Marine-derived Fungi and Its Biological
Degradation Ability*
CHEN Huiying(陈慧英), WANG Mingxia(王明霞), SHEN Yubin(沈煜斌)and YAO Shanjing(姚善泾)**
Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
Mycelia pellet formed spontaneously in the process of cultivation was exploited as a biological carrier for whole-cell immobilization due to its unique structural characteristic. An innovative two-species whole-cell immobilization system was achieved by inoculating the marine-derived fungus Pestalotiopsis sp. J63 spores into culture medium containing another fungus Penicillium janthinellum P1 pre-grown mycelia pellets for 2 days without any pretreatment. In order to evaluate the biological degradation capacity of this novel constructed immobilization system, the immobilized pellets were applied to treat paper mill effluent and decolorize dye Azure B. The use of the constructed immobilization system in the effluent resulted in successful and rapid biodegradation of numerous insoluble fine fibers. The optimum conditions of immobilized procedure for maximum biodegradation capacity were determined using orthogonal design with biomass of P1 pellets 10 g (wet mass), concentration of J63 spore 2×109ml−1, and immobilization time 2 d. The results demonstrate that immobilized pellets have more than 99% biodegradation capacity in a ten-hour treatment process. The kinetics of biodegradation fits the Michaelis-Menten equation well. Besides, the decolorization capability of immobilized pellets is more superior than that of P1 mycelia pellets. Overall, the present study offers a simple and reproducible way to construct a two-species whole-cell immobilization system for sewage treatment.
whole-cell immobilization, mycelia pellet, Pestalotiopsis sp., Penicillium janthinellum, biodegradation kinetics, biological wastewater treatment
1 INTRODUCTION
With an ever-accelerating tide of human impact and rapid development of petroleum industry over the last few decades, the environment has been changed more in the last 30 years than in all of human history before. The large-scale production, usage and transportation of petroleum products result in hazardous contaminants in soil and groundwater system. The presence of various pollutants in the ecosystem has a great impact on environment, public health, as well as on the economic development.
Among the available technologies for wastewater treatment, traditional techniques, such as physical and chemical methods—adsorption, filtration, centrifugation and chemical oxidation, are generally effective, but not so often cost-effective [1, 2]. On the other hand, biotechnological approaches have been proven to be potentially effective in dealing with these pollutants in an environment-friendly and cost-competitively manner [3]. White rot fungi are considered as the most efficient microorganisms in degrading industrial pollutants in nature [4-7]. Their outstanding property is mainly attributed to the production of extracellular lignolytic enzymes, which are capable to degrade wide varieties of xenobiotic compounds due to their low substrate specificity and highly oxidative capability [8, 9].
Recently, there is ever-growing interest in the use of immobilizing microbial cells for the biological treatment of chemical wastes, especially the immobilized bioaugmentation technology [10-13]. In comparison with free-living cells, the immobilized microbial cells not only promote a biodegradation process, but also provide many other advantages, such as high cell density, feasibility of continuous processing, cell stability and lower costs of recovery [14, 15].
Besides these studies by use of single-species cultures, the idea that a combination of two or more microorganisms is better and more effective than a single microorganism is widely recognized in many fields [16]. Combining complementary metabolic pathways into single functional community and maintaining multiple cell types, multi-species immobilization system could benefit the applied processes, including degradation of mixed wastes, production of various biological value-added products, drug or fuel biosynthesis, etc [17-21].
The present work describes the construction of a novel two-species whole-cell immobilization system and subsequently proves that this system possesses excellent biodegradation ability in the biological treatment of industrial wastewater-paper mill effluent and in the decolorization of dye Azure B. In those two processes (paper mill effluent and decolorization of dye Azure B) mentioned previously, mycelia pellets are used as carrier. Mycelia pellet, spontaneouslyformed under a certain specific cultivation condition, possesses a unique structural characteristic, poly-porous network and high specific area, which is beneficial to mass transportation. In addition, the mycelia pellet has many other advantages, such as short generation time, rapid colonization, and low production costs. Regarding its potential merits, the mycelia pellet as carrier will add new dimensions to the rapidly advancing immobilized microorganism technology. Above all, comparing with other synthetic polymers, mycelia pellet is of environmentally friendly advantage without secondary pollution. Thus the idea of taking mycelia pellet as a novel immobilized biological support has been proposed and practiced [22-25].
In current work, we investigate the capability of the white rot fungus, with a marine-derived Pestalotiopsis sp. J63 secreting higher laccase immobilized into another marine-derived fungus Penicillium janthinellum P1 mycelial pellet producing higher cellulase, to biodegrade paper mill effluent and biodecolorize dye Azure B. In this constructed two-species whole-cell immobilization system, P1 mycelia pellet not only acts as a carrier but also represents an essential element of this system, playing dual roles. Our investigation mainly focuses on obtaining very active immobilized pellets as biocatalysts. The immobilization efficiency is optimized with orthogonal design.
