A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
2014-03-25
1College of Biology and Environment, Graduate School, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
2College of Life and Environment Sciences, Huangshan University, Huangshan 245041, Anhui, China
3State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, Yunnan, China
4Department of Life Sciences and Food Engineering, Yibin University, Yibin 644000, Sichuan, China
A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
Lifang PENG1,2#, Changhu LU1#, Song HUANG2,3*, Peng GUO4and Yaping ZHANG3
1College of Biology and Environment, Graduate School, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
2College of Life and Environment Sciences, Huangshan University, Huangshan 245041, Anhui, China
3State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, Yunnan, China
4Department of Life Sciences and Food Engineering, Yibin University, Yibin 644000, Sichuan, China
The hot-spring snakes, Thermophis, were previously known only from the Tibet Autonomous Region and Western Sichuan, China. During the past two years, three adult hot-spring snakes (2 females, 1 male) were sampled in Shangri-La, northern Yunnan, China, thus expanding their known distribution region towards the southeast. This site is the southeastern-most corner of the Tibetan Plateau and the southernmost tip of the Hengduan Mountains (Mts.). Phylogenetic analysis of mitochondrial (mt) and nuclear (n) DNA segments suggested that the three specimens belong to the genus of Thermophis. Morphologically, the new species is more similar to T. zhaoermii. However, it is distinguished from T. zhaoermii in the number of maxillary teeth (15), distance between the two eyes/head width, rostral width/ height, mental width/height, in one character limited to female: head width/length, and in four characters restricted to male: occurrence of the reduction from 10 to 8 (8 to 6, 6 to 4) scales in each dorsal row on the tail. There are differences in morphology, genetics (mtDNA, nDNA), and geography between the putative new species and T. zhaoermii, the new species meets our proposed eclectic and feasible “four-differences” rule.
morphology, phylogenetics, “four-differences” rule, Thermophis Shangrila sp. nov.
1. Introduction
Despite more than one century of effort, taxonomists still have been trying to reach a consensus on the concept of species and methods of species delimitation (Mayden, 1997; de Queiroz, 1998; Fu and Zeng, 2008; Yang and Rannala, 2010; Chen et al., 2013b). To determine the validity of a species, relaxed criteria may result in confusion, whereas overly strict criteria may not facilitate consistent communications and actions in the practice of taxonomy and conservation biology. Here, we propose an eclectic and feasible rule—viz., the “four-differences” rule—to determine the validity of a species, also apply to identify a new species. First and foremost, the morphological difference(s) compared with the closest species should be perceptible. To further confi rm that the morphological difference(s) possess taxonomic significance rather than representing intraspecific polymorphism, we also need evidence of mitochondrial DNA (matrilineal divergence) and nuclear DNA differences (patrilineal divergence). Finally, the presence of geographical or ecological difference represents the potential for natural reproductive isolation. In this paper, we illustrate the utility of this rule in relation to the taxonomy of hot-spring snakes.
Hot-spring snakes, Thermophis, a relict genus endemic to the Tibetan Plateau, achieve the world’s highest altitude distribution among all snakes, commonly restricted to the proximity of geothermal sites (Zhao, 2006; Dorge et al.,
2007; Huang et al., 2009; He et al., 2009; Hofmann, 2012). This genus was erected by Malnate (1953), and only included one species (Thermophis baileyi Wall, 1907) at that time, which was restricted to the Tibet Autonomous Region (TAR). The species was listed as‘‘vulnerable’’ (IUCN, 2010).
Dorge et al. (2007) reported 13 new distribution records of T. baileyi in TAR, as well as predicting that if suitable habitats were available, Thermophis could also occur in hot springs in Sichuan and probably even in the northern parts of Yunnan. Guo et al. (2008) described a new species, T. zhaoermii in Cogsum, western Sichuan.
During investigations of biogeography and taxonomy of snakes in Western China in summer for the past two years (2011, 2012), we sampled three adult snakes (2 females, 1 male) in the vicinity of a hot spring in Shangri-La, northern Yunnan (Figure 1 A). Based on morphological, molecular and geographical analyses, these three specimens, while clearly belonging to the genus Thermophis, differ from the other two known species and should be considered a new species of this genus.
