Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
2014-03-25*
*
1Department of Biology, Kangwon National University, Chuncheon, Kangwon 200-701, South Korea
2Division of Science Education, Kangwon National University, Chuncheon, Kangwon 200-701, South Korea
Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
Jun-Haeng HEO1#, Heon-Joo LEE1#, Il-Hun KIM1, Jonathan J. FONG2, Ja-Kyeong KIM1, Sumin JEONG1and Daesik PARK1*
1Department of Biology, Kangwon National University, Chuncheon, Kangwon 200-701, South Korea
2Division of Science Education, Kangwon National University, Chuncheon, Kangwon 200-701, South Korea
Introduction of an invasive prey species into an ecosystem may affect an endemic predator’s fitness by altering the prey-predator system. Successful adaptation may allow the endemic predator to eat and control the invasive species, while unsuccessful adaptation may result in extinction of the predator. We examine the possible effects of the invasive North American bullfrog (Rana [Lithobates] catesbeiana) on the endemic Red-backed rat snake (Oocatochus rufodorsatus) in South Korea. We do so by comparing the morphology and behavior of adult and hatchling snakes from bullfrog-exposed (Taean) and bullfrog-unexposed (Hongcheon) populations. Among the seven morphological characteristics investigated, relative tail length (tail length/snout-vent length) of both adults and hatchlings from Taean was significantly greater than that of adults and hatchlings from Hongcheon. Also, adult snakes from Taean had a signifi cantly shorter latency of fi rst tongue fl ick in response to prey compared to adults from Hongcheon. This difference was not observed in hatchlings. In other snake species, a longer relative tail length and shorter latency of fi rst tongue fl ick are known to improve foraging effi ciency, and these characters may be adaptations of O. rufodorsatus to prey on bullfrogs. This study provides preliminary evidence that the presence of an invasive prey species may cause morphological and behavioral changes in an endemic predator.
invasive prey, bullfrog, Rana catesbeiana, Oocatochus rufodorsatus, predator response
1. Introduction
Invasive species can disrupt an ecosystem (Gurevitch and Padilla, 2004; Strauss et al., 2006; Vellend et al., 2007; Strayer, 2012) by altering the prey-predator system (Carlsson et al., 2009; Anson et al., 2013). Often, endemic prey are more affected by an altered prey-predator system than endemic predators (Lima, 2002; Johnson and Agrawal, 2003; Nuismer and Thompson, 2006). However, invasive prey can also affect endemic predators (Phillips and Shine, 2004, 2006; De Rivera et al., 2005; King et al., 2006; Langkilde, 2009; Wanger et al., 2011; Harley et al., 2013; Llewelyn et al., 2013).
Invasive prey species may directly or indirectly decrease the fitness of endemic predators, such as by altering prey composition (Suarez and Case, 2002; Pothoven and Madenjian, 2008; Heinonen and Auster, 2012). As a response, morphological and behavioral changes due to developmental phenotypic plasticity and/ or evolutionary adaptation can appear in the endemic predators (Mittelbach et al., 1999; Aubret et al., 2004; Phillips and Shine, 2004; Langkilde, 2009; Li et al., 2011a; Wanger et al., 2011). Populations of successfully adapted predators often decrease in the early stages of a prey’s invasion, but soon recover to stable levels and play a key role in the long-term control of the invasive prey species (Brodie and Brodie, 1999; Phillips and Shine, 2004; Kishida et al., 2006). On the contrary, populations of unsuccessful endemic predators continuously decline and are often extirpated (Case and Bolger, 1991).
Bullfrogs (Rana [Lithobates] catesbeiana), native
to North America, are now found in more than 45 countries worldwide. Due to its negative impacts in introduced areas, R. catesbeiana was designated one of the 100 worst invasive species by IUCN (Lowe et al., 2000). R. catesbeiana often causes the decline of endemic insects and frogs directly through predation or indirectly through foraging competition (Kang and Youn, 1994; Kiesecker et al., 2001; Hirai, 2004; Wu et al., 2005; Wang et al., 2007; Da Silva and Filho, 2009; Ra et al., 2010; Li et al., 2011a, 2011b; Jancowski and Orchard, 2013). Such effects result in altered prey-predator systems.
In Korea, R. catesbeiana was first introduced from Japan for commercial purposes in the early 1970s and dispersed to most parts of South Korea in the mid-1990s (Kim, 1972; Kang and Youn, 1994; Choi et al., 1998; Kim and Ko, 1998). R. catesbeiana has been observed feeding on insects, amphibians, fi sh, and juvenile snakes in freshwater habitats (Kim and Ko, 1998; Chung, 2005; Oh and Hong, 2007). The distribution of R. catesbeiana in South Korea was frequently monitored (Kim and Ko, 1998; Jang and Suh, 2010; Figure 1). The Red-backed rat snake, Oocatochus rufodorsatus, is a predator of R. catesbeiana and provides a good opportunity to study the effects of prey introduction on an endemic predator because they share the same habitat (Sung et al., 2006). A negative correlation between the presence of Pelophylax nigromaculatus, a prey item of O. rufodorsatus, and R. catesbeiana (Ra et al., 2010) provides evidence that R. catesbeiana is altering the prey-predator system. In this study, we compared morphological characteristics and prey response behaviors of adult and hatchling O. rufodorsatus from bullfrog-exposed and unexposed populations in South Korea to study the effect of an altered prey-predator system.
2. Materials and Methods
2.1 Collection and housingAnimal handling and experimental procedures were conducted in accordance with guidelines established by the Kangwon National University Institutional Animal Care and Use Committee. For the bullfrog-exposed populations, we selected two sites in Taean County, Chungcheongnam Province, South Korea (populations hereafter referred to as “Taean”), where R. catesbeiana were introduced before 1994 and are currently found (Kim and Ko, 1998; Jang and Suh, 2010; Jang et al., 2011; Figure 1). The two sites in Taean are approximately 40 km apart in Wonbuk (Taean 1) (36°49′17.15" N, 126°14′40.44" E) and Anmyeon (Taean 2) (36°28′53.37" N, 126°20′0.42" E) (Figure 1).