2 MATERIALS AND METHODS
2.1 Microorganism
Penicillium janthinellum P1 was isolated from the sediment samples collected from Nanji Island off the east coast of China in the Pacific Ocean, then deposited in the China Center for Type Culture Collection CCTCC M 2012006. The accession numbers of the fungus in GenBank is JQ727998. The filamentous Pestalotiopsis sp. J63 [26] and P1 were maintained on Potato Dextrose Agar (PDA) slant, stored at 4 °C and subcultured every 2 months.
2.2 Preparation of pellet
Spore suspension of fungus P1 was prepared from freshly grown (7 days at 28 °C) fungus. For pellet preparation, 5 ml of spore suspension was inoculated into 250 ml Erlenmeyer flasks containing 100 ml of modified Takashio medium [(g·L−1): glucose, 10; ammonium tartrate, 2; KH2PO4, 2; MgSO4·7H2O, 0.5; yeast extract, 2; pH 5.0]. Cultures were incubated on a rotary shaker (160 r·min−1) at 28 °C for 4 days. After cultivation, mycelia pellets were harvested, washed with sterile distilled water and then stored at 4 °C for further use.
2.3 Immobilization process
For immobilization, Pestalotiopsis sp. J63 spores from PDA plates were suspended in pre-sterilized water and then 10 ml (107ml−1) of this spore suspension was used for the inoculation of 100 ml of immobilization medium in 250 ml Erlenmeyer flasks. The immobilization medium consisted of (g·L−1): glucose, 20; ammonium tartrate, 4; KH2PO4, 2; MgSO4·7H2O, 0.5; yeast extract, 2; pH 5.0. Subsequently, the pre-grown P1 mycelia pellets (as mentioned as Section 2.2) were poured into the flasks containing immobilization media and a certain amount of J63 spores. For co-immobilization of fungi J63 and P1 to obtain immobilized pellets, the spore suspension and mycelia pellets were co-cultivated in the same immobilization medium on the rotary shaker at 28 °C and 160 r·min−1for designed time period. The immobilized pellets were washed with tape water for biodegradation experiments.
2.4 Orthogonal design
In order to obtain high efficiency of immobilization, the orthogonal design [orthogonal array L9(34)] was carried out. The parameters and their levels of orthogonal test are shown in Table 1.
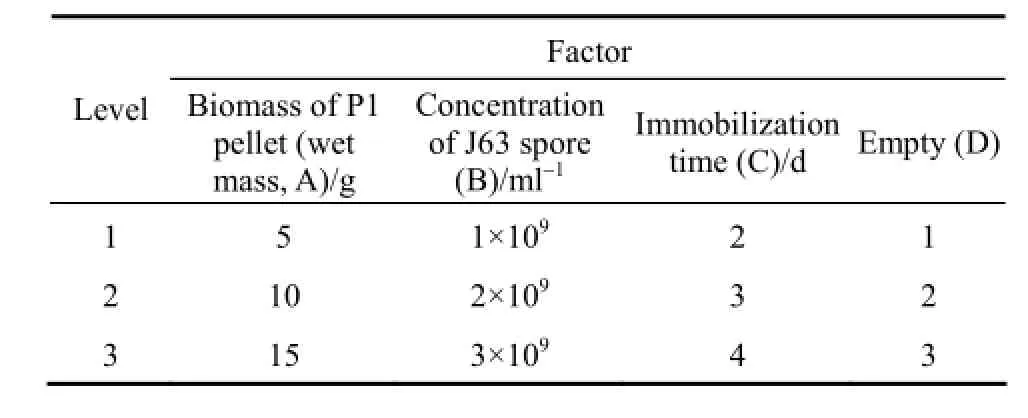
Table 1 Factors and levels of the orthogonal design
2.5 Removal and biodegradation of insoluble fine fiber
The resulting immobilized pellets were employed in the elimination and biodegradation of insoluble suspended fine fiber in the wastewater from waste paper-recycling. Immobilized pellets and mycelia pellets were transferred to 250 ml flasks containing 100 ml wastewater suspension separately. In addition, the wastewater suspension used comprised of diluted wastewater 20 % (by volume) and 5 g·L−1glucose and 1 g·L−1ammonium tartrate. The ratio of pellets to wastewater suspension used stood at 5%. Then, the flasks were kept on a rotary shaker at 28 °C and 160 r·min−1for 10 h. The biodegradation capacity of immobilized pellets and mycelia pellets was determined by the change of turbidity (expressed as NTU). The measurement of turbidity was conducted by Model CAS-S56 Portable Turbidity Meter (China). On the other hand, the wastewater suspension without immobilized pellets and mycelia pellets was used as control and kept under the same condition. The biodegradation efficiency was calculated by the following equation:
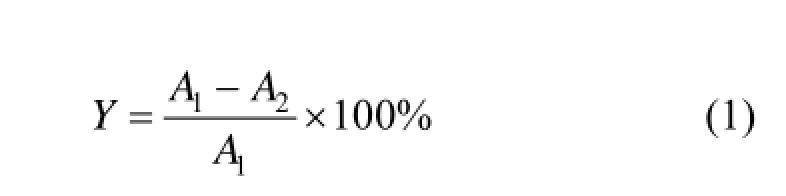
where A1is the final turbidity value of wastewater suspension of control, and A2is the final turbidity value of wastewater suspension sample.
