Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
2014-03-25朱伟娜常春马晨杜风光
(朱伟娜)(常春),**(马晨)(杜风光)
1School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, China
2Shanghai Jiao Tong University Library, Shanghai Jiao Tong University, Shanghai 200240, China
3State Key Laboratory of Motor Vehicle Biofuel Technology, Nanyang 473000, China
Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
ZHU Weina(朱伟娜)1, CHANG Chun(常春)1,**, MA Chen(马晨)2and DU Fengguang(杜风光)3
1School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, China
2Shanghai Jiao Tong University Library, Shanghai Jiao Tong University, Shanghai 200240, China
3State Key Laboratory of Motor Vehicle Biofuel Technology, Nanyang 473000, China
The kinetics for production of ethyl levulinate from glucose in ethanol medium was investigated. The experiments were performed in various temperatures (433-473 K) and initial glucose concentrations (0.056-0.168 mol·L−1) with extremely low sulfuric acid as the catalyst. The results show that higher temperature can improve the conversion of glucose to ethyl levulinate, with higher yield of ethyl levulinate (44.79%, by mole) obtained at 473 K for 210 min. The kinetics follows a simplified first-order kinetic model. For the main and side reactions, the values of activation energy are 122.64 and 70.97 kJ·mol−1, and the reaction orders are 0.985 and 0.998, respectively.
ethyl levulinate, glucose, kinetics, biomass
1 INTRODUCTION
Nowadays, production of new platform chemicals from biomass is gaining more attention around the world. Among the explorations, one attractive approach is the direct conversion of hexose carbohydrates into ethyl levulinate (EL) [1]. Ethyl levulinate is a versatile chemical feedstock with numerous potential industrial applications, which is regarded as a new kind of platform chemicals from biomass [2]. Besides current commercial usage in the food industry and fragrance industry, it can be used as octane booster for gasoline and fuel extender of diesel [3, 4].
Several researches have been reported on the direct conversion of carbohydrates into ethyl levulinate in ethanol medium [5-8]. Although the ethanolysis reaction of carbohydrates is effective, the process has several limitations. The yields of ethyl levulinate are low, most of which are less than 40%. Many by-products form in the process [9]. Sulfuric acid is widely used as the catalyst in the reaction due to its low price and high activity. When sulfuric acid is in higher concentrations, it has serious drawbacks in separation and recycling, as well as equipment corrosion [10]. Thus it is necessary to develop new and green processes for production of ethyl levulinate.
One of the promising methods for conversion of biomass is the usage of extremely low acid (ELA), which means the acid concentration lower than 0.01mol·L−1, with minimal impact on the environment. In addition, the corrosion is close to that in a neutral reaction, so that general stainless steel equipment can be used, which is significant in cost advantage. Several studies have showed the advantages of ELA in the conversion process of biomass. Chang et al. developed an efficient procedure for the conversion of rice huck into levulinic acid [11]. Zhuang et al. reported that ELA is good for the glucose production from biomass [12]. Peng et al. also reported that the ELA system is efficient, economical and environmentally benign for the conversion of carbohydrates into high value-added chemicals and fuels [13]. However, to our knowledge, little has been reported on the usage of ELA for production of ethyl levulinate from biomass, and there is no related report on the study of the kinetics. Therefore, the objective of present work is to study the kinetics of ethyl levulinate production from glucose under ELA conditions, which is helpful to develop new and green processes.
2 EXPERIMENTAL
2.1 Materials
Glucose and ethyl levulinate used for calibration (purity over 99%) were obtained from Aladdin Reagent (Shanghai, China). Other reagents and chemicals were of analytical grade from Kermel Chemical Reagent (Tianjin, China). Deionized water was used in all experiments. All chemicals were used without further purification.