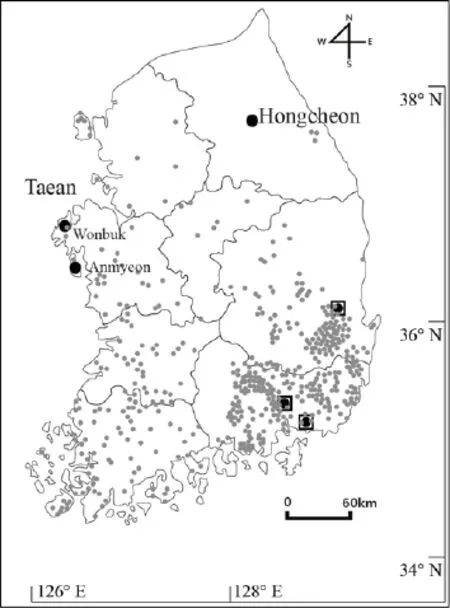
Figure 1 Locations (black filled circles) of sites where adult Oocatochus rufodorsatus were collected. The frogs used as prey items, Rana (Lithobates) catesbeiana and Pelophylax nigromaculatus, were collected from the same sites. Distribution of R. catesbeiana in South Korea reported in 2010 (Jang and Suh, 2010), represented by gray dots. Three locations where R. catesbeiana was fi rst introduced are indicated by black fi lled circles surrounded by a square.
For the bullfrog-unexposed population, one site was selected in Hongcheon County, Gangwon Province, South Korea (population hereafter referred to as “Hongcheon”) (37°28′53.37" N, 128°04′33.67" E), where there are neither historical nor modern records of R. catesbeiana (Kim and Ko, 1998; Suh, 2005; Jang and Suh, 2010; Jang et al., 2011; Figure 1).
All sampling was conducted in July of 2013. At Taean 1, we collected 9 adult snakes (9 females) from an unused rice paddy with an agricultural pond. This rice paddy had other rice paddies on one side and low mountains on the other three sides. The size of the paddy was 2188 m2and the nearest rural town (> 5 houses) was 800 m away. During sampling, we observed one O. rufodorsatus foraging for R. catesbeiana. At Taean 2, we collected 15 adult snakes (10 females, 5 males). The habitat consisted of an agricultural pond adjoined with rice paddies on one
side and pine tree windbreaks on the other three sides. The size of the pond was 1732 m2and approximately half of the perimeter was occupied by fully-grown Oriental cattails (Typha orientalis). The nearest rural town was 630 m away. Although we did not observe R. catesbeiana during our sampling they have been found in neighboring areas (< 3 km) (Jang et al., 2011). At Hongcheon, we collected 22 adult snakes (15 females, 7 males). This site consisted of an agricultural pond adjoined to rice paddies on two sides and low mountains on the other two sides. The size of the pond was 1675 m2and most of the perimeter was occupied by T. orientalis. The nearest rural town was 170 m away. During the sampling, we did not observe any R. catesbeiana.
After capture, adult snakes were immediately transported to the laboratory at Kangwon University (37°52′06.06" N, 127°44′33.32" E; 180 km away from Taean and 30 km away from Hongcheon). Individuals were marked by inserting a passive integrated transponder tag (TX1411L, Biomark, Boise, Idaho, USA) under the skin. All snakes were individually kept in plastic boxes (39 cm × 26 cm × 17 cm) and fed one mouse (1.4 g –2.3 g) every two weeks. A bowl with 200 ml of water was placed inside the box and changed every two days. For shelter and effective skin shedding, paper towels and a plastic T-shape pipe were put inside the box. Temperature and light–dark cycles were kept at ambient levels throughout the study. After completing behavioral experiments, adult and hatchling snakes were all released at their respective collection sites after health examination based on skin color, body condition, and locomotion.
2.2 Morphological characteristics of adult snakesBefore conducting experiments, we measured seven morphological characteristics of 22 adult snakes (18 females and 4 males) from Taean and 21 (15 females and 6 males) from Hongcheon; three snakes with partially damaged tails were excluded (1 female/1 male from Taean, 1 male from Hongcheon). We measured the snoutvent length (SVL; tip of the snout to the posterior of the vent), tail length (TAL; posterior of the vent to the tip of the tail) to the nearest 0.1 cm using a measuring tape and the body weight (BW) to the nearest 0.1 g using a digital balance (SI-132, Excel Precision Co., New Taipei City, the People’s Republic of China). From these data, we calculated the relative tail length (RTL) by dividing the TAL by SVL. In addition, we measured the head length (HL; tip of the snout to the quadrate-articular jaw joint), head width (HW; at the widest point of the head), chin length (CL; tip of low jaw to the line which connects two points of the most posterior end of the mouth) and chin width (CW; width between the two points of the most posterior end of the mouth) to the nearest 0.01 mm using digital vernier calipers (IP66, Mitutoyo, Kawasaki, Japan). Using the data of CL and CW, we calculated the gape index (GI) of each snake following GI = π(CL) (CW)/4 (King, 2002).
2.3 Adult snake response to frog prey
2.3.1 Preparing preyThree different frog prey were used for trials: 1) metamorphosed R. catesbeiana from Taean 1, 2) P. nigromaculatus from Taean 1, and 3) P. nigromaculatus from Hongcheon. All prey items were caught in rice paddies near where the adult snakes were collected. The frogs were transported to the laboratory and kept at a density of 20 individuals in aquaria (53 cm × 40 cm × 28 cm) that had both aquatic and terrestrial parts. For food, we provided three crickets per frog once every three days. To facilitate successful prey foraging of adult snakes, only recently metamorphosed frogs (~2 weeks) were used.
2.3.2 Experimental proceduresExperiments were conducted with adult O. rufodorsatus, 16 (12 females, 4 males) from Taean and 16 (11 females, 5 males) from Hongcheon between 10:00 and 19:00 from August 6 to 11, 2013. We presented pithed, unconscious frogs to the snakes. None of the frogs were recovered during the experiment and the uneaten frogs were fi nally sacrifi ced by guillotine.
To acclimate the snakes, each individual was placed in an opaque experimental box (30 cm × 30 cm × 30 cm) 1 hour prior to starting the trial. During the experiments, light and temperature followed local, ambient conditions. All behaviors were recorded using a digital video camcorder (DCR-SR65, Sony, Tokyo, Japan) positioned 30 cm above the arena. We presented a frog in the center of the box using forceps (30.5 cm long), and following previous studies (Cooper and Burghardt, 1990; Labra et al., 2001), the trial was complete when the snake ate the frog or after 10 min. The time taken to eat the prey was measured from the snake’s fi rst touch to completely swallowing the frog. The snake and prey type for each trial were randomly assigned using an online random number generator (http://www.random.org/). Each snake was exposed to each type of prey once, and was given two days rest between trials. After each experiment, the experimental boxes were washed with hot water and dried before using again.
2.4 Hatchling snake response to frog odors
2.4.1 Obtaining hatchlings and preparing frog odorsAfter the prey response experiments, female adult snakes
were individually kept in plastic boxes (39 cm × 26 cm × 17 cm) and fed one mouse once a week. Most females gave birth between August 8 and 20. The clutch size of the 13 pregnant females (7 in Taean, 6 in Hongcheon) was 9.6 ± 3.3 SD (range: 5–14). We kept each clutch in separate aquaria (45 cm × 32 cm × 35 cm), placing paper towels and a T-shaped plastic pipe for shelter. We marked each hatchling with a number on the dorsal plate. The birth day and the day of skin shedding were recorded for each individual. After the prey odor experiments, we measured the SVL, BW, TAL, and RTL of the hatchlings using the same method for adult snakes. Since the hatchlings were immature (7– 10 days old), we could not determine their sex.
In these experiments, we used prey odor instead of frogs, as hatchling O. rufodorsatus have a smaller gape than the body size of metamorphosed R. catesbeiana. To prepare prey odors, individuals of the three prey types (R. catesbeiana Taean, P. nigromaculatus Taean, P. nigromaculatus Hongcheon) were weighed, pithed and sacrificed using a guillotine, and cut into small pieces using scissors. To prevent possible contamination of odor sources, we did not use euthanizing chemicals. The pieces were ground using a mortar and pestle, adding 100 ml distilled water per 10 g BW of the frog. The solution was centrifuged for 5 min at 3500 rpm (NF-80, Hanil Science Co., Seoul, South Korea). The supernatant was decanted into 50 ml tubes and preserved at –20oC until use. As a control odor, we used distilled water.
2.4.2 Experimental proceduresTo know if exposure to invasive R. catesbeiana affects the response of newborn O. rufodorsatus, prey odors were sequentially presented to the hatchlings. The experiment was conducted with each of the 25 O. rufodorsatus hatchlings from each five adult females from both Taean and Hongcheon, between 10:00 and 19:00 from August 17 to 28, 2013. As five individuals was the smallest clutch size, we arbitrarily selected five hatchlings from each female for experimentation. At approximately 7 days old, O. rufodorsatus hatchlings show tongue-flicking behavior towards prey odors (personal observation), so we used only 7–10 day old hatchlings for this experiment.
During experiments, light and temperature followed ambient conditions. We recorded all behaviors with a digital video camcorder. For acclimation, each hatchling was placed in an opaque experimental box (10 cm × 10 cm × 10 cm) 1 hour prior to starting each trial. We presented gauze (2 cm × 2 cm) soaked with 1.5 ml frog odor solution in the center of the box using forceps and the trial was considered complete after 10 min. The frog odor type and individual hatchling used were randomly assigned for each trial. As in previous olfactory tests of reptiles (Cooper and Burghardt, 1990; Labra and Niemeyer, 1999), only one odor type was presented per day. After each experiment, the boxes were washed with hot water and dried before using again.
2.5 Data analyses
2.5.1 Comparison of the morphological characteristics of adult snakesSVL, BW, TAL, RTL, HL, HW, and GI of adult O. rufodorsatus were compared using a General Linear Model (GLM). In the analysis, we used the locality as the independent variable, sex as a covariable, and the seven morphological characteristics as dependent variables. In each analysis, we assessed the interaction of locality * sex. In addition, we compared the morphological characteristics of the adult female snakes caught from the Taean 1 (8 females) and Taean 2 (10 females) using an independent t-test or Mann-Whitney U test based on the normal distribution of the data (Shapiro-Wilk test). Since males were only collected from Taean 2, we could not perform any comparison between males.
2.5.2 Adult snake response to frog preyThe number of adult O. rufodorsatus that responded by tongue fl icking, touching prey, and foraging prey were compared between Taean and Hongcheon using a Chi-square test (Preacher, 2001) and between each pair of prey types within each population using the Fisher exact test (Preacher and Briggs, 2001). The significance of the Fisher exact test was set at P = 0.017 after Bonferroni correction (Zar, 1999). The prey response of adult O. rufodorsatus was analyzed using a GLM. We used locality, sex, and prey type as independent variables, SVL and BW as covariables, and the latency of the fi rst tongue fl ick, time to the first touch of prey, the number of tongue flicks within the fi rst 1 min after the fi rst tongue fl ick, number of tongue flicks during the 10 min experiment, and the time taken to eat a prey item from the fi rst touch of prey as dependent variables (Cowles and Phelan, 1958; Cooper and Burghardt, 1990; Labra et al., 2001; Saviola et al., 2011). In each analysis, we also assessed the interactions of locality * sex, locality * prey type, sex * prey type, and locality * sex * prey type.
When the prey type signifi cantly affected the dependent variables, a Turkey post-hoc test in the GLM was applied to compare the differences between each pair of prey type. Relationships between the SVL and BW of frog prey and the snake’s response variables were analyzed using a Spearman correlation. We did not compare the responses of the two Taean populations because the sample sizes were too small.
2.5.3 Hatchling snake response to frog odorsSVL, BW, TAL, RTL, and age of hatchlings were compared between Taean and Hongcheon using an independent t-test or Mann-Whitney U test based on the normal distribution of the data (Shapiro-Wilk test). Due to the small sample size (n = 5), the characteristics of hatchlings’ mothers were only compared between Taean and Hongcheon using the Mann-Whitney U test.
The number of the O. rufodorsatus hatchlings that responded by tongue flicking and touching prey odors were compared between Taean and Hongcheon using a Chi-square test (Preacher, 2001) and between each pair of odor type within each population using the Fisher exact test (Preacher and Briggs, 2001). The signifi cance of the Fisher exact test was determined at P = 0.008 after Bonferroni correction (Zar, 1999).
The responses of the O. rufodorsatus hatchlings to prey odors were analyzed using a GLM. We used the locality and the prey odor type as independent variables, the SVL and BW of the hatchlings and their ages as covariables, and the latency to the first tongue flick, time to the fi rst touch of prey and the number of tongue fl icks during 10 min as dependent variables. In each analysis, we also assessed the interaction of locality * prey odor type.
We performed the Chi-square test and Fisher exact test online (http://www.quantpsy.org; Preacher, 2001; Preacher and Briggs, 2001). For the remaining analyses, we used software SPSS v. 20.0 (SPSS Inc., Chicago, IL, USA) . All data are presented as mean ± SE.
