Unhatched and Hatched Eggshells of the Chinese Cobra Naja atra
2014-03-25*
*
1College of Biological and Environmental Sciences, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
2Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210023, Jiangsu, China
3Hangzhou Key Laboratory for Animal Adaptation and Evolution, School of Life Sciences, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China
Unhatched and Hatched Eggshells of the Chinese Cobra Naja atra
Zheng WANG1,2, Longhui LIN3and Xiang JI2*
1College of Biological and Environmental Sciences, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
2Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210023, Jiangsu, China
3Hangzhou Key Laboratory for Animal Adaptation and Evolution, School of Life Sciences, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China
Changes in structure and composition of the eggshell resulting from embryonic mobilization of minerals from the eggshell are found in all oviparous reptiles studied thus far. In this study, we measured samples of unhatched and hatched eggshells of the Chinese cobra Naja atra to determine the percentage of ash and the phase composition of calcium carbonate. The mean percentage of ash was signifi cantly higher in unhatched eggshells (24.6%) than in hatched eggshells (22.3%). The dominant phase in unhatched eggshells was the calcite form of calcium carbonate. In addition to the peaks of calcite, a few small peaks were found to be caused by the aragonite and vaterite phases of calcium carbonate, implying that there are small amounts of aragonite and vaterite in the eggshell. The concentration of the various phases calculated from the intensity of the X-ray diffraction spectra allowed the estimation that percentages of calcite, aragonite and vaterite were about 92%, 4% and 4%, respectively. Hatched eggshells produced similar spectral characteristics as unhatched eggshells, with one exception. The dominant phase composition in the hatched eggshell was also calcite, but the amount of the aragonite phase had a marked increase. Our study adds evidence that embryonic mobilization of minerals from the eggshell may result in changes in structure of the eggshell.
Elapidae, Naja atra, eggshell structure, egg incubation, X-ray diffraction spectra, minerals
1. Introduction
The majority of extant reptiles are oviparous and produce different eggs with three major components: embryo (plus extra embryonic membranes), yolk and eggshell (Andrews and Mathies, 2000). The embryonic stage at oviposition is species-dependent and more variable in squamates than in other reptilian taxa. For example, turtles and tuataras oviposit eggs when embryos are at the gastrula stage, crocodilians lay eggs at the neurula stage, and squamates exhibit nearly the entire gamut of possible embryonic stages at oviposition (Shine, 1983; Andrews and Mathies, 2000). Yolk is deposited in theoocyte during vitellogenesis prior to ovulation, and is used by embryos as the source of all organic and most inorganic nutrients (Ji and Braña, 1999; Du et al., 2001; Lu et al., 2009). The eggshell, containing both organic and inorganic components, is secreted by the oviduct after ovulation (Packard and DeMarco, 1991; Heulin et al., 2005; Stewart et al., 2010). Eggshells are flexible or rigid and function as an outer protective layer of the egg and a secondary source of minerals (mainly calcium) for embryos (Packard and Packard, 1984; Packard, 1994; Lu et al., 2009; Stewart and Ecay, 2010; Stewart et al., 2010, 2011). Available data have shown that the proportional amounts of calcium mobilized by embryos from the eggshell range 50%−80% in crocodilians and turtles (Bustard et al., 1969; Jenkins, 1975; Packard and Packard, 1984, 1989), and 14%−81% in squamates (Packard et al., 1984; Packard and Packard, 1988; Shadrix et al., 1994; Ji et al., 1997a,b, 1999a; Ji and Braña, 1999; Stewart et al.,
2009). Variation in the structure and quantity of each shell component was found to be correlated with phylogeny and with functional roles of the eggshell (Packard and Packard, 1988; Packard and DeMarco, 1991).