2.6 Decolorization test
The immobilized pellets and mycelia pellets were also applied to decolorize Azure B. A certain amount of immobilized pellets and mycelia pellets were transferred to two 150 ml flasks containing 50 ml of decolorization media separately. The decolorization medium used comprised of 200 mg·L−1Azure B, 5 g·L−1glucose and 1 g·L−1ammonium tartrate. The flasks were then kept on a rotary shaker at 28 °C and 160 r·min−1for 10 d. The control experiment without immobilized pellets and mycelia pellets was carried out under the same condition. The decolorization capacity of the dye was determined as a relative decrease of absorbance at wavelength of its maximum absorbance.
2.7 Analytical methods
To determine the growth characteristics of Penicillium janthinellum P1 during fermentation period, the culture samples were withdrawn from flasks at certain intervals and filtered with filter papers (Whatman No. 1) in vacuum [27]. After filtration, the mycelia pellets were washed with distilled water and then dried to a constant weight.
To evaluate the capability of production of cellulase by fungus Penicillium janthinellum P1 under liquid cultivation, the total cellulase activity (filter paper activity, FPA) was assayed according to IUPAC recommendations by using filter paper as the substrate [28]. A reaction mixture containing a string of filter paper (Whatman No. 1), 0.5 ml of a 50 mmol·L−1citrate buffer (pH 5.0) and 0.5 ml appropriately diluted supernatant was incubated at 50 °C for 60 min. Laccase activity was determined by using 2,2-azinobisethylbenthiazoline-6-sulfonate (ABTS) as substrate according to Soares et al [29].
Reducing sugar was measured by 3,5-dinitrosalicylic acid assay using D-glucose as a standard according to Breuil and Saddler [30].
3 RESULTS AND DISCUSSION
3.1 The growth characteristics of marine-derived fungus Penicillium janthinellum P1
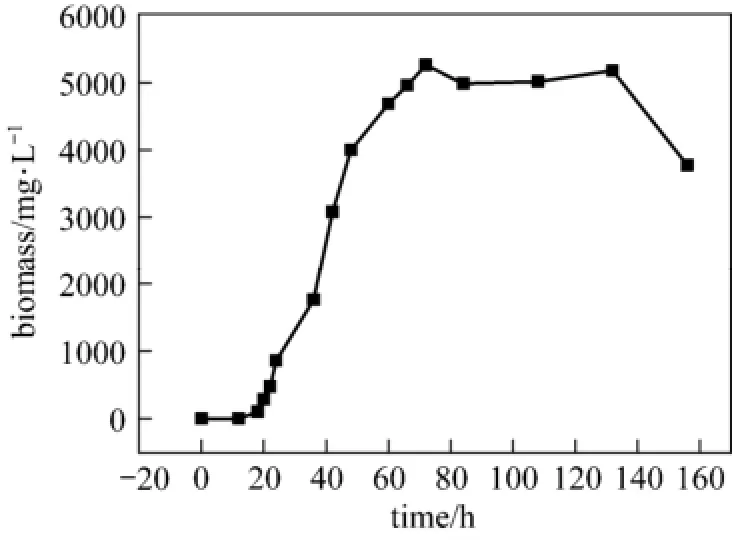
Figure 1 The curve of biomass by Penicillium janthinellum P1
P1 spores (107ml−1) were inoculated into growth medium for investigation of biomass. The results are recorded in Fig. 1. The whole process can be divided into four phases. The first phase, from inoculation to 24th hour, biomass increased, especially in the initial 12 hours of cultivation, P1 pellet can hardly be detected because P1 spores were just in the process of germination. The second stage began from 24th hour to 72th hour, in which fungus P1 propagated very fast, hence biomass soared dramatically. The maximum biomass reached 5259 mg·L−1in the fermentation of 72th hour. This should be the exponential phase. The third stage was from 72th hour to 132th hour. In this period, the overall biomass almost kept the same. This was the stationary phase. Subsequently, the effect of self-lysis occurred due to the lack of nutrients, decreasing biomass in the latter period of fermentation.
3.2 The property of enzyme production by Penicillium janthinellum P1
Fungus Penicillium janthinellum P1 was evaluated for extra-cellular production of cellulase in shake flask containing growth medium with wheat bran (1.5%) as substrate. The time course of enzyme production by P1 in liquid culture is shown in Fig. 2. Maximum FPase activity was found on 6.5th day of fermentation, peaking at 0.15 U·ml−1.