2. Materials and Methods
All sampling and procedures involving live snakes were in accordance with the Wild Animals Protection Law of the People’s Republic of China and approved by the Animal Ethics Committee at Huangshan University. The 3 adult snakes (2 females, 1 male) were humanely euthanized using lethal injection at our laboratory. Fresh liver tissues were removed and immediately preserved in 95% ethanol. The holotype (Collection number: HS11192) and paratypes (Collection number: HS12120–21) were preserved and deposited in the Museum of Huangshan University (Voucher numbers: HUM20120001–3).
We examined 23 characters from the 3 specimens, 12 T. zhaoermii and 11 T. baileyi. Fourteen scale counts were taken, for the numbers of preoculars, postoculars, temporals, supralabials, infralabials, loreal, dorsals, ventrals, subcaudals, nasals, subcaudal scale position of the reduction from 10 to 8 (8 to 6, 6 to 4) scale rows in tail, and the number of maxillary teeth. Nine mensural characters were represented as: total length, tail length, distance between the two eyes, head width, head length, rostral height, rostral width, mental height, and mental width. Body and tail lengths were measured using a ruler to the closest 1 mm; other measurements were measured with an electronic caliper to 0.1 mm. Symmetric mensural head characters were measured only on the right, while asymmetric characters were recorded on both sides.
Total genomic DNA was extracted according to the phenol/chloroform extraction procedure (Sambrook et al., 1989). Three mtDNA and one nDNA sequences of the three specimens, four T. zhaoermii and five T. baileyi were obtained by polymerase chain reaction (PCR) and direct sequencing using the primers and methods described in Che et al. (2012) for COI (611 bp), Burbrink et al. (2000) for cytb (1095 bp), Arévalo et al. (1994) for ND4 (651 bp), and Lawson et al. (2005) for c-mos (567 bp). The four DNA segments are frequently used in studies of snake phylogeny, and have been shown to
resolve relationships well in every taxonomic category of snakes (Kraus and Brown, 1998; Burbrink et al., 2000; Kelly et al., 2003; Malhotra and Thorpe, 2004; Lawson et al., 2005; de Queiroz and Lawson, 2008; Dawson et al., 2008; Huang et al., 2009; Pyron et al., 2011, 2013; Chen et al., 2013a).
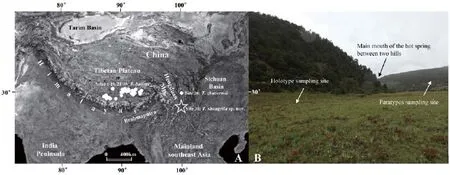
Figure 1 Sampling sites of Thermophis and habitat of T. shangrila sp. nov. A: The type locality (site 31, see Table 3) of T. shangrila sp. nov. is indicated by an asterisk, located at Shangrila, Northern Yunnan, China (N 27º28', E 99º29'; 3362 m a.s.l.). The other known sampling sites (1–30, see Table 3) of the genus Thermophis are shown by white circles. B: Habitat of type locality of T. shangrila sp. nov. Photo by S. HUANG.
In order to find the molecular systematic position of the three snakes of this study, we retrieved cytb, ND4 and c-mos sequences from GenBank for 85 genera (Table 1) of Alethinophidia (primitive + advanced snakes). Among these, 24 species (from 12 genera and two different species each genus) only had cytb and/or ND4 and/or c-mos sequences, while 79 species (from 73 genera) had all three genes. In order to maximize our dataset of combined genes, three different sequences from two congeneric species were combined to form supraspecifi c terminals at the generic level (according to Bininda-Emonds et al., 1998; Kelly et al., 2003; Huang et al., 2009). We aligned the new cytb, ND4 and c-mos sequences of Thermophis, as well as other sequences retrieved from GenBank using Clustal X (Thompson et al., 1997). Bayesian Inference (BI) and Maximum likelihood (ML) methods were used to construct phylogenetic trees as described in Huang et al. (2009). In order to detect mtDNA differences and nDNA differences, uncorrected pair-wise distances and unrooted trees of all sequences of each of those four genes of Thermophis (from this study and Genbank) were calculated using PAUP* v4b10 (Swofford, 2002) and MEGA 4.0 (Tamura et al., 2007).