Embryonic reptiles mobilize minerals from the eggshell in late developmental stages when they undergo signifi cant skeletal ossifi cation (Shadrix et al., 1994; Du et al., 2001; Xu et al., 2004; Cai et al., 2007; Lu et al., 2009), and changes in structure of the eggshell resulting from embryonic mobilization of minerals from the eggshell may occur in eggshells of all oviparous reptiles (Packard and DeMarco, 1991). These findings directed our study hypothesis that shells from unhatched and hatched eggs should differ in structure in any species where embryos use the eggshell as an important source of minerals. To test this idea, we conducted a study examining differences in structure between unhatched and hatched eggshells of the Chinese cobra Naja atra.
2. Materials and Methods
Naja atra is a large-sized and highly venomous elapid snake that is widely distributed in southeastern China, including Taiwan, Hongkong and Hainan, southward to Vietnam (Wüster et al., 1997). From previous studies on N. atra we have known the following. First, females larger than 800 mm snout-vent length can lay a single clutch of 5−28 pliable-shelled eggs per breeding season (Ji and Wang, 2005). Second, temperatures optimal for embryonic development fall within the range of 26°C−30 °C (Ji and Du, 2001; Lin et al., 2008). Third, nearly 14% of hatchling calcium is mobilized by embryos from the eggshell (Ji et al., 1997b).
We obtained 17 gravid females in mid-June 2005 from a private collector in Quanzhou, Guangxi, southern China. Females were transported to our laboratory in Hangzhou, where one or two were housed in each 500 mm × 450 mm × 350 mm (length × width × height) egglaying cage. All cages were placed in a room inside which ambient temperatures were controlled within the range of 26°C−30°C optimal for embryonic development (Ji and Du, 2001; Lin et al., 2008). Food [common toads (Bufo gargarizans) and Chinese skinks (Plestiodon chinensis)] and water enriched with vitamins and minerals were provided ad libitum. Egg-laying cages were checked at least thrice daily. Eggs laid directly on the cage fl oor were always collected, measured (to the nearest 0.1 mm) and weighed (to the nearest 1 mg) less than 3 h after being layed to minimize the uncertainty about the egg mass at laying due to loss or gain of water (Ji and Du, 2001). Post-oviposition body mass (to the nearest 0.1 g) and SVL (to the nearest 1 mm) were taken for each female.
Females laid eggs between 2nd to 12th July. Of the 185 eggs collected, 160 were fertile and viable at oviposition. One freshly laid egg, randomly selected from each clutch, was dissected to identify Zehr’s (1962) embryonic stage at oviposition. Shells from the dissected freshly laid eggs were rinsed briefly, dried on blotting paper, weighed on a Mettler balance, and then lyophilized to a constant mass. The remaining eggs were incubated under multiple thermal regimes, and data will be reported elsewhere. Shells from the hatched eggs were handled in the same way described above for those from the dissected freshly laid eggs.
Dried eggshells were burned in a muffle furnace at 700°C for 24 h to determine ash mass. Samples of six unhatched eggshells and six hatched eggshells were used to analyze the phase composition of calcium carbonate using a D5005 X-ray diffractometer (XRD, Siemens, Germany) in a θ-2θ mode with an incident X-ray wavelength of 1.540 Å (Cu Kα line) (Supplementary Materials).
3. Results and Discussion
Shells from the 17 dissected unhatched eggs averaged 75.4 ± 0.4% organic material (range = 72.8%−78.2%) and 24.6 ± 0.4% ash by dry mass (range = 21.9%−27.2%). Shells from 17 clutches of hatched eggs averaged 77.7 ± 0.3% organic material (range = 75.3%−79.6%) and 22.3 ± 0.3% ash by dry mass (range = 20.4%−24.7%). The mean percentage of ash was significantly higher in unhatched eggshells than in hatched eggshells (paired-sample t-test; t = 6.89, df = 16, P < 0.0001). This result is consistent with studies of oviparous snakes studied in China thus far, including N. atra from an island population in Dinghai, Zhejiang, China (Table 1). Data from these studies show that embryonic mobilization of minerals from the eggshell results in a reduction of ash contents in hatched eggshells (Table 1), and that percentage of ash in the unhatched eggshell explains approximately 92% of variation in percentage of ash in the hatched eggshell and is thus a good predictor of the latter variable (linear regression analysis; F1,6= 65.58, P < 0.0002; Figure 1). Proportional amounts of ash mobilized by embryos from the eggshell may vary considerably among species and among populations of the same species. For example, the percentage of ash was reduced by approximately 4% in hatched eggshells of Dinodon rufozonatum, Elaphe carinata, Xenochrophis piscator and Rhabdophis tigrinus
lateralis, but only by approximately 1% in hatched eggshells of Ptyas korros (Table 1). In this study, the percentage of ash was reduced by 2.5% in hatched eggshells, approximately 1% lower than the value (3.6%) reported for an island population of N. atra in Dinghai, Zhejiang, eastern China (Table 1).
Figure 2 displays the X-ray diffraction spectra of the unhatched specimen 67 (a) and the hatched specimen Q2 (b), respectively. It could be seen in the spectrum (a) that most of the peaks and all the intensive peaks such as the peaks at 29.4°, 39.4°, 43.1°, 47.5° and 48.5° matched exactly the peaks (104), (113), (202), (018) and (116) in the diffraction pattern of calcite, revealing that the main content in the unhatched eggshell is calcite. Besides the peaks of calcite, a few small peaks were found to be caused by the aragonite and vaterite phases of calcium carbonate. In fact, the concentration of the various phases calculated from the intensity of the XRD spectra allowed the estimation that percentages of calcite, aragonite and vaterite were about 92%, 4% and 4%, respectively. That there were small amounts of aragonite and vaterite in the unhatched eggshell is somewhat inconsistent with earlier studies of oviparous squamates in which the inorganic layer of the eggshell consists of calcium carbonate in the form of calcite. However, this inconsistency is not unexpected because even environmental factors such as diet and captivity may affect the morph of calcium carbonate in the eggshell (Deeming, 1988; Packard and DeMarco, 1991).

Figure 2 X-ray diffraction spectra of Naja atra eggshells. (a) Unhatched eggshell (specimen 67); (b) Hatched eggshell (specimen Q2).
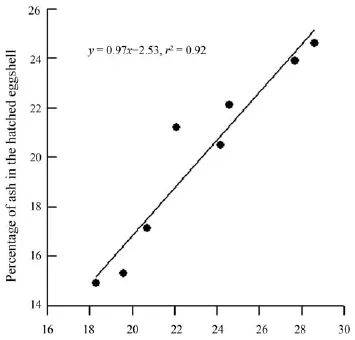
Figure 1 Percentage of ash in the hatched eggshell (y) in relation to percentage of ash in the unhatched eggshell (x) in oviparous snakes so far studied in China, according to data shown in Table 1. The regression equation and coeffi cient are given in the fi gure.
Hatched eggshells produced similar spectral characteristics as unhatched eggshells, with one exception. The dominant phase composition in the hatched eggshells was also calcite. However, the amount of the aragonite phase had a marked increase (Supplementary materials). In most of the spectra of the hatched eggshells, an unknown broad peak appeared at around 32°. This peak may result from a new phase produced during the last trimester of the hatching process. The mineral phase is probably CaCO3·6H2O, which has a peak at 32°. It may have resulted from a chemical reaction between calcite and the egg albumin contents during the hatching process, but this cannot be confi rmed by using XRD measurements only. Further investigation using other characterization techniques, such as X-ray photoelectron spectrum analysis or energy dispersive X-ray analysis may explain the nature of this broad phase seen in fi ve of six hatched eggshell specimens analysed.