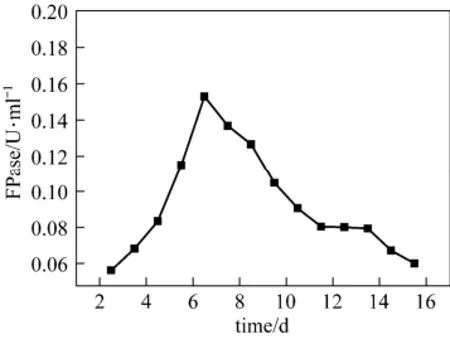
Figure 2 Time course of production of cellulase by Penicillium janthinellum P1
Penicillium janthinellum has been reported to secrete large amount of cellulase [31] and has been broadly studied for exploring its potential applications[32, 33]. In a nutshell, filamentous fungus Penicillium janthinellum is a promising fungus in practical applications. Not only can it produce a large amount of cellulase but also can secrete many other biologically active metabolites, such as evodiamine [34], janthinellin, indole alkaloid [35], carboxypeptidase and protease [32].
3.3 Optimization of immobilization process
Attempts have been made to optimize the immobilization of Pestalotiopsis sp. J63 hyphae into Penicillium janthinellum P1 mycelium pellets. Using orthogonal method, three factors, biomass of P1 pellets, concentration of J63 spore and immobilization time, are chosen to determine their effects on biodegradation capabilities of immobilized pellets and the results are shown in Table 2. Owing to its limitation, range analysis is not adopted here. However, univariate analysis of SPSS software is ultimately applied for ANOVA analysis and the results are shown in Table 3. The effect on biodegradation capacity is ordered by immobilization time, concentration of J63 spore, and biomass of P1 pellet. The immobilization time has statistically significant effect for biodegradation, while the other two factors are considered as statistically insignificant variables. Hence, the optimum immobilization conditions are as follows: biomass of P1 pellets, 10 g (wet mass); concentration of J63 spore, 2×109ml−1; and immobilization time, 2 d.
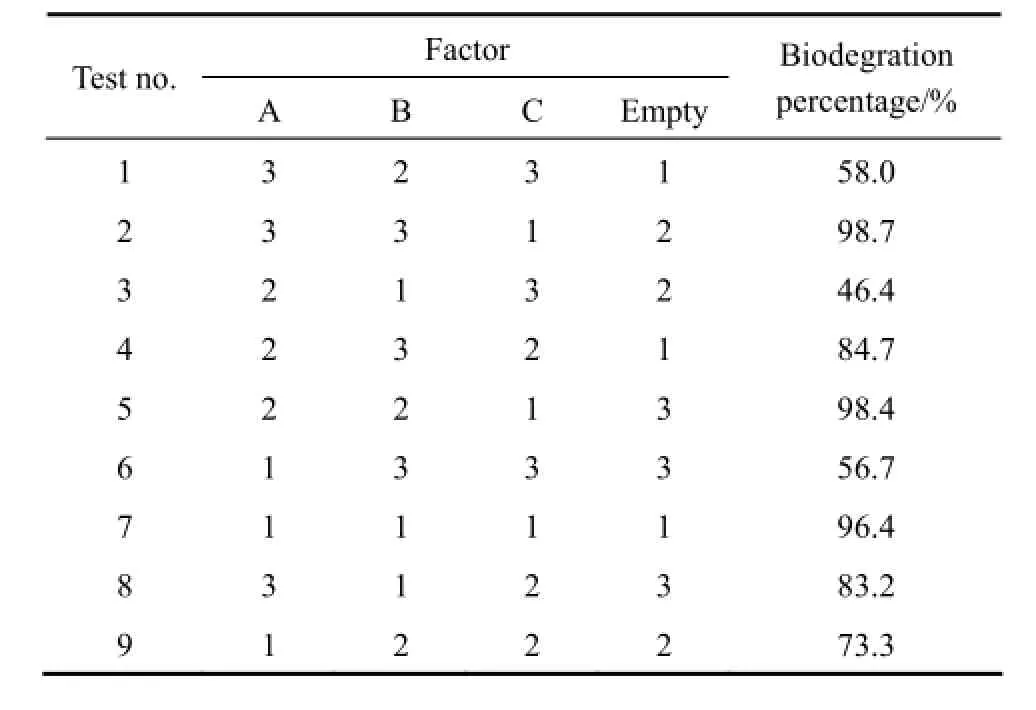
Table 2 Orthogonal table L9(34) and experimental results
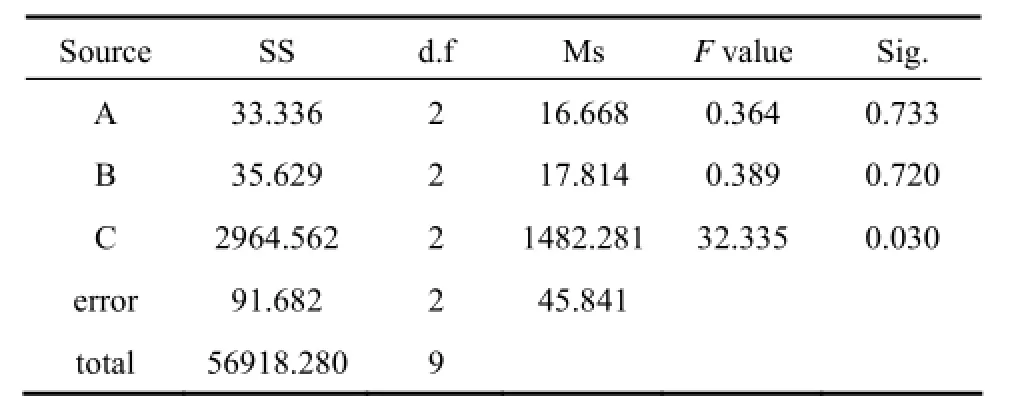
Table 3 Variance analysis of the orthogonal design
3.4 Biodegradation test
The constructed immobilized pellets were applied to treat paper mill effluent. A rapid biodegradation of effluent was observed in the first 4 h of treatment, reaching 85% of their total biodegradation capacity (Fig. 3). At the end of the treatment period, i.e., at 10 h, the biodegradation capacity of immobilized pellets nearly approached 99%. Simultaneously, consumption of reducing sugar, mainly referring to glucose, was also evaluated during the biodegradation of the effluent. Same