3. Results
3.1 Comparison of the morphological characteristics of adult snakesRTL of adult snakes was signifi cantly greater in Taean than Hongcheon (F1,43=14.97, P < 0.01; Table 1; Figure 2A). The remaining six morphological characteristics were not statistically different between Taean and Hongcheon (P > 0.05 for all cases; Table 1). Comparing males and females, all morphological characteristics except TAL (P = 0.97) were signifi cantly different between the sexes (P < 0.01 for all cases; Table 1). RTL was greater in males than that of females, but the remaining fi ve characteristics were greater in females than in males (Table 1). None of the characteristics were signifi cantly different between the two Taean populations (P > 0.05 for all cases; data not shown).
3.2 Adult snake response to frog preyThe number of adults that responded by tongue fl icking, touching prey, and eating prey was not significantly different between Taean and Hongcheon (Chi-square test, P > 0.05 for all cases; Table 2) and between each pair of prey type within each population (Fisher exact test, P > 0.017 for all cases; Table 2).
Adults from Taean had a signifi cantly shorter latency to the fi rst tongue fl ick than adults from Hongcheon (F1,69= 8.88, P = 0.004; Figure 3), but the latency time was not different between sexes (F1,69= 0.32, P = 0.58) and among different prey types (F2,69= 0.29, P = 0.75). None of the interactions between locality-sex, locality-prey type, sexprey type, and locality-sex-prey type were signifi cant (P >0.05 for all cases). The number of tongue fl icks within the fi rst 1 min, during the fi rst 10 min, and the time to the fi rst touch of the prey were not signifi cantly different between Taean and Hongcheon (P = 0.78, P = 0.13, and P = 0.76, respectively), between the sexes (P = 0.41, P = 0.14, and P = 0.37, respectively), and among different prey types (P = 0.63, P = 0.31, and P = 0.13, respectively). None of the interactions between variables were signifi cant (P> 0.05 for all cases). The time taken to eat prey was not different between Taean and Hongcheon (F1,45= 0.04, P = 0.84) and between the sexes (F1,45= 0.53, P = 0.47), but was different among different prey types (F2,45= 6.66, P = 0.004). In particular, snakes took more time to eat R. catesbeiana than P. nigromaculatus from both Taean (P< 0.01) and Hongcheon (P = 0.008), but the time to eat P. nigromaculatus from Taean and Hongcheon (P = 0.32). The SVL and BW of adult snakes were not signifi cantly correlated with any of the response measurements (P >0.05 for all cases).
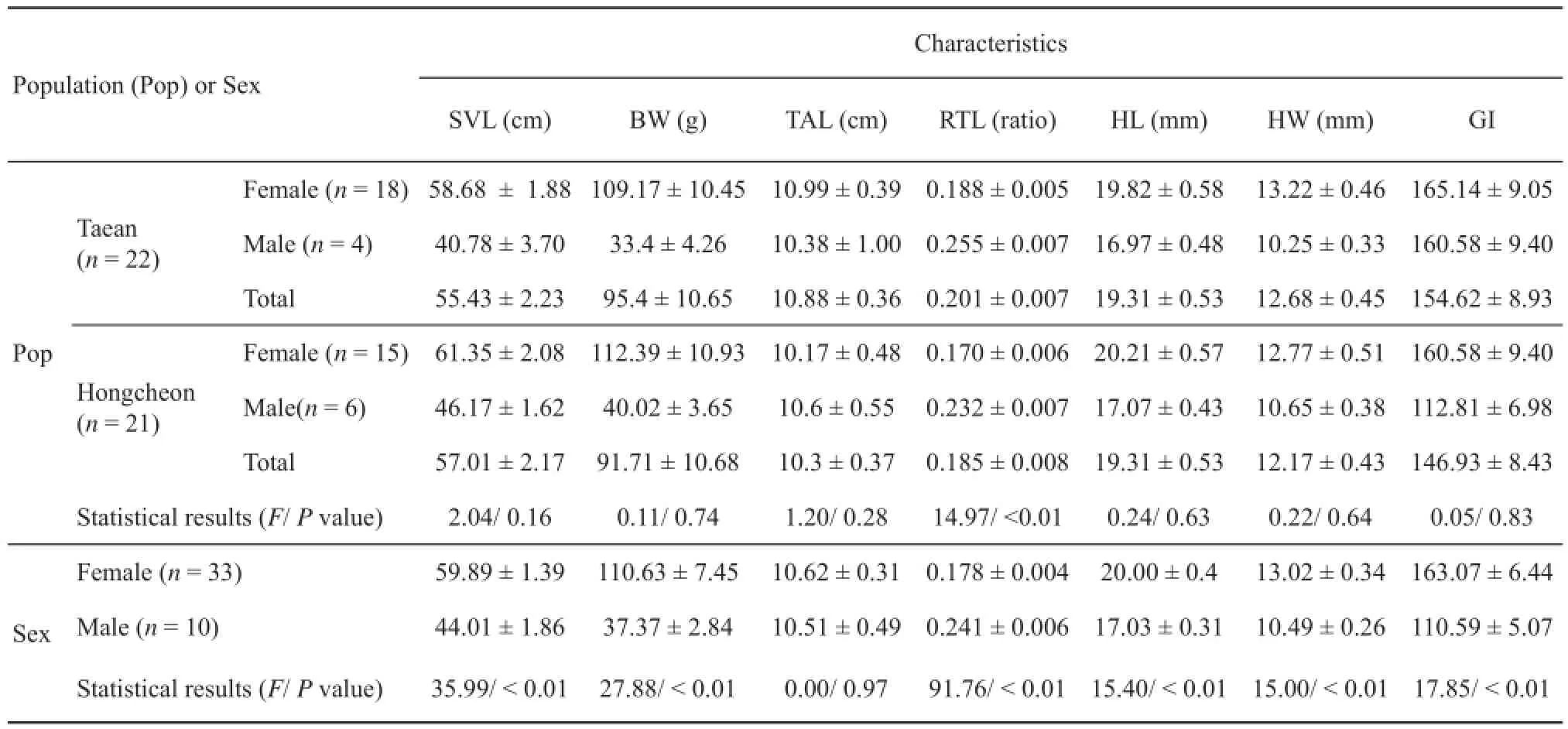
Table 1 Morphological characteristics of adult Oocatochus rufodorsatus caught from bullfrog-exposed populations at Taean and bullfrogunexposed population at Hongcheon. Data are presented as mean ± SE based on the populations and the sex. Gape index (GI) = π (chin length) (chin width) /4. SVL, snout-vent length; BW, body weight; TAL, tail length; RTL, relative tail length (TAL/ SVL); HL, head length; HW, head width.

Table 2 The number of adult Oocatochus rufodorsatus from bullfrog-exposed populations at Taean and bullfrog-unexposed population at Hongcheon and their hatchlings that responded in each response measurements to frog prey and to frog prey odors, respectively. CatTA: Rana (Lithobates) catesbeiana or their odors from Taean; NigTA and NigHC: Pelophylax nigromaculatus or their odors from Taean and Hongcheon; DH2O: distilled water-used as control odor.
The SVL and BW of the prey were positively correlated with the number of tongue flicks during the fi rst 10 min (r = 0.263, P = 0.029 for the SVL; r = 0.265, P = 0.028 for the BW) and the time taken to eat prey (r = 0.609, P = 0.004 for the SVL; r = 0.642, P = 0.002 for the BW), but were not correlated with the time to the fi rst tongue flick and the number of tongue flicks within the fi rst 1 min (P > 0.05 for the cases).
3.3 Hatchling snake response to frog odorsTAL and RTL of hatchlings from Taean were greater than that from Hongcheon (TAL: t = 2.58, df = 48, P = 0.013; RTL: U = 153.5, P = 0.002; Table 3, Figure 2B), but the SVL, BW, and age were not different (P > 0.05 for the cases, Table 3). Comparing between hatchlings’ mothers, RTL was greater in Taean than Hongcheon (U = 1.00, P = 0.016), but the SVL, BW, TAL and GI were not different (P >0.05 for the cases, Table 3).
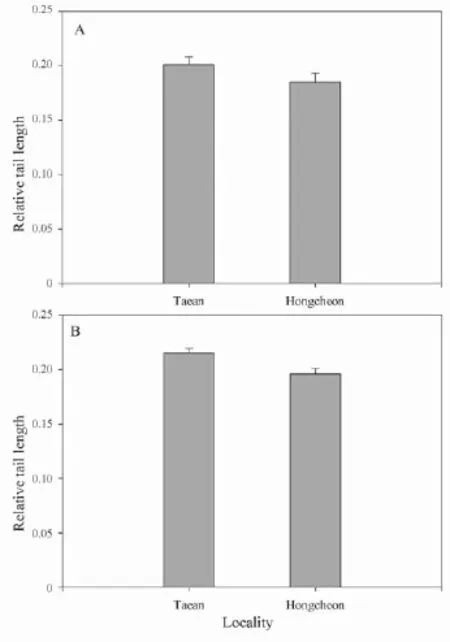
Figure 2 Relative tail length (tail length/snout-vent length) of (A) adult female Oocatochus rufodorsatus and (B) hatchlings used in the study. Females and hatchlings from bullfrog-exposed populations in Taean had a greater relative tail length than those from bullfrog-unexposed population in Hongcheon (P < 0.01 for females; P = 0.002 for hatchlings).
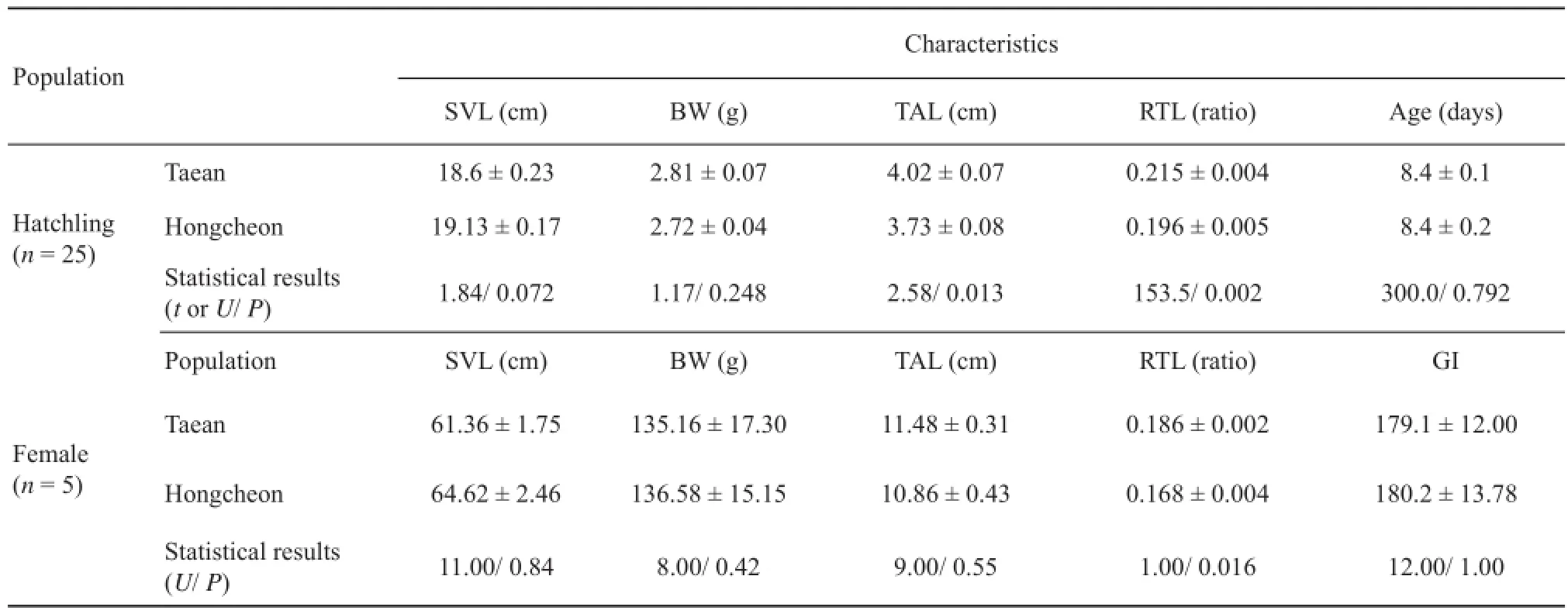
Table 3 Morphological characteristics of hatchling Oocatochus rufodorsatus used in the behavioral experiment and their mothers caught from bullfrog-exposed populations at Taean and bullfrog-unexposed population at Hongcheon. Data are presented as mean ± SE. GI (Gape index) = π(chin length) (chin width) /4. SVL, snout-vent length; BW, body weight; TAL, tail length; RTL, relative tail length (TAL/ SVL).