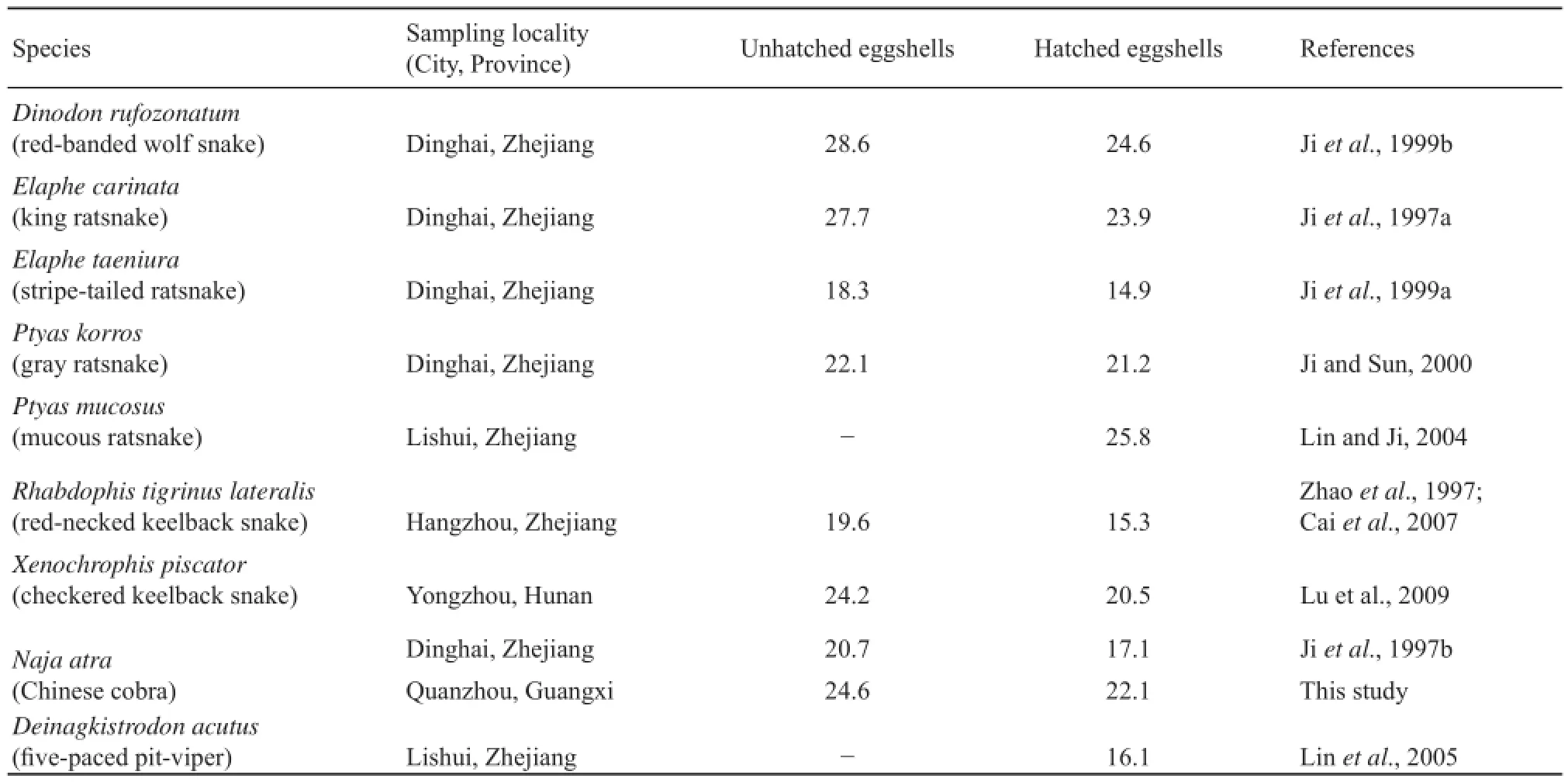
Table 1 Mean values for percentage of ash in unhatched and/or hatched eggshells of oviparous snakes studied in China thus far.
AcknowledgementsThe work was supported by grants from the National Natural Science Foundation of China (30370229 and 31272294), Priority Academic Program Development of Jiangsu Higher Education Institutions and High Academic Talent Foundation of Nanjing Forestry University (GXL201306). We thank Cheong-Hoong DIONG for arranging XRD analysis in Singapore, and Ling ZHANG for collecting and preparing eggshell specimens.