as biodegradation capacity, the rate of consumption of glucose was fairly fast in the initial 4 h of treatment with gradual decline later. Furthermore, after treatment, the wastewater, originally feculent, turned out to be clarified and odorless, without visible particles. In order to better understanding the essence of biodegradation by immobilized pellets, laccase and cellulose were also detected during wastewater treatment. Fig. 4 demonstrates that immobilized pellets can secrete not only laccase but also cellulase, though their productivities are not high.
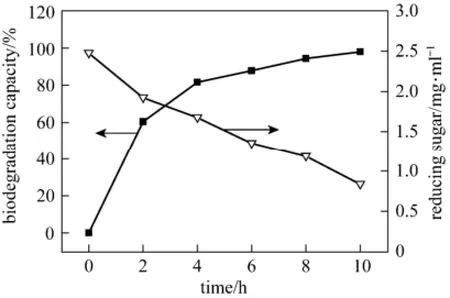
Figure 3 Biodegradation capacity of immobilized pellets and concentration of reducing sugar in wastewater treatment process
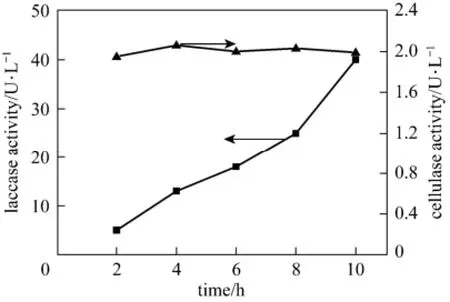
Figure 4 Time course of production of laccase and cellulase by immobilized pellets in wastewater treatment process
Therefore, there is a sound reason to explain the biodegradation process. Our immobilized pellets are composed of white-rot fungus Pestalotiopsis sp. J63 and filamentous fungus Penicillium janthinellum P1. Among them, J63 has been revealed in our previous work that it can secrete high capacity of ligninolyticenzyme—laccase, which is considered as a potential alternative applied in many industrial sectors, such as environmental protection, bioremediation, and wastewater treatment, due to its low substrate specificity and highly oxidative capability [35-41], while P1 can produce a large amount of cellulase. Thus this two-species immobilization system developed here assembles cellulose-degrading metabolic pathway and lignin-degrading metabolic pathway together. The immobilized pellets do work in the wastewater treatment, leading to degradation and removal of insoluble fine fibers suspended in the effluent.
3.5 Kinetics of biodegradation
In most of industrial wastewater biological treatment, Michaelis-Menten equation is frequently used to describe the first-order kinetic equation. In this study, 5 g of immobilized pellets were used for biological treatment of paper mill effluent. The process of wastewater biodegradation is reflected by the change of turbidity (Fig. 5). Then Menten equation is applied to fit the curve of turbidity. The kinetic equation for biodegradation is expressed as lnC=−0.3649t+6.60 (Fig. 6). The first-order model gives a good fitting, with R2= 0.9811.