3. Results
Molecular genetic analyses
All three specimens shared one haplotype for each of the four genes. These sequences have been submitted to GenBank. Their accession numbers are KF038429 (CO1), KF038430 (cytb), KF038435 (ND4), KF514883 (c-mos). We also sequenced the four genes of four T. baileyi and five T. zhaoermii. For CO1 gene, four T. baileyi shared 3 haplotypes (accession numbers: KF038425–27), five T. zhaoermii shared 1 haplotype (accession number: KF038428). The sequences of Cytb, ND4 and c-mos all shared the same haplotypes with the sequences previously reported (Huang et al., 2009; He et al., 2009, 2010; Hofmann, 2012).
Bayesian Inference (BI) and Maximum likelihood (ML) methods were used to construct phylogenetic trees. The topologies of separate analyses of each mitochondrial gene (trees not shown), and combined genes (Figure 2) were nearly identical. The node supports of the trees based on single gene were generally low, especially for internal nodes. The topologies of combined genes of BI and ML bifurcating trees were almost identical. The basic relationships of the commonly recognized families and/or subfamilies are similar to Kelly et al. (2003), Pinou et al. (2004), Lawson et al. (2005) and Huang et al. (2009). The three snakes clustered in the genus Thermophis with clear internal structure and high supports.
For mitochondrial genes, the minimums of interspecifi c divergences are 4.1 times (CO1), 38 times (cytb) and 2.3 times (ND4) more than the maximums of intraspecific divergences (Figure 3 B, C, D). For nuclear gene c-mos, 3 haplotypes (from 14 sequences) represent 3 species respectively. No intraspecifi c divergence was found. The numerical values of pair-wise distances of 3 species seem well-distributed (Figure 3 A).
Taxonomy
Thermophis shangrilasp. nov.(Figures 4, 5)
Shangri-La hot-spring snake,Xianggelila Wenquanshe (Bopomofo)
Holotype:HUM20120001, adult female, was captured on a grassland at the forest edge (see Figure 1 B) near a hot spring about 500 m in Shangri-La, Northern Yunnan, China, at 12:30 on 23 August, 2011, when it was fast moving towards the forest.
Paratypes:HUM20120002, adult male, was captured on the forest path of the opposite hill (see Figure 1 B) at 15: 25, on 20 July, 2012, when it was basking on the path. HUM20120003, adult female, was captured on the same path at 18:29, on same day, when it was passing through the path.
Diagnosis:Thermophis shangrila sp. nov. is distinguished from all other species of Thermophis by number of maxillary teeth and external characters (Table 2). These three individuals all have 15 maxillary teeth, are distinguished from T. baileyi (21–24) and T. zhaoermii (16–17). T. shangrila sp. nov. is morphologically distinguished from similar species T. zhaoermii by several characters: a closer distance between the two eyes (distance between the two eyes/head width is 0.56 in females, 0.58 in male, vs. 0.59–0.61 in females, 0.64–0.73 in males), a wider rostral (rostral width/height is 1.70–2.40 in females, 2.10 in male, vs. 1.00–1.50 in females, 1.72–1.86 in males), and a wider mental (mental width/height is 1.85–2.05 in females, 1.45 in male vs. 1.45–1.77 in females, 1.15–1.30 in males). In females, a slightly longer head (head width/length is 0.62–0.65 vs. 0.68–0.71). In males, a more anterior occurrence of the reduction from 10 (8, 6) to 8 (6, 4) scales in each dorsal
row on the tail (30 vs. 34.5–35, 49 vs. 52–61.5, 75 vs. 76.5–77).

Figure 2 The 50% majority-rule consensus tree from Bayesian Inference analysis based on cytb, ND4 and c-mos combined sequences. The values on the corresponding branches indicate posterior probability support (BI)/bootstrap support (ML), under 50% from both analyses omitted. Likelihood settings from best-fi t model (GTR + I + G) selected by AIC in jModeltest 0.1 (–ln L = 66699.4633). Rates = gamma, Shape = 0.4560, Pinvar = 0.3310. Supraspecifi c terminals are labeled with generic names + 2sp.