The number of hatchlings that responded by tongue flicking and touching odor gauze was not different between the two populations (Chi-square test, P > 0.05 for all cases) and between each pair of prey odor types (Fisher exact test, P > 0.008 for all cases; Table 2).
The latency time to the first tongue flick, time to the first touch of prey odor gauze, and number of tongue flicks during 10 min were not different between hatchlings from Taean and Hongcheon (P = 0.53, P = 0.10, P = 0.99, respectively) and among different prey odor types (P = 0.33, P = 0.38, P = 0.17, respectively). None of the interactions between locality and odor type were signifi cant (P > 0.05 for all cases).
4. Discussion
Adult and hatchling O. rufodorsatus from bullfrogexposed populations in Taean had a signifi cantly greater relative tail length (RTL) than those from bullfrogunexposed population in Hongcheon. In addition, adult snakes from Taean responded more rapidly to prey with tongue flicking compared to Hongcheon. These differences tentatively demonstrate that invasive R. catesbeiana affect the morphology and behavior of endemic predator snakes. In the case of O. rufodorsatus, both of these changes may be adaptations to increased foraging effi ciency. Discussion of both characters follows below.
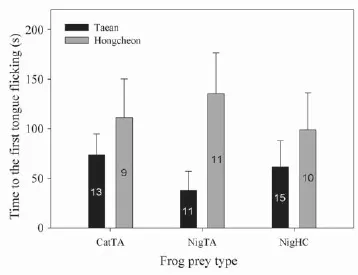
Figure 3 Comparison of time to the first tongue flick of adult Oocatochus rufodorsatus from bullfrog-exposed populations in Taean and bullfrog-unexposed population in Hongcheon. Experiments were run with three different prey types: Rana (Lithobates) catesbeiana from Taean (CatTA), Pelophylax nigromaculatus from Taean (NigTA), and P. nigromaculatus from Hongcheon (NigHC). Snakes from Taean always had faster times compared to Hongcheon (P = 0.004). The numbers on the bars are the number of the snakes which responded to tongue fl icking of the frogs out of a total of 16 snakes investigated.
A snake’s tail causes forward propulsive forces and balances the body during movement, and the loss of tail tip significantly decreased the burst speed of snakes in aquatic habitats (Aubret et al., 2005). In our study, adult snakes from Taean had a greater RTL than those from Hongcheon. RTL is probably heritable as female snakes in our study with greater RTL gave birth to hatchlings with a greater RTL. We propose three main explanations for the greater RTL in Taean: 1) an adaptation for prey capture (Clark, 1966; King, 1986)—bullfrogs are more agile and common than endemic frogs in Taean, 2) an adaptation
to habitat (Clark, 1966; King, 1986)—aquatic habitats are more common in Taean, or 3) pre-existing geographic variation between Taean and Hongcheon before bullfrog introductions. When comparing the two adaptation explanations, we believe RTL variation due to habitat is less likely than prey capture. Although the absolute and percentage area of marsh habitat was greater in Taean County (23 978 055 m2, 4.6% of total county area; Taean, 2013) than Hongcheon County (1 597 040 m2, 0.9% of total county area; Hongcheon, 2012), the time an individual O. rufodorsatus is exposed to aquatic habitats should be similar across sites because except hibernating on land in winter, O. rufodorsatus stays in or near aquatic habitats (Lee et al., 2011). Our results suggest that a greater RTL in Taean improves foraging efficiency of preying upon frogs and freshwater fi sh (Kang and Yoon, 1975; Ji et al., 1997), through better burst locomotion. To evaluate this preliminary finding and contrast it against the alternative of pre-existing intraspecific variation, a time-series comparison of historical specimens is still needed.
Rapid response to prey in adult snakes from bullfrogexposed populations at Taean might also increase foraging effi ciency. Snakes smell by fl icking their tongue to deliver airborne odors to the vomeronasal organ (Halpern, 1992; Cooper, 1994). Snakes that have an earlier as opposed to late tongue flicking response can detect odor sources sooner (Cowles and Phelan, 1958) and at farther distance (Burghardt and Abeshaheen, 1971). In our study, adult snakes from Taean had a significantly shorter latency to the first tongue flick than those from Hongcheon, regardless of prey type. We propose two hypotheses for the shorter latency in Taean populations. First, a shorter latency could be due to increased motivation to capture prey. This phenomenon was observed in the lizard Liolaemus bellii and explained by decreased prey availability (Labra et al., 2001). The same phenomenon may be possible in O. rufodorsatus if endemic prey (frogs and fish) are reduced by bullfrogs (Wu et al., 2005; Wang et al., 2007; Ra et al., 2010; Da Silva et al., 2011; Jancowski and Orchard 2013). Second, a changed preference for prey may infl uence latency. In China, the Red-banded snake (Dinodon rufozonatum) preferred the introduced bullfrogs over native frog species because bullfrogs showed less avoidance behaviors (Li et al., 2011a). Data supporting each hypothesis should differ—if the shorter latency to fi rst tongue fl ick is due to a shift in prey preference, we expect O. rufodorsatus response time to differ for R. catesbeianus and P. nigromaculatus.
We did not find a difference in response to the two prey species, making the second explanation less likely. However it is still possible that a common, fast response to different prey may be learned. Our results suggest that bullfrog presence has influenced the foraging behavior of snakes from Taean. The faster reaction of individuals to prey in Taean may increase foraging success, allowing them to detect frog prey faster and at farther distances.
Unlike adult O. rufodorsatus, hatchlings did not show any difference in prey odor response. These results might be explained in four ways. First, frog odor sources used might be less stimulating than frog prey, resulting in less distinctive olfactory responses. It is possible that some of airborne odor stimulus might be lost during odor preparing process. Second, as shown in the study of Eastern indigo snake (Drymarchon couperi) (Saviola et al., 2011), both olfactory and visual cues are needed. Third, hatchlings used in this study may be still undergoing olfactory developments. In general, olfactory development in hatchling snakes are modulated over time based on early odor exposure and genetically determined factors (Mushinsky and Lotz, 1980). Finally, frogs may not be a major prey of O. rufodorsatus hatchlings, resulting in low responses to frog odors. As far as we know, detailed diets of the hatchlings have not been studied.