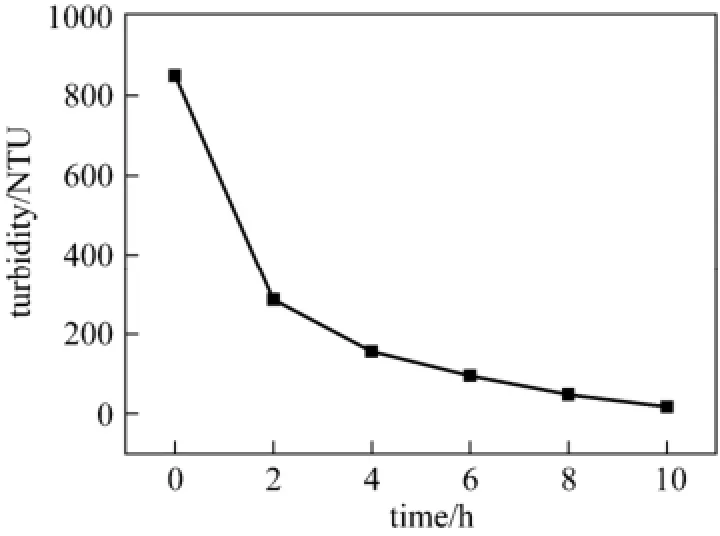
Figure 5 Turbidity change during wastewater treatment process
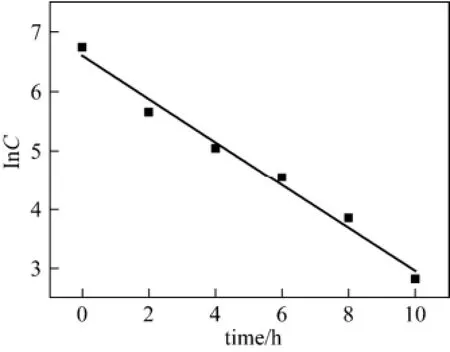
Figure 6 Kinetics of wastewater biodegradation by immobilized pellets
3.6 Decolorization test
Figure 7 displays the difference between immobilized pellets (IP) and mycelia pellets (MP) in decolorization capability. Azure B, known as trimethylthionin chloride, commonly used in biological stain, is recalcitrant to environment [42]. Both P1 mycelia pellets and immobilized pellets can effectively decolorize Azure B. However, the decolorization capability of immobilized pellets is improved notably, by 26%, compared with mycelia pellets during a 10-day treatment. It confirms that this co-immobilization system is more advantageous than single mycelia pellets in industrial applications.
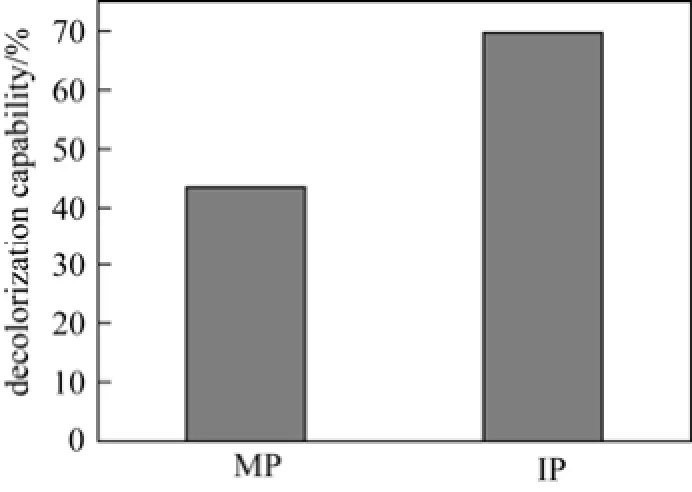
Figure 7 Comparison of decolorization capability of immobilized pellets (IP) and mycelia pellets (MP)
4 CONCLUSIONS
Whole-cell immobilization technology has drawn extensive interest for application in several areas such as biodegradation of mixed wastes and drug or fuel biosynthesis. Mycelia pellet, which is considered as a promising biological carrier, has been employed in many industrial sectors. The constructed novel two-species whole-cell immobilization system presents several advantages. First and most importantly, the co-immobilization method is safe for cells than those previously reported methods. This technique circumvents the use of chemicals, such as chelating agents and cross-linker, which can be potentially toxic to cells. Because this system involves immobilization simply via entrapment without any chemical treatment, it can maintain the viability of cells in the maximum extent. Second, it offers stronger biocatalytic capability than those of single-cell immobilization systems due to its combination of complementary functions into a single community. Third, the carrier, mycelia pellet, is more environmentally-friendly and cost-effective than those reported carriers. Since mycelia pellet itself is a microbe, it can yield needed enzymes or other biological active substances during cultivation, so it possesses dual properties, carrier as well as production of enzymes. Also, this co-immobilization system can be applied in wider range of substrates. The present work is to investigate the effect of wastewater treatment by these immobilized pellets. Results show more than 99% biodegradation percentage of immobilized pellets in the batch run, indicating that this constructed two-species whole-cell immobilization system can serve as a potential alternative for sewage treatment.
REFERENCES
1 Robinson, T., McMullan, G., Marchant, R., Nigam, P., “Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative”, Bioresour. Technol., 77 (3), 247-255 (2001).
2 Gogate, P.R., Pandit, A.B., “A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions”, Adv. Environ. Res., 8 (3), 501-551 (2004).
3 Kuhad, R.C., Sood, N., Tripathi, K.K., Singh, A., Ward, O.P., “Developments in microbial methods for the treatment of dye effluents”, Adv. Appl. Microbiol., 56, 185-213 (2004).