2.2 Equipment and procedure
The experiments were carried out in a 200 ml cylindrical pressurized stainless reactor equipped with an electrical heating jacket and a mechanical stirrer. The reaction temperature was monitored by a thermocouple connected to the reactor. For each experiment, a given amount of glucose and the mixture of ethanol with sulfuric acid were mixed and poured into the reactor, with the total volume of 80 ml. The acid concentration was in the range of 0.0025-0.01 mol·L−1.Then, the reactor was sealed and heated to the desired temperature by external heating with a stirring rate of 250 r·min−1. The temperature range was 433-473 K according to our previous study [14]. When the desired temperature was reached, the stirring rate was adjusted to 500 r·min−1. After desired reaction duration, the reactor was quenched in an ice cool water bath to terminate the reaction. The reaction liquid was filtered and collected for analysis. The amounts of diethyl ether from ethanol were calculated according to the mass loss of liquid phase before and after the reaction [10]. All the experiments were performed in duplicate and average values were reported.
2.3 Analytical methods
Ethyl levulinate concentration was analyzed using a gas chromatography equipped with a FFAP capillary column (30 m×0.32 mm×0.33 μm) and a flame ionization detector operated at 523 K. The carrier gas was nitrogen. The ethyl levulinate amount in the liquid was determined using calibration curves obtained by standard solutions, and reaction products were detected and confirmed by GC-MS (Thermo Fisher Scientific).
The yield of ethyl levulinate (%, by mole) is calculated by
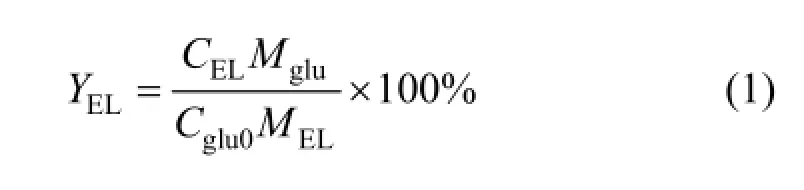
3 RESULTS AND DISCUSSION
3.1 Effect of sulfuric acid concentration on ethanol self-condensation reaction
In the reaction, the presence of sulfuric acid will promote the conversion of ethanol to diethyl ether, an unavoidable side reaction under the experimental conditions [15]. For practical applications, it is necessary to investigate the influence of sulfuric acid concentration on ethanol self-condensation reaction.
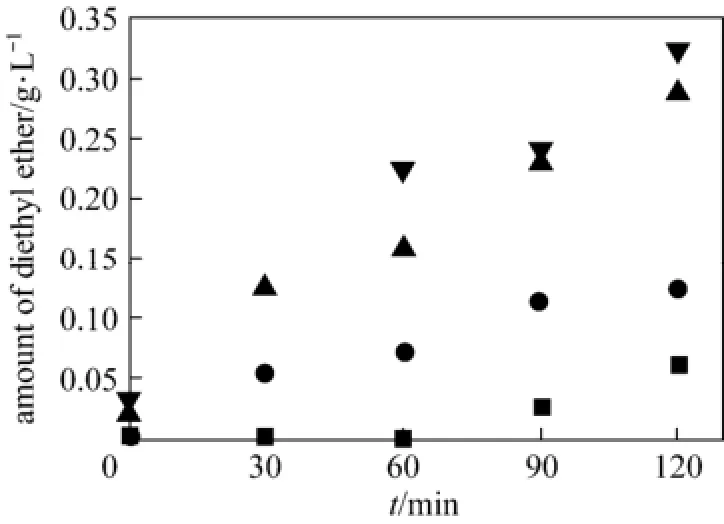
Figure 1 Effect of sulfuric acid concentration on the production of diethyl ether (T=473 K)sulfuric acid/mol·L−1: ■ 0; ● 0.0025; ▲ 0.005; ▼ 0.01
Figure 1 shows the amount of diethyl ether per unit volume of ethanol versus reaction time. Without sulfuric acid, only negligible amount of diethyl ether can be detected. With the addition of sulfuric acid, the amount of diethyl ether increases significantly with reaction time. Since the concentration of sulfuric acid is less than 0.01 mol·L−1, the amount of diethyl ether is limited. In our previous experiments, at the acid concentration of 0.1 mol·L−1, the amount of diethyl ether can reach 1.88 g·L−1at 453 K for 30 min (data not shown). These results show that extremely low acid is benefit to suppress the self-condensation of ethanol and reduce the amount of diethyl ether [13].
3.2 Effect of initial glucose concentration on EL yield
Figure 2 shows the effect of initial glucose concentration on the yield of ethyl levulinate. The yield decreases slightly as the initial glucose concentration increases from 0.056 mol·L−1to 0.168 mol·L−1. It suggests that lower glucose concentration is benefit to the accumulation of product [16]. On the contrary, the ethyl levulinate concentration increases with the initial glucose concentration. Considering higher EL concentration is favorable for the separation of product, the initial glucose concentration of 0.112 mol·L−1was adopted for the further experiments.
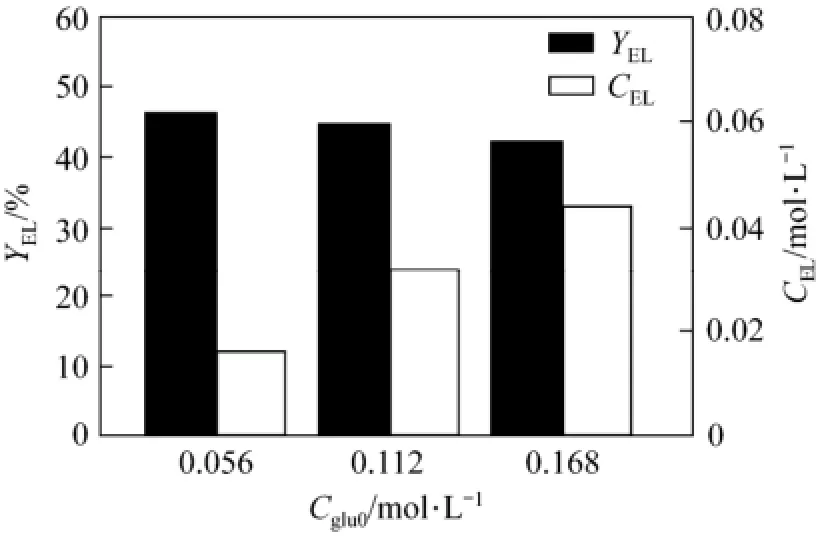
Figure 2 Effect of initial glucose concentration on EL yield (T=473 K, t=120 min, CH2SO4=0.01 mol·L−1)
3.3 Effect of temperature on EL yield
Reaction temperature plays a significant role in the reaction process. Fig. 3 shows the effect of reaction temperature on the yield of ethyl levulinate. At 433 K, the yield maintains at a low level. At 453 K, the yield reaches 27.93% (by mole) in 210 min. At 473 K, the yield increases with time more quickly at first and then gradually, reaching higher yield (44.79%, by mole). However, it was found that more humic solid accumulated in the reactor at higher temperature, which may diminish the efficiency of conversion. These results indicate that higher temperature accelerates the reaction rate, but unwanted side reactions appear [17]. In terms of the selectivity of the reaction, appropriate temperature is 473 K in this study.