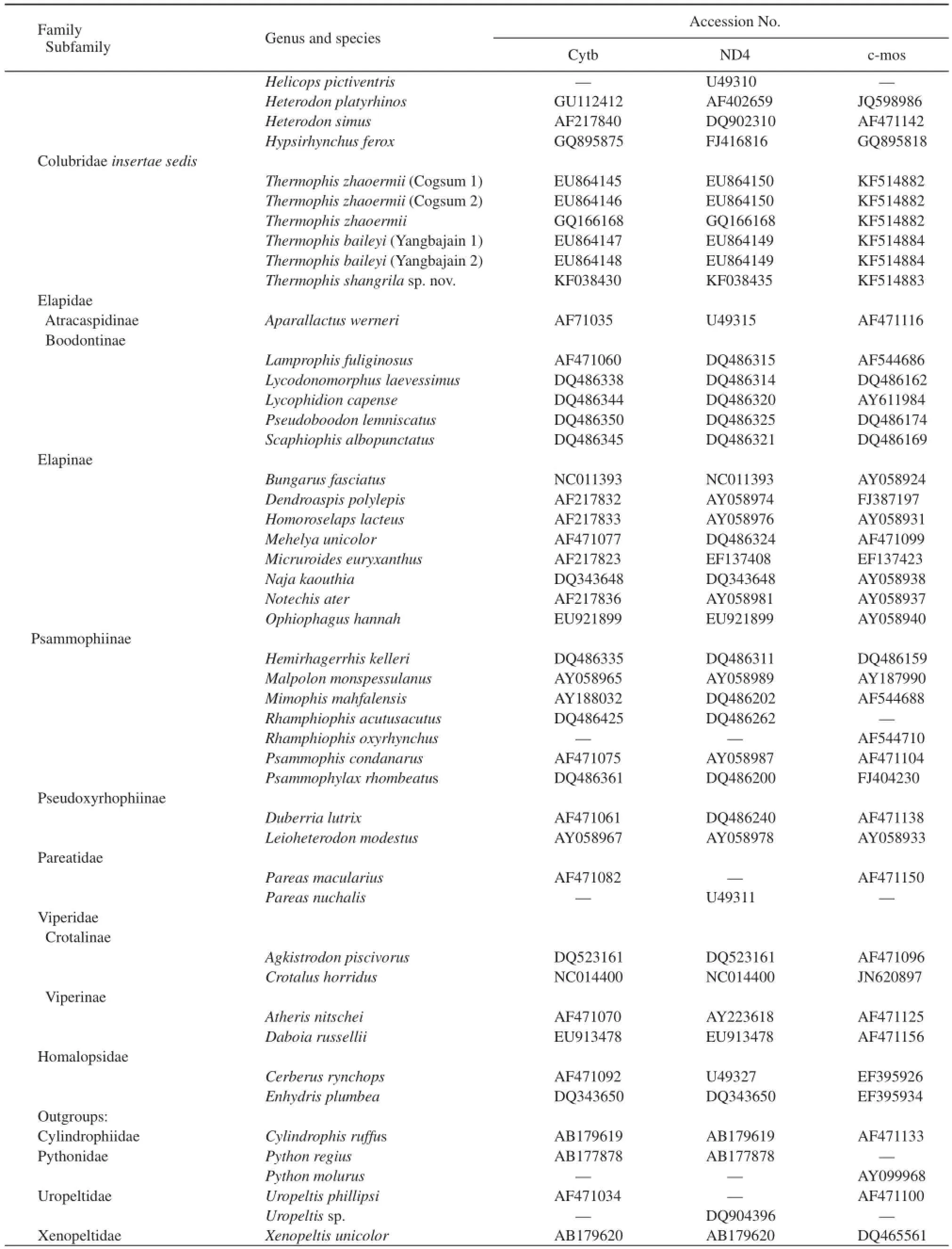
Table 1 Accession numbers of the sequences retrieved from GenBank and sequenced in this study. Systematic nomenclature follows the updated classifi cation recommended by Lawson et al. (2005).