In summary, our results suggest that invasive R. catesbeiana can affect the morphological characteristics and behaviors of the endemic predator O. rufodorsatus. When we compared bullfrog-exposed and unexposed populations, we found signifi cant differences in characters that may increase foraging effi ciency—relative tail length and time to fi rst tongue fl ick. Studies of more populations, O. rufodorsatus prey items, and time series comparisons of characters during the history of bullfrog-exposed and unexposed populations are needed to elucidate the full effect of invasive prey species on endemic predators.
Anson J. R., Dickman C. R., Boonstra R., Jessop T. S.2013. Stress triangle: Do introduced predators exert indirect costs on native predators and prey? PLoS ONE, 8: e60916.doi:10.1371/ journal.pone.0060916
Aubret F., Bonnet X., Maumelat S.2005. Tail loss, body condition and swimming performances in Tiger snakes, Notechis ater occidentalis. J Exp Zool, 303A: 894–903
Aubret F., Shine R., Bonnet X.2004. Evolutionary biology: adaptive developmental plasticity in snakes. Nature, 431: 261–262
Brodie III E. D., Brodie Jr.E. D.1999. Costs of exploiting poisonous prey: Evolutionary trade-offs in a predator–prey arms race. Evolution, 53: 626–631
Burghardt G. M., Abeshaheen J. P.1971. Response to chemical stimuli of prey in newly hatched snakes of the genus Elaphe. Anim Behav, 19: 486–489
Carlsson N. O., Sarnelle O., Strayer D. L.2009. Native predators and exotic prey–an acquired taste? Front Ecol Environ, 7: 525–532
Case T. J., Bolger D. T.1991. The role of introduced species in shaping the distribution and abundance of island reptiles. Evol Ecol, 5: 272–290
Choi D. S., Ko S. K., Chung H. H.1998. Report on the use of and on the ecological characteristics and capture of American Bullfrogs. Gwacheon, South Korea: Ministry of Environment of Korea (In Korean)
Chung M. H.2005. Factors involved in the decline of the Bullfrogs, Rana catesbeiana. Gwacheon, South Korea: Ministry of Environment of Korea (In Korean)
Clark Jr D. R.1966. Notes on sexual dimorphism in tail-length in American snakes. Trans Kans Acad Sci, 69: 226–232
Cooper Jr W. E., Burghardt G. M.1990. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J Chem Ecol, 16: 45–65
Cooper Jr W. E.1994. Chemical discrimination by tongueflicking in lizards: A review with hypothesis on its origin and its ecological and phylogenetic relationships. J Chem Ecol, 20: 439–487
Cowles R. B., Phelan R. L.1958. Olfaction in rattlesnakes. Copeia, 1958: 77–83
Da Silva E. T., Filho O. P. R.2009. Predation on juveniles of the invasive American Bullfrog Lithobates catesbeianus (Anura, Ranidae) by native frog and snake species in South-eastern Brazil. Herpetol Notes, 2: 215–218
Da Silva E. T, Filho O. P. R., Feio R. N.2011. Predation of native anurans by invasive Bullfrogs in Southeastern Brazil: Spatial variation and effect of microhabitat use by prey. S Amer J Herpetol, 6: 1–10
De Rivera C. E., Ruiz G. M., Hines A. H., Jivoff P.2005. Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology, 86: 3364–3376
Gurevitch J., Padilla D. K.2004. Are invasive species a major cause of extinctions? Trends Ecol Evol, 19: 470–474
Halpern M.1992. Nasal chemical senses in reptiles: structure and function. pp. 423–523 In Gans C., Crews D. (Eds.), Biology of the Reptilia, Vol. 18, Physiology E: Hormones, Brain, and Behavior. Chicago: University of Chicago Press, 423–523
Harley C. D. G., Anderson K. M., Lebreton C. A. M., MacKay A., Ayala-Díz M., Chong S. L., Pond L. M., Amerongen Maddison J. H., Hung B. H. C., Iverson S. L., Wong D. C. M.2013. The introduction of Littorina littorea to British Columbia, Canada: potential impacts and the importance of biotic resistance by native predators. Mar Biol, 160: 1529–1541
Heinonen K. B., Auster P. J.2012. Prey selection in crustaceaneating fishes following the invasion of crab Hemigrapsus sanguineus in a marine temperate community. J Exp Mar Biol Ecol, 413: 177–183
Hirai T.2004. Diet composition of introduced bullfrog Rana catesbeiana the Mizorogaike pond of Kyoto, Japan. Ecol Res, 19: 375–380
Hongcheon.2012. Hongcheon statistical yearbook. Hongcheon County, Kangwon Province, South Korea; Hongcheon County (In Korean)
Jancowski K., Orchard S. A.2013. Stomach contents from invasive American Bullfrogs Rana catesbeianus (Lithobates catesbeianus) on southern Vancouver island, British Columbia, Canada. Neo Biota, 16: 17–37
Jang H. J., Suh J. H.2010. Distribution of amphibian species in South Korea. Korean J Herpetol, 2: 45–51 (In Korean)
Jang M. H., Koo K. S., Song J. Y.2011. The list of amphibian species in 66 islands in Korea. Korean J Herpetol, 3: 19–24 (In Korean)
Ji X., Xie Y. Y., Sun P. Y., Zheng X. Z.1997.Sexual dimorphism and female reproduction in a viviparous snake, Elaphe rufodorsata. J Herpetol, 31: 420–422
Johnson M. T. J., Agrawal A. A.2003.The ecological play of predator-prey dynamics in an evolutionary theatre. Trends Ecol Evol, 18: 549–591
Kang Y. S., Yoon I. B.1975. Illustrated encyclopedia of fauna and fl ora of Korea, Vol.17, Amphibia-Reptilia. Seoul, South Korea: Samhwa Publisher (In Korean)
Kang E. J., Youn C. H.1994.The settlement and distribution of the introduced Bullfrog, Rana catesbeianus, in Korea. Korean Assoc Conserv Nat, 13: 231–250 (In Korean)
Kiesecker J. M., Blaustein A. R., Miller C. L.2001. Potential mechanisms underlying the displacement of native Red-legged frogs by introduced Bullfrogs. Ecology, 82: 1964–1970
Kim H. K.1972. Biology of the Bullfrog (Rana catesbeiana). J Korean Res Ins Better Living (Ewha Womans University), 8: 67–92 (In Korean)
Kim H. S., Ko S. K.1998. Distribution, food habit and seasonal cycles of germ cell activity in the introduced Bullfrog, Rana catesbeianus in Korea. FRI J Forest Sci, 57: 165–177 (In Korean)