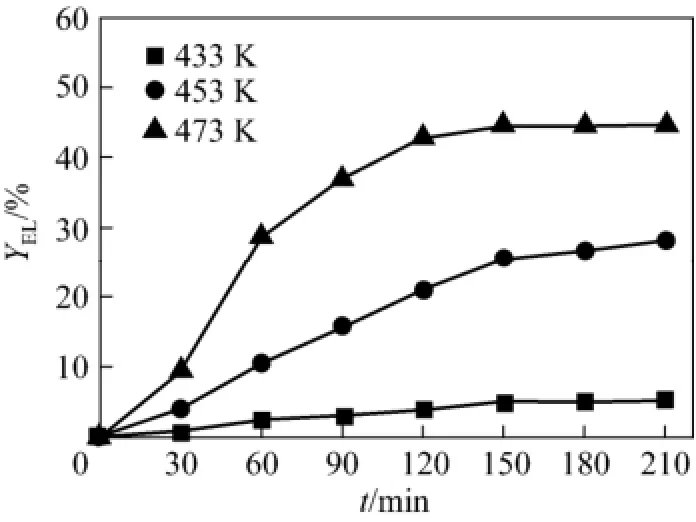
Figure 3 Effect of temperature on EL yield ( CH2SO4=0.01 mol·L−1, Cglu0=0.112 mol·L−1)
3.4 Effect of acid concentration on EL yield
The effect of acid concentration on the yield of ethyl levulinate is presented in Fig. 4. The curves are similar to those in Fig. 3, and the yield increases with reaction time. At the acid concentration of 0.01 mol·L−1, less reaction time is needed to reach the equilibrium, and the yield reaches 44.79% (by mole) at 210 min. These results indicate that the conversion of glucose is accelerated by increasing the acid concentration [9]. The acid concentration of 0.01 mol·L−1is recommended in practical production.

Figure 4 Effect of acid concentration on EL yield (T=473 K, Cglu0=0.112 mol·L−1)
3.5 Kinetic model of EL production from glucose
Besides the main product, some dark-brown insoluble substance known as humins was observed, which may be the products of side-reactions [17]. According to the experimental results and related literature [18], a plausible reaction pathway of the acid-catalyzed conversion of glucose to ethyl levulinate in ethanol medium is proposed, as summarized in Fig. 5.
According to previous research, a simplified model is used to determine the kinetics of glucose ethanolysis [13], as given in Fig. 6.
Based on this model, the concentration of ethyl levulinate as a function of time is represented as

Solving the differential equations, analytic expressions for concentration and yield of ethyl levulinate are obtained
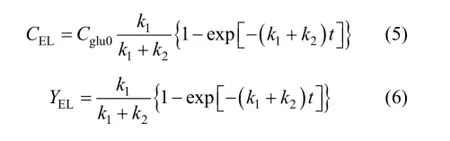
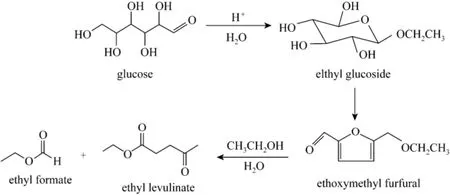
Figure 5 Reaction pathway of the conversion of glucose to EL in ethanol medium
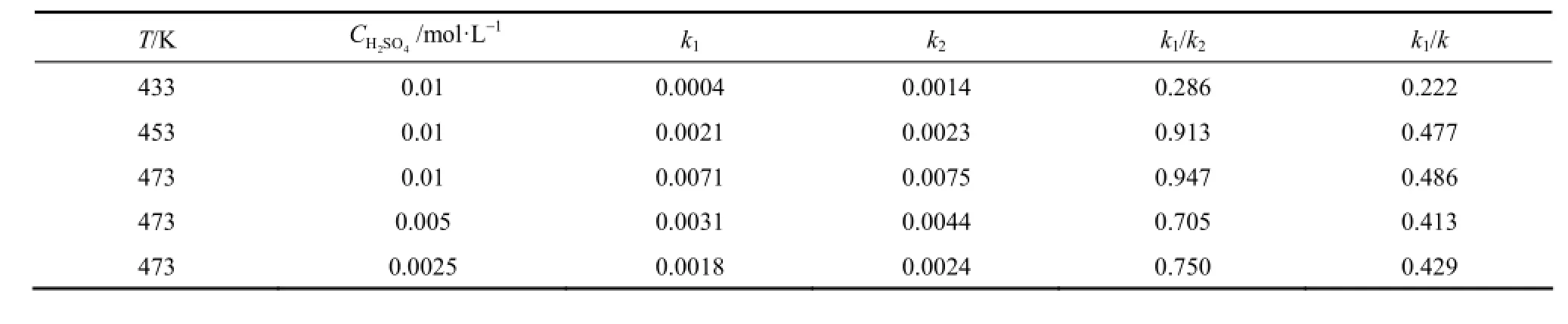
Table 1 Rate constants of glucose ethanolysis under different reaction conditions
We assume that the reaction order of main reaction is α and that of side reaction is β. The kinetic coefficients are correlated with temperature by applying modified Arrhenius equations for the effect of acid concentration
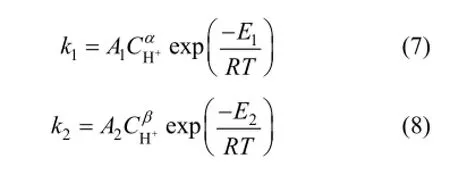