(Continued Table 1)

Table 2 The comparison of distinguishing characters of the three species of Thermophis.
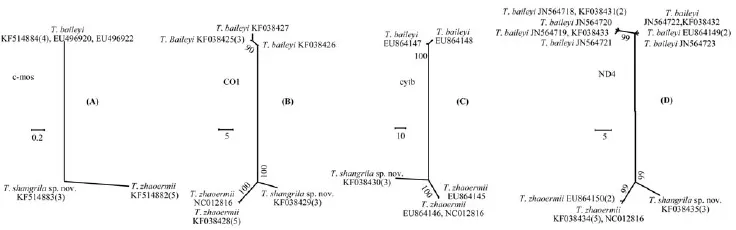
Figure 3 Unrooted trees and genetic distances of Thermophis were calculated based on the partial c-mos, CO1, cytb, and ND4 gene sequences derived from MP analyses. Numbers above or below branches are MP bootstraps values, only showing those higher than 50%. The number in parentheses indicate that the number of specimens that share this haplotype. (A) The p-distances of partial c-mos gene of 3 haplotypes of 14 sequences (each haplotype represents a species) of Thermophis ranged between 0.2%–0.5% among species. (B) The p-distances of partial CO1 gene of 6 haplotypes of 15 sequences of Thermophis ranged between 2.9%–11.2% among species, the maximum within species is 0.7%. (C) The p-distances of partial cytb gene of 5 haplotypes of 17 sequences of Thermophis ranged between 3.8%–12.6% among species, the maximum within species is 0.1%. (D) The p-distances of partial ND4 gene of 10 haplotypes of 23 sequences of Thermophis ranged between 2.5%–11.2% among species, the maximum within species is 1.1%. Sequences from this study; Huang et al., 2009; He et al., 2009; He et al., 2010; and Hofmann, 2012.
Description of holotype and variation:When differing from the holotype, features of the paratypes follow in parentheses (in the order HUM20120002, HUM20120003). Holotype an adult female (adult male, adult female) with body weight 121 g (83 g, 105 g), snout-vent length 743 mm (589 mm, 659 mm), tail length 208 mm (206 mm, 186 mm). Scutellation: 2 preoculars; 2 postoculars; 2 + 3 temporals; 8 supralabials, the 4th and 5th bordering the eye; 10 infralabials; 1 loreal; 19–19–17 forebody-midbody-hindbody transverse dorsal scale rows, dosal scales well keeled, outer row smooth or faintly keeled posteriorly; 223 ventrals (212, 222); 88 pairs subcaudals (95, 92); anal plate divided. Body and headshape: body relatively slender; venter round; head narrow, elongate, not strongly distinct from the neck; eye large, pupils round; Coloration: the dorsal ground color is light-brown marked with dark chocolate spots and stripes. A vertebral stripe of dark gray-brown spots; posteriorly on the body the spots become obscure and the stripe more prominent. The scales of the first three rows are darker centrally and form a broad, smoky lateral stripe. Venter olive green; Central line on the ventral surface is
prominent. Paler anteriorly and grayer posteriorly, the ventral plates narrowly edged with yellow laterally and along their free edges; the yellow lateral edge forms, with the yellow edge of the outer scale row, a narrow, irregular, ventro-lateral line. Each ventral with a pair of irregularly semicircular, black spots, which are more prominent on the posterior part of the belly (these are connected across the belly but the connecting areas of black are hidden by the overlapping edge of the preceding plate). Head brown, mottled with black-gray, lighter laterally and toward the snout. Supralabials creamy yellow, their sutures clouded dusky gray. Chin and throat yellow, the mental and anterior labials clouded gray-brown, the posterior labials narrowly edged with gray-green. Caudal pattern similar to that of the body, but much reduced.

Figure 4 Body of Holotype (HUM20120001, adult female). A: Dorsolateral; B: Back. Photos by L. F. PENG.

Figure 5 Head of holotype (HUM20120001, adult female). A: Ventral; B: Dorsal; C: rostral and Right; D: Left. Photos by L. F. PENG.