4 Wesenberg, D., Kyriakides, I., Agathos, S.N., “White-rot fungi and their enzymes for the treatment of industrial dye effluents”, Biotechnol. Adv., 22, 161-187 (2003).
5 Reddy, C.A., “The potential for white-rot fungi in the treatment of pollutants”, Curr. Opin. Biotech., 6 (3), 320-328 (1995).
6 Rubilar, O., Elgueta, S., Tortella, G., Gianfreda, L., Diez, M.C.,“Pelletization of Anthracophyllum discolor for water and soil treatment contaminated with organic pollutants”, Revista de la ciencia del suelo y nutrición vegetal, 9 (3), 161-175 (2009).
7 Robinson, T., Nigam, P.S., “Remediation of textile dye waste water using a white-rot fungus Bjerkandera adusta through solid-state fermentation (SSF)”, Appl. Biochem. Biotech., 151 (2), 618-628 (2008).
8 Diwaniyan, S., Kharb, D., Raghukumar, C., Kuhad, R. C., “Decolorization of synthetic dyes and textile effluents by basidiomycetous fungi”, Water, Air & Soil Pollution, 210 (1), 409-419 (2010).
9 Casieri, L., Varese, G.C., Anastasi, A., Prigione, V., Svobodova, K., Filippelo Marchisio, V., Novotný, Č., “Decolorization and detoxication of reactive industrial dyes by immobilized fungi Trametes pubescens and Pleurotus ostreatus”, Folia Microbiol., 53 (1), 44-52 (2008).
10 Park, C., Lee, B., Han, E.J., Lee, J., Kim, S., “Decolorization of acid black 52 by fungal immobilization”, Enzyme Microb. Tech., 39 (3), 371-374 (2006).
11 Iqbal, M., Edyvean, R.G., “Loofa sponge immobilized fungal biosorbent: a robust system for cadmium and other dissolved metal removal from aqueous solution”, Chemosphere, 61 (4), 510-518 (2005).
12 Enayatzamir, K., Alikhani, H.A., Yakhchali, B., Tabandeh, F., Rodríguez-Couto, S., “Decolouration of azo dyes by Phanerochaete chrysosporium immobilised into alginate beads”, Environ. Sci. Pollut. R., 17 (1), 145-153 (2010).
13 Gao, Q.T., Wong, Y.S., Tam, N., “Removal and biodegradation of nonylphenol by immobilized Chlorella vulgaris”, Bioresour. Technol., (102), 10230-10238 (2011).
14 Kourkoutas, Y., Bekatorou, A., Banat, I.M., Marchant, R., Koutinas, A.A., “Immobilization technologies and support materials suitable in alcohol beverages production: a review”, Food Microbiol., 21 (4), 377-397 (2004).
15 Cohen, Y., “Biofiltration—the treatment of fluids by microorganisms immobilized into the filter bedding material: a review”, Bioresour. Technol., 77 (3), 257-274 (2001).
16 De-Bashan, L.E., Bashan, Y., “Immobilized microalgae for removing pollutants: review of practical aspects”, Bioresource Technol., 101 (6), 1611-1627 (2010).
17 Anisha, G.S., Prema, P., “Cell immobilization technique for the enhanced production of α-galactosidase by Streptomyces griseoloalbus”, Bioresour. Technol., 99 (9), 3325-3330 (2008).
18 Fukuda, H., Hama, S., Tamalampudi, S., Noda, H., “Whole-cell biocatalysts for biodiesel fuel production”, Trends Biotechnol., 26 (12), 668-673 (2008).
19 Koda, R., Numata, T., Hama, S., Tamalampudi, S., Nakashima, K., Tanaka, T., Ogino, C., Fukuda, H., Kondo, A., “Ethanolysis of rapeseed oil to produce biodiesel fuel catalyzed by Fusarium heterosporum lipase-expressing fungus immobilized whole-cell biocatalysts”, Journal of Molecular Catalysis B: Enzymatic, 66 (1), 101-104 (2010).
20 Zhao, L., Cao, G., Wang, A., Guo, W., Liu, B., Ren, H., Ren, N., Ma, F., “Enhanced bio-hydrogen production by immobilized Clostridium sp. T2 on a new biological carrier”, Int. J. Hydrogen Energ., 37, 162-166 (2012)
21 García Martínez, T., Puig Pujol, A., Peinado, R.A., Moreno, J., Mauricio, J.C., “Potential use of wine yeasts immobilized on Penicillium chrysogenum for ethanol production”, J. Chem. Technol. Biot., (87), 351-359 (2012).
22 Cing, S., Yesilada, O., “Astrazon red dye decolorization by growing cells and pellets of Funalia trogii”, J. Basic Microb., 44 (4), 263-269 (2004).
23 Yesilada, O., Asma, D., Cing, S., “Decolorization of textile dyes by fungal pellets”, Process Biochem., 38 (6), 933-938 (2003).