Equation (6) is used to correlate the experimental data in Figs. 3 and 4. The kinetic parameters evaluated by using MATLAB 7.0 are given in Table 1. The rate constants are associated with the Arrhenius formula at different temperatures at the initial glucose concentration of 0.112 mol·L−1and the acid concentration of 0.01 mol·L−1. The activation energy of main reaction E1is 122.64 kJ·mol−1and that of side reaction E2is 70.97 kJ·mol−1. Since the hydrogen ion concentration of sulfuric acid in the high temperature ethanol is difficult to be measured accurately, it is assumed that the sulfuric acid ionized completely at high temperature and pressure. For the same temperature (473 K) and different concentrations of sulfuric acid, the reaction order of main reaction α is 0.985 and that of side reaction β is 0.998 from Eqs. (7) and (8). On this basis, the pre-exponential factor of main reaction A1is 2.6185×1013mol−0.985·L0.985·min−1and that of side reaction A2is 4.5136×107mol−0.998·L0.998·min−1.
As shown in Table 1, the rate constants of main and side reactions increase with the increase of reaction temperature and acid concentration, suggesting that higher temperature and acid concentration promote the chemical reaction rate. The increase of k1/k2with acid concentration indicates that the formation rate of ethyl levulinate relative to that of humins can be enhanced by increasing acid concentration [8]. At the acid concentration of 0.01 mol·L−1, the value of k1/k2increases as the temperature increases from 433 K to 473 K. This indicates that high temperature also improves the formation of ethyl levulinate under the experimental conditions.
Figure 7 shows that the predicted yields of ethyl levulinate according to the model are in good agreement with experimental data. Thus the kinetic model is appropriate.
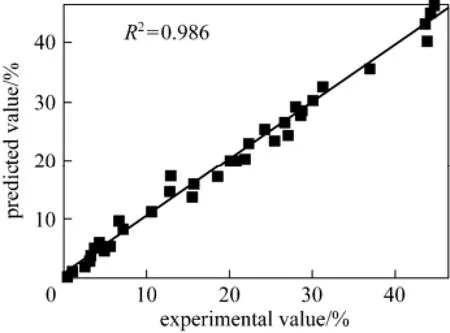
Figure 7 Comparison of predicted values of EL yield with experimental data
The kinetic model allows the determination of the optimum reaction conditions to achieve high yield of ethyl levulinate. For this purpose, t is assumed as infinite in Eq. (6), and the combination of Eq. (1) lead to
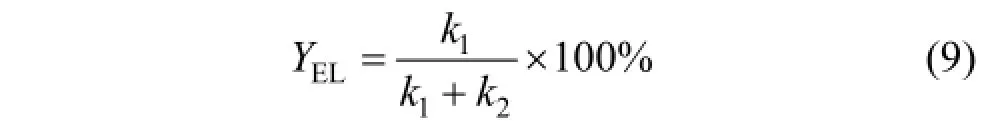
Substituting the kinetic coefficients expressed as Eqs. (7) and (8), we obtain the yield of ethyl levulinate as a function of reaction temperature and acid concentration