Ecology:The species is a diurnal terrestrial snake.
Etymology:The specifi c name refers to the type locality, Shangri-La County, Yunnan, China.
4. Discussion
The coordinates and altitudes of the 31 currently known distribution sites of hot-spring snakes (according to the literature and our expeditions) are listed in Table 3. The discovery reported here expands the distribution region of hot-spring snakes in a southeasterly direction. The new site is the southeastern-most corner of the Tibetan Plateau and the southern-most tip of the Hengduan Mts. (Figure 1 A). The name “Hengduan” means “to transect”and “cut downward” in Chinese. Topographically, in this area, parallel mountain ranges are separated by deep, narrowly incised river valleys. With strongly geographic
heterogeneity, Hengduan Mts. had naturally resulted in vicariance restricting gene flow (Huang et al., 2009). Such geographical difference could represent the potential for natural reproductive isolation. There are differences in morphology, mtDNA, nDNA, and geography between the new species and T. zhaoermii. This new species meets our proposed eclectic and feasible “four-differences” rule.

Table 3 Geographic coordinates and Temperature of thermal springs of the known sampling sites of the genus Thermophis.
In the Late Pliocene, the Qinghai-Xizang region (Tibetan Plateau), with an elevation of only about 1000 m a.s.l. (Li et al., 1979) was characterized by landscapes of subtropical montane forest and forest-steppe (Zheng and Li, 1990). At that time, the environment of this region should have facilitated the existence of many species of snakes. This region underwent intensive uplifting, beginning at the end of Pliocene and Early Pleistocene, and the environment changed gradually from warm-humid to cold-arid (Zheng and Li, 1990), reducing its ability to support populations of ectotherms. In the central region of the Tibetan Plateau (CRTP, between Transhimalaya and Tanggula Mts., west of Sejila Mts.), Thermophis baileyi is the only snake survivor currently known, and is exclusively restricted to habitats with hot springs (Dorge et al., 2007; Huang et al., 2009), thus making it an ideal model for the study of adaptive evolution in Serpentes.
The temperatures recorded at the mouths of hot springs at the 31 known sampling sites of Thermophis exceed 40°C (see Table 3, the temperature data cited from Guo et al., 1994 and Tong et al., 2000). There are 229 hot springs with the temperature hotter than 40°C in the CRTP (counted from Tong et al., 2000), therefore begging the question why only a few hot springs are utilized by Thermophis. Why did snakes sympatric with Thermophis in earlier geologic periods fail to survive in these regions? These observations raise many issues of considerable interest.
Uplifting of the Tibetan Plateau and incision of river valleys on its eastern margin must have occured simultaneously. Differing considerably from the CRTP, the deep incised valleys of the eastern margin of Tibetan Plateau have strong habitat heterogeneity, and offered refuges for many species (e.g., the famous Hengduan Mts.). This region harbors about 30 species of snakes, including T. zhaoermii and T. shangrila sp. nov. There are more than 400 hot springs hotter than 40°C in Hengduan Mts. (based on Guo et al., 1994), yet Thermophis have been found at only two of them.
Several fieldworks had been conducted from 2011 to 2014 in northern Yunnan and southern Sichuan. We found that the quality of habitats around the hot springs has declined because of the increasingly serious exploitation of geothermal energy. The habitat faces an extremely high risk of extinction in the wild. During these fieldworks, only one extent of occurrence of hot-spring snakes had been found, where we had got three samples (in 2011 and 2012). The number of individuals considered to be facing a continuing decline. According to IUCN Red List Categories and Criteria (Version 3.1, Second edition, 2001), the new species should be listed as “Critically Endangered” (A2cd+3cd+4cd; B1ab (i, iii, v)).
Taxonomy is an important foundation of biology. However, there are few taxonomists in China. Therefore, the National Natural Science Foundation of China has a focus on taxonomy in order to encourage its development (Chen et al., 2010). This is also a global phenomenon (Dr. Anita Malhotra, personal communication) where there are millions of species still undescribed and there are far too few taxonomists to do the job, especially in biodiversityrich but economically poorer countries (http://www.cbd. int/gti/problem.shtml, web of Convention on Biological Diversity). Once it has undergone comprehensive discussion and improvement, we hope that it will help to promote the development of taxonomy and accelerate the description of new species.