24 Yesilada, O., Yildirim, S. C., Birhanli, E., Apohan, E., Asma, D., Kuru, F., “The evaluation of pre-grown mycelial pellets in decolorization of textile dyes during repeated batch process”, World Journal of Microbiology and Biotechnology, 26 (1), 33-39 (2010).
25 Yang, Y., Hu, H., Wang, G., Li, Z., Wang, B., Jia, X., Zhao, Y., “Removal of malachite green from aqueous solution by immobilized Pseudomonas sp. DY1 with Aspergillus oryzae”, Int. Biodeter. Biodegr., (65), 429-434 (2011).
26 Chen, H.Y., Xue, D.S., Feng, X.Y., Yao, S.J., “Screening and production of ligninolytic enzyme by a marine-derived fungal Pestalotiopsis sp. J63”, Appl. Biochem. Biotech., (165), 1754-1769 (2011).
27 Xiao, K., Zeng, H. Y., Jiang, H., Cai, L. H., Huang, L., “Induced mutagenesis of high lipase productivity strain and immobilization of produced lipase”, China Biotech., 29 (5), 88-94 (2002).
28 Ghose, T.K., “Measurement of cellulase activities”, Pure Appl. Chem., 59 (2), 257-268 (1987).
29 Soares, G.M.B., Pessoa de Amorim, M.T., Costa-Ferreira, M., “Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R”, J. Biotech., 89, 123-129 (2001).
30 Breuil, C., Saddler, J.N., “Comparison of the 3, 5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity”, Enzyme Microb. Tech., 7 (7), 327-332 (1985).
31 Rapp, P., Grote, E., Wagner, F., “Formation and location of 1, 4-β-glucanases and 1, 4-β-glucosidases from Penicillium janthinellum”, Appl. Environ. Microb., 41 (4), 857-866 (1981).
32 Oliveira, L.A., Porto, A.L.F., Tambourgi, E.B., “Production of xylanase and protease by Penicillium janthinellum CRC 87M-115 from different agricultural wastes”, Bioresour. Technol., 97 (6), 862-867 (2006).
33 Adsul, M.G., Bastawde, K.B., Varma, A.J., Gokhale, D.V., “Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production”, Bioresour. Technol., 98 (7), 1467-1473 (2007).
34 Li, L., Liu, R., Ye, M., Hu, X., Wang, Q., Bi, K., Guo, D., “Microbial metabolism of evodiamine by Penicillium janthinellum and its application for metabolite identification in rat urine”, Enzyme Microb. Tech., 39 (4), 561-567 (2006).
35 Smetanina, O.F., Kalinovsky, A.I., Khudyakova, Y.V., Pivkin, M.V., Dmitrenok, P.S., Fedorov, S.N., Ji, H., Kwak, J.Y., Kuznetsova, T.A.,“Indole alkaloids produced by a marine fungus isolate of Penicillium janthinellum Biourge”, J. Nat. Prod., 70 (6), 906-909 (2007)
36 Šušla, M., Novotný, C., Svobodová, K., “The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures”, Bioresour. Technol., 98 (11), 2109-2115 (2007).
37 Wang, P., Fan, X.R., Cui, L., Wang, Q., Zhou, A.H., “Decolorization of reactive dyes by laccase immobilized in alginate/gelatin blent with PEG”, Journal of Environ. Sci., 21, 1519-1522 (2008).
38 Muhammad, A., Haq, N. B., Muhammad, A., Raymond, L., “Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system”, Biodegr., 19, 771-783 (2008).
39 Bayramoglu, G., Yakup ArIca, M., “Immobilization of laccase onto poly (glycidyl methacrylate) brush grafted poly (hydroxyethylmethacrylate) films: Enzymatic oxidation of phenolic compounds”, Mater. Sci. Eng. C, 29 (6), 1990-1997 (2009).
40 Makas, Y.G., Kalkan, N.A., Aksoy, S., Altinok, H., Hasirci, N., “Immobilization of laccase in kappa-carrageenan based semi-interpenetrating polymer networks”, J. Biotechnol., 148 (4), 216-220 (2010).
41 Tastan, E., Önder, S., Kok, F.N., “Immobilization of laccase on polymer grafted polytetrafluoroethylene membranes for biosensor construction”, Talanta, 84 (2), 524-530 (2011).
42 Fan, H., Yang, J.S., Gao, T.G., Yuan, H.L., “Removal of a low-molecular basic dye (Azure Blue) from aqueous solutions by a native biomass of a newly isolated Cladosporium sp.: Kinetics, equilibrium and biosorption simulation”, Taiwan Insti. Chem. Engrs., 43 (3), 386-392 (2012).
Received 2012-10-25, accepted 2013-01-25.
* Supported by the National Natural Science Foundation of China (21036005), Scientific Technology Program of Zhejiang Province (2011C33016).
** To whom correspondence should be addressed. E-mail: yaosj@zju.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
- Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
- Hydrogenation of Silicon Tetrachloride in Microwave Plasma
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