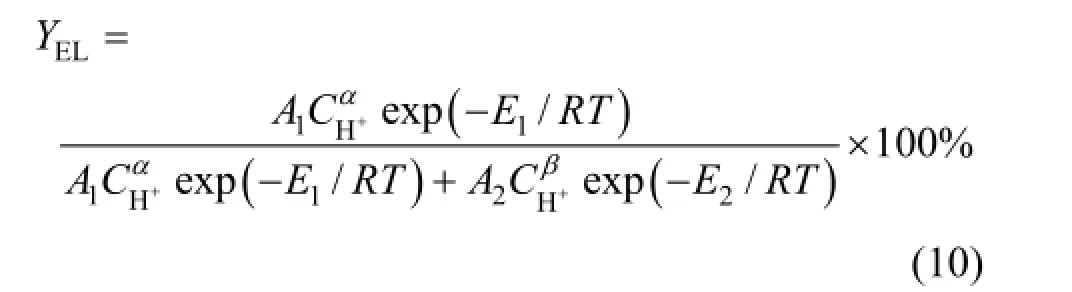
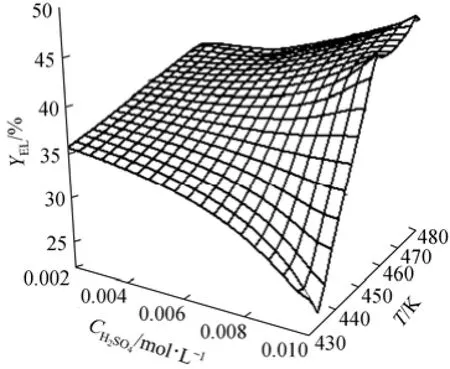
Figure 8 EL yield versus temperature and acid concentration

Table 2 Kinetic parameters under different conditions
Figure 8 shows a three-dimensional surface to predict the yield of ethyl levulinate at different temperatures and acid concentrations. At the temperature lower than 453 K, the acid concentration has little effect on the yield. When the temperature is higher than 453 K, higher acid concentration improves the production of ethyl levulinate significantly. At the acid concentration of 0.01 mol·L−1, the highest yield (48.24%, by mole) can be obtained at 472 K.
3.6 Comparison with other investigations
The results of this study and other investigations are compared in Table 2. The activation energy and reaction order for alcoholysis of glucose are similar.
NOMENCLATURE
A1pre-exponential factor, mol−α·Lα·min−1
A2pre-exponential factor, mol−β·Lβ·min−1
CELethyl levulinate concentration, mol·L−1
Cgluglucose concentration, mol·L−1
Cglu0initial glucose concentration, mol·L−1
CH+H+concentration, mol·L−1
CH2SO4sulfuric acid concentration, mol·L−1
E activation energy, kJ·mol−1
k rate constant, min−1
MELmolecular mass of ethyl levulinate, 144 g·mol−1
Mglumolecular mass of glucose, 180 g·mol−1
R gas constant, 8.3143 J·mol−1·K−1
T temperature, K
t time, min
YELyield of ethyl levulinate (by mole), %
α reaction order of main reaction
β reaction order of side reaction
Subscripts
1 main reaction
2 side reaction
REFERENCES
1 Joshi, H., Moser, B.R., Toler, J., Smith, W.F., Walker, T., “Ethyl levulinate: A potential bio-based diluent for biodiesel which improves cold flow properties”, Biomass Bioenergy, 35, 3262-3266 (2011).
2 Mascal, M., Nikitin, E.B., “High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl)furfural, levulinic acid and levulinic esters via 5-(chloromethyl)furfural”, Green Chem., 12, 370-373 (2010).
3 Gurbuz, E.I., Alonso, D.M., Bond, J.Q., Dumesic, J.A., “Reactive extraction of levulinate esters and conversion to gamma-valerolactone for production of liquid fuels”, Chem. Sus. Chem., 4, 357-361 (2011).