Andrews R. M., Mathies T.2000. Natural history of reptilian development: constraints on the evolution of viviparity. BioScience, 50: 227–238
Bustard H. R., Jenkins N. K., Simkiss K.1969. Some analyses of artificially incubated eggs and hatchlings of green and loggerhead sea turtles. J Zool, 158: 311−315
Cai Y., Zhou T., Ji X.2007. Embryonic growth and mobilization of energy and material in oviposited eggs of the red-necked keelback, Rhabdophis tigrinus lateralis. Comp Biochem Physiol A, 147: 57−63
Deeming D. C.1988. Eggshell structure of lizards of two subfamilies of the Gekkonidae. Herpetol J, 1: 230−234
Du W. G., Ji X., Xu W. Q.2001. Dynamics of material and energy during incubation in the soft-shelled turtle (Pelodiscus sinensis). Acta Zool Sin, 47: 371−375
Heulin B., Stewart J. R., Surget-Groba Y., Bellaus P., Jouan F., Lancien G., Deunff J.2005. Development of the uterine shell glands during the preovulatory and early gestation periods in oviparous and viviparous Lacerta vivipara. J Morphol, 266: 80−93
Jenkins N. K.1975. Chemical composition of the eggs of the crocodile (Crocodylus novaeguineae). Comp Biochem Physiol A, 51: 891−895
Ji X., Braña F.1999. The influence of thermal and hydric environments on incubating eggs and embryonic use of energy and nutrients in the wall lizard Podarcis muralis. Comp Biochem Physiol A, 124: 205−213
Ji X., Du W. G.2001. Effects of thermal and hydric conditions on incubating eggs and hatchling traits in the cobra, Naja naja atra. J Herpetol, 35: 186−194
Ji X., Sun P. Y.2000. Embryonic use of energy and post-hatching yolk in the gray rat snake, Ptyas korros (Colubridae). Herpetol J, 10: 13−17
Ji X., Sun P. Y., Fu S. Y., Zhang H. S.1997a. Utilization of energy and nutrients in incubating eggs and post-hatching yolk in a colubrid snake, Elaphe carinata. Herpetol J, 7: 7−12
Ji X., Sun P. Y., Fu S. Y., Zhang H. S.1999a. Utilization of egg energy and material during incubation and post-hatching yolk in a colubrid snake, Elaphe taeniura. Asiatic Herpetol Res, 8: 53−59
Ji X., Sun P. Y., Zhang H. S., Fu S. Y.1997b. Incubation and utilization of energy and material during embryonic development in eggs of Naja naja atra. J Herpetol, 31: 302−306
Ji X., Wang Z. W.2005. Geographic variation in reproductive traits and trade-offs between size and number of eggs of the Chinese cobra, Naja atra. Biol J Linn Soc, 85: 27−40
Ji X., Xu X. F., Lin Z. H.1999b. Influence of incubation temperature on characteristics of Dinodon rufozonatum (Reptilia: Colubridae) hatchlings, with comments on the function of residual yolk. Zool Res, 20: 342−346
Lin L. H., Li H., An H., Ji X.2008. Do temperature fl uctuations during incubation always play an important role in shaping the phenotype of hatchling reptiles? J Therm Biol, 33: 193−199
Lin Z. H., Ji X.2004. Reproductive output and effects of incubation thermal environments on hatchling phenotypes of
mucous ratsnake (Ptyas mucous). Acta Zool Sin, 50: 541−550
Lin Z. H., Ji X., Luo L. G., Ma X. M.2005. Incubation temperature affects hatching success, embryonic expenditure of energy and hatchling phenotypes of a prolonged egg-retaining snake, Deinagkistrodon acutus (Viperidae). J Therm Biol, 30: 289−297
Lu H. L., Hu R. B., Ji X.2009. Embryonic growth and mobilization of energy and material during incubation in the checkered keelback snake, Xenochrophis piscator. Comp Biochem Physiol A, 152: 214−218