AcknowledgementsWe thank Dr. Anita Malhotra (Bangor University, UK) for helpful comments and correction of English on a draft of this manuscript. We also thank Tianqi ZHOU, Xin ZHENG, Jun-Sheng CUI, Diancheng YANG and Tengfeng NI (Huangshan University) for helping in collecting samples, and Jinmin CHEN, Baolin ZAHNG (Yunnan University) and Xiaoyu SUN (Chengdu Institute of Biology, Chinese Academy of Sciences) for helping in data processing. This research was funded by the National Natural Science Foundation of China (31471968, 31090250, 31372152). This work was also supported by grants from the Ministry of Science and Technology of China (MOST Grant 2011FY120200), the Chinese Academy of Sciences (CAS; KSCX2-EW-Z-2; KSCX2-EW-Q-9), the Bureau of Science and Technology of Yunnan Province to ZYP, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Department of education of Sichuan Province (13TD0027).
Reference
Arévalo E., Davis S. K., Sites Jr., J. W.1994. Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in Central Mexico Syst Biol, 43: 387–418
Bininda-Emonds O. R. P., Bryant H. N., Russell A. P.1998.
Supraspecific taxa as terminals in cladistic analysis: implicit assumptions of monophyly and a comparison of methods. Biol J Linn Soc, 64: 101–133
Burbrink F. T., Lawson R., Slowinski J. B.2000. Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsoleta): a critique of the subspecies concept. Evolution, 54: 2107–2118
Che J., Chen H., Yang J., Jin J., Jiang K., Yuan Z., Murphy W., Zhang Y. P.2012. Universal COI primers for DNA barcoding amphibians. Mol Ecol Res, 12: 247–258
Chen L., Chen Y., Hu J. J.2010. Summarization and suggestions for the preference support of NSFC (National Natural Science Foundation of China) to classical animal taxonomy. China Basic Science, 3: 55–57 (In Chinese)
Chen X., Huang S., Guo P., Colli G. R., Montes de Oca A. N., Vitt L. J., Pyron R. A., Burbrink F. T.2013a. Understanding the formation of ancient intertropical disjunct distributions using Asian and Neotropical hinged-teeth snakes (Sibynophis and Scaphiodontophis: Serpentes: Colubridae). Mol Phylogenet Evol, 66: 254–261
Chen X., Jiang K., Guo P., Huang S., Rao D. Q., Ding L., Takeuchi H., Che J., Zhang Y., Myers E. A., Burbrink F. T.2013b. Assessing species boundaries and the phylogenetic position of the rare Szechwan ratsnake, Euprepiophis perlacea (Serpentes: Colubridae), using coalescent-based methods, Mol Phylogenet Evol, doi: http://dx.doi.org/10.1016/ j.ympev.2013.09.003
Dawson K., Malhotra A., Thorpe R. S., Guo P., Mrinalini, Ziegler T.2008. Mitochondrial DNA analysis reveals a new member of the Asian pitviper genus Viridovipera (Serpentes: Viperidae: Crotalinae). Mol Phylogenet Evol, 49(1): 356–61
de Queiroz A., Lawson R.2008. A peninsula as an island: multiple forms of evidence for overwater colonization of Baja California by the gartersnake Thamnophis validus Biol J Linn Soc Lond, 95 (2): 409–424
de Queiroz K.1998. The general lineage concept of species, species criteria, and the process of speciation. In: Endless Forms: Species and Speciation (eds Howard DJ, Berlocher SH). Oxford, UK: Oxford University Press, 57–75 pp
Dorge T., Hofmann S., Wangdwei M., Duoje L., Solhoy T., Miehe G.2007. The ecological specialist, Thermophis baileyi (Wall, 1907)–new records, distribution and biogeographic conclusions. Br Herpetol Soc, 101: 8–12