4 Lee, A., Chaibakhsh, N., Rahman, M.B.A., Basri, M., Tejo, B.A.,“Optimized enzymatic synthesis of levulinate ester in solvent-free system”, Ind. Crops Prod., 32, 246-251 (2010).
5 Garves, K., “Acid catalyzed degradation of cellulose in alcohos”, J. Wood Chem. Technol., 8, 121-134 (1988).
6 Olson, E.S., Kielden, M.R., Schlag, A.J., Sharma, R.K., “Levulinate esters from biomass wastes”, A.C.S. Symp. Ser., 784, 51-63 (2001).
7 Mao, R.L.V., Zhao, Q., Dima, G., “New process for the acid-catalyzed conversion of cellulosic biomass (AC3B) into alkyl levulinates and other esters using a unique one-pot system of reaction and product extraction”, Catal. Lett., 141, 271-276 (2011).
8 Wu, X.Y., Liu, X.Y., Chen, T., Chen, Z.N., “Alcoholysis kinetics of glucose catalyzed by dilute sulfuric acid in near-critical methanol”, CIESC J., 61, 2585-2589 (2010). (in Chinese)
9 Mascal, M., Nikitin, E.B., “Comment on processes for the direct conversion of cellulose or cellulosic biomass into levulinate esters”, Chem. Sus. Chem., 3, 1349-1351 (2010).
10 Peng, L.C., Lin, L., Zhang, J.H., Shi, J.B., Liu, S.J., “Solid acid catalyzed glucose conversion to ethyl levulinate”, Appl. Catal. A: Gen., 397, 259-265 (2011).
11 Chang, C., Wang, D., Wei, W., Jiang, X.X., “Effects of extremely-low-concentration acid hydrolysis on levulinic acid production from rice husk and characterization of cellulosic structure”, Chem. Ind. Forest Prod., 31, 23-27 (2011).
12 Zhuang, X.S., Wang, S.R., An, H., “Cellulose hydrolysis research for liquid fuelproduction under low concentration acids biomass hydrolysis under extremely low acids for fuel ethanol production”, J. Zhejiang Univ. (Eng. Sci.), 40, 997-1001 (2006).
13 Peng, L.C., Lin, L., Li, H., “Extremely low sulfuric acid catalyst system for synthesis of methyl levulinate from glucose”, Ind. Crops and Prod., 40, 136-144 (2012).
14 Chang, C., Ma, X.J., Cen, P.L., “Kinetics of levulinic acid formation from glucose decomposition at high temperature”, Chin. J. Chem. Eng., 14 (5), 708-712 (2006).
15 Liu, D., Lin, L., Peng, L.C., “Conversion of sucrose to ethyl levulinate catalyzed by solid acidMod. Chem. Ind., 31, 45-49 (2011).
16 Girisuta, B., Janssen, L.P.B.M., Heeres, H.J., “A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid”, Green Chem., 8, 701-709 (2006).
17 Hu, X., Lievens, C., Larcher, A., Li, C.Z., “Reaction pathways of glucose during esterification: Effects of reaction parameters on the formation of humin type polymers”, Bioresour. Technol., 102, 10104-10113 (2011).
18 Peng, L.C., Lin, L., Yang, Q.L., “Conversion of carbohydrates biomass into levulinate esters using heterogeneous catalysts”, Appl. Energy, 88, 4590-4596 (2011).
Received 2013-04-08, accepted 2013-08-24.
* Supported by the National Natural Science Foundation of China (21176227) and the State Key Laboratory of Motor Vehicle Biofuel Technology (2013011).
** To whom correspondence should be addressed. E-mail: chunchang@zzu.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
- Hydrogenation of Silicon Tetrachloride in Microwave Plasma
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
- Performance of EDAB-HCl Acid Blended System as Fracturing Fluids in Oil Fields*