Packard G. C., Packard M. J.1988. The physiological ecology of reptilian eggs and embryos. In Gans C., Huey R. B. (Eds.), Biology of the Reptilia, Vol. 16, Ecology B, Defense and Life History. New York: Alan R. Liss, 523–605
Packard M. J.1994. Patterns of mobilization and deposition of calcium in embryos of oviparous, amniotic vertebrates. Isr J Zool, 40: 481–492
Packard M. J., DeMarco V. G.1991. Eggshell structure and formation in eggs of oviparous reptiles. In Deeming D. C., Ferguson M. W. J. (Eds.), Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge: Cambridge University Press, 53–69
Packard M. J., Packard G. C.1984. Comparative aspects of calcium metabolism in embryonic reptiles and birds. In Seymour R. S. (Ed.), Respiration and Metabolism of Embryonic Vertebrates. Dordrecht, The Netherlands: W. Junk Publishing, 155–179
Packard M. J., Packard G. C.1988. Sources of calcium and phosphorus during embryogenesis in bullsnakes (Pituophis melanoleucus). J Exp Zool, 246: 132–138
Packard M. J., Packard G. C.1989. Mobilization of calcium, phosphorus, and magnesium by embryonic alligators (Alligator mississippiensis). Am J Physiol, 257: R1541–R1547
Packard M. J., Packard G. C., Gutzke W. H. N.1984. Calcium metabolism in embryos of the oviparous snake Coluber constrictor. J Exp Biol, 110: 99–112
Shine R.1983. Reptilian reproductive modes: the oviparityviviparity continuum. Herpetologica, 39: 1–8
Shadrix C. A., Crotzer D. R., MaKinney S. L., Stewart J. R.1994. Embryonic growth and calcium mobilization in oviposited eggs of the scincid lizard, Eumeces fasciatus. Copeia, 1994: 493−498
Stewart J. R., Ecay T. W.2010. Patterns of maternal provision and embryonic mobilization of calcium in oviparous and viviparous squamate reptiles. Herpetol Conserv Biol, 5: 341–359
Stewart J. R., Ecay T. W., Heulin B.2009. Calcium provision to oviparous and viviparous embryos of the reproductively bimodal lizard Lacerta (Zootoca) vivipara. J Exp Biol, 212: 2520–2524
Stewart J. R., Ecay T. W., Heulin B., Fregoso S. P., Linville B. J.2011. Developmental expression of calcium transport proteins in extraembryonic membranes of oviparous and viviparous Zootoca vivipara (Lacertilia, Lacertidae). J Exp Biol, 214: 2999–3004
Stewart J. R., Mathieson A. N., Ecay T. W. , Herbert J. F., Parker S. L., Thompson M. B.2010. Uterine and eggshell structure and histochemistry in a lizard with prolonged uterine egg retention (Lacertilia, Scincidae, Saiphos). J Morphol, 271: 1342–1351
Wüster W., Golay P., Warrell D. A.1997. Synopsis of recent developments in venomous snake systematics. Toxicon, 35: 319–340
Xu X. F., Wu Y. L., Zhang J. L.2004. Dynamics of material and energy during incubation in the grass lizards Takydromus septentrionalis. Acta Zool Sin, 50: 37–42
Zehr D. R.1962. Stages in the normal development of the common garter snake, Thamnophis sirtalis sirtalis. Copeia, 1962: 322–329
Zhao Q., Zhang J. Q., Huang H. Y., Ji X.1997. Utilization of egg energy and material by Rhabdophis tigrinus lateralis during incubation. J Hangzhou Normal Coll (Nat Sci), 14(3): 60−64
*Corresponding author: Prof. Xiang JI, from College of Life Sciences, Nanjing Normal University, Jiangsu, China, with his research focusing on physiological and evolutionary ecology of reptiles.
E-mail: xji@mail.hz.zj.cn
Received: 24 August 2014 Accepted: 2 December 2014
杂志排行
Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
- Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
- Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Body Size and Reproductive Tactics in Varanid lizards