Fu J. Z., Zeng X. M.2008. How many species are in the genus Batrachuperus? A phylogeographical analysis of the stream salamanders (family Hynobiidae) from southwestern China. Mol Ecol, 17: 1469–1488
Guo G. Y., Liao Z. J., Liu S. B., Shen M. Z., Tong W., Wang D. X., Zhang B. S., Zhang Z. F., Zhao F. S., Zhu M. X., Wu C. Z., Zhang M. T., Zhou C. J., Kan R. J., Yan F. T., Han Z. S., Bi D. Z., Li G. X., Zhang Z. X., Zhao H., Zhao S. M., Zhang, G. W.1994. Thermal Springs in Hengduan Mountains. Beijing: Science Press, 1–326 (In Chinese)
Guo P., Liu S. Y., Huang S., He M., Sun Z. Y., Feng J. C., Zhao E. M.2009. Morphological variation in Thermophis Malnate (Serpentes: Colubridae), with an expanded description of T. zhaoermii. Zootaxa, 1973: 51–60
Guo P., Liu S., Feng J., He M.2008. The description of a new species of Thermophis (Serpentes: Colubridae). Sichuan J Zool, 27: 321
He M., Feng J., Liu S., Guo P., Zhao E. M.2009. The phylogenetic position of Thermophis (Serpentes: Colubridae), an endemic snake from the Qinghai-Xizang Plateau, China. J Nat Hist, 43: 479–488
He M., Feng J., Zhao E. M.2010. The complete mitochondrial genome of the Sichuan hot-spring keel-back (Thermophis zhaoermii; Serpentes: Colubridae) and a mitogenomic phylogeny of the snakes. Mitochondrial DNA, 21: 8–18
Hofmann S.2012. Population genetic structure and geographic differentiation in the hot spring snake Thermophis baileyi (Serpentes, Colubridae): Indications for glacial refuges in southern-central Tibet. Mol Phylogenet Evol, 63: 396–406
Huang S., Liu S. Y., Guo P., Zhang Y. P., Zhao E. M.2009. What are the closest relatives of the hot-spring snakes (Colubridae, Thermophis), the relict species endemic to the Tibetan Plateau? Mol Phylogenet Evol, 51: 438–446
IUCN.2010. IUCN Red List of Threatened Animals. Retrieved from www.iucnredlist.org
Kelly C. M. R., Barker N. P., Villet M. H.2003. Phylogenetics of advanced snakes (Caenophidia) based on four mitochondrial genes Syst Biol, 52: 439–459
Kraus F., Brown W. M.1998. Phylogenetic relationships of colubroid snakes based on mitochondrial DNA sequences. Zool J Linn Soc, 122: 455–487
Lawson R., Slowinski J. B., Crother B. I., Burbrink F. T.2005. Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Mol Phylogenet Evol, 37 (2): 581–601
Li J. J., Wen S. X., Zhang Q. S., Wang F. B., Zheng B. X., Li B. Y.1979. A discussion on the period, amplitude and type of the uplift of the Qinghai-Xizang Plateau. Scientia Sinica, 22: 608–616 (In Chinese)
Malhotra A., Thorpe R. S.2004. A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pitvipers (Trimeresurus and Ovophis) Mol Phylogenet Evol, 32: 83–100
Malnate E. V.1953. The taxonomic status of the Tibetan colubrid snake Natrix baileyi. Copeia, 2: 92–96
Mayden R. L.1997. A hierarchy of species concepts: the denouement in the saga of the species problem. In Claridge M., Darwah H. A, Wilson M. R. (Eds.), Species: the Units of Biodiversity. London: Chapman & Hall, 381–424
Pinou T., Vicarioa S., Marschner M., Caccone A. 2004. Relict snakes of North America and their relationships within Caenophidia, using likelihood-based Bayesian methods on mitochondrial sequences. Mol Phylogenet Evol, 32: 563–574
Pyron R. A., Burbrink F. T., Colli G. R., de Oca A. N., Vitt L. J., Kuczynski C. A., Wiens J. J.2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol Phylogenet Evol, 58 (2): 329–342
Pyron R. A., Kandambi D., Hendry C. R., Pushpamal V., Burbrink F. T., Somaweera