King R. B.1986. Population ecology of the Lake Erie water snakes, Nerodia sipedon insularum. Copeia, 1986: 757–772
King R. B.2002. Predicted and observed maximum prey size-snake size allometry. Funct Ecol, 16: 766–772
King R. B., Ray J. M., Stanford K. M.2006. Gorging on gobies: Benefi cial effects of alien prey on a threatened vertebrate. Can J Zool, 84: 108–15
Kishida O., Mizuta Y., Nishimura K.2006. Reciprocal phenotypic plasticity in a predator–prey interaction between larval amphibians. Ecology, 87: 1599–1604
Labra A., Beltrán S., Niemeyer H. M.2001.Chemical exploratory behavior in the lizard Liolaemus bellii. J Herpetol, 35: 51–55
Langkilde T.2009. Invasive fire ants alter behavior and morphology of native lizards. Ecology, 90: 208–217
Lee H.J., Lee J.H., Park D.2011. Habitat use and movement patterns of the viviparous aquatic snake, Oocatochus rufodorsatus, from Northeast Asia. Zool Sci, 28: 593-599
Li Y., Ke Z., Wang S., Smith G. R., Liu X.2011a. An exotic species is the favorite prey of a native enemy. PLoS ONE, 6(9): e24299. doi:10.1371/ journal.pone.0024299
Li Y., Zhunwei K. E., Wang Y., Blaackburn T. M.2011b. Frog community responses to recent American Bullfrog invasions. Curr Zool, 57: 83–92
Lima S. L.2002. Putting predators back into behavioral predatorprey interactions. Trends Ecol Evol, 17: 70–75
Llewelyn J., Schwarzkopf L., Phillips B. L., Shine R.2013. After the crash: How do predators adjust following invasion of a novel toxic prey type? Austral Ecol, doi:10.1111/aec.12058
Lowe S., Browne M., Boudjelas S., De Poorter M.2000. 100 of the world’s worst invasive alien species a selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN)
Mittelbach G. G., Osenberg C. W., Wainwright P. C.1999. Variation in feeding morphology between pumpkinseed populations: phenotypic plasticity or evolution? Evol Ecol Res, 1: 111–128
Mushinsky H. R., Lotz K. H.1980. Chemoreceptive responses of two sympatric water snakes to extracts of commonly ingested prey species. J Chem Ecol, 6: 523–535
Nuismer S. L., Thompson J. N.2006. Coevolutionary alternation in antagonistic interactions. Evolution, 60: 2207–2217
Oh H. S., Hong C.2007. Current conditions of habitat for Rana catesbeianus and Trachemys scripta elegans imported to Jejudo, including proposed management plans. Korean J Env Eco, 21: 311–317 (In Korean)
Phillips B. L., Shine R.2004. Adapting to an invasive species: Toxic cane toads induce morphological change in Australian snakes. Proc Natl Acad Sci USA, 101: 17150–17155
Phillips B. L., Shine R.2006. An invasive species induces rapid adaptive change in a native predator: Cane toads and Black snakes in Australia. P R Soc B, 273: 1545–1550
Pothoven S. A., Madenjian C. P.2008. Changes in consumption by alewives and lake Whitefi sh after Dreissenid mussel invasion in lakes Michigan and Huron. N Am J Fish Manag, 28: 308–320
Preacher K. J.2001. Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fi t and independence [Computer software]. Retrieved from http:// quantpsy.org.
Preacher K. J., Briggs N. E.2001. Calculation for Fisher’s exact test: An interactive calculation tool for Fisher’s exact probability test for 2 × 2 tables [Computer software]. Retrieved from http:// quantpsy.org
Ra N. Y., Park D., Cheong S., Kim N. S., Sung H. C.2010. Habitat associations of the endangered Gold-spotted pond frog (Rana chosenica). Zool Sci, 27: 396–401
Saviola A. J., Lamoreaux W. E., Opferman R., Chiszar D.2011. Chemosensory response of the threatened Eastern indigo snake (Drymarchon couperi) to chemical and visual stimuli of Mus musculus. Herpetol Conserv Biol, 6: 449–454
Strayer D. L.2012. Eight questions about invasions and ecosystem functioning. Ecol Lett, 15: 1199–1210
Strauss S. Y., Lau J. A., Carroll S. P.2006. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol Lett, 9: 357–374
Suarez A. V., Case T. J.2002. Bottom-up effects on persistence of a specialist predator: Ant invasions and Horned lizards. Ecol Appl, 12: 291–298
Suh J. H.2005. Natural resource survey in Taean coastal national park: Herpetofauna. Seoul: Korea National Park Research Institute (In Korean)
Sung H. C., Kim S. K., Cheong S. W., Park S. R., Roh D. C., Baek K. W., LeeJ. H., Park D.2006.Estimating detection probabilities and site occupancy rates of three anuran species using call surveys in Haenam Gun. Korean J Ecol Field Biol, 29: 331–335
Taean.2013. Taean statistical yearbook. Taean County, Chungnam Province, South Korea; Taean County (In Korean)
Vellend M., Harmon L. J., Lockwood J. L., Mayfield M. M., Hughes A. R., Wares J. P., Sax D. F.2007. Effects of exotic species on evolutionary diversification. Trends Ecol Evol, 22: 481–488
Wang Y. P., Guo Z. W., Pearl C. A., Li Y. M.2007. Body size affects the predatory interactions between introduced American Bullfrogs Rana catesbeianus and native anurans in China: An experimental study. J Herpetol, 41: 514–520
Wanger T. C., Wielgoss A. C., Motzke I., Clough Y., Brook B. W., Sodhi N. S., Tschamtke T.2011. Endemic predators, invasive prey and native diversity. P R Soc B, 278: doi: 10.1098/ rspb.2010.1512
Wu Z., Li Y., Wang Y., Adams M. J.2005. Diet of introduced Bullfrogs Rana catesbeianus: predation on and diet overlap with native frogs on Daishan island, China. J Herpetol, 39: 668–674
Zar J. H.1999. Biostatistical analysis. Upper Saddle River, NJ, USA: Prentice Hall
#These authors contributed equally to this work.
*Corresponding author: Dr. Daesik PARK, from Kangwon National University, Chuncheon, South Korea, with his research focusing on the basic and conservation ecology of Korean amphibians and reptiles.
E-mail: parkda@kangwon.ac.kr
Received: 11 April 2014 Accepted: 25 August 2014
杂志排行
Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
- Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Body Size and Reproductive Tactics in Varanid lizards
- Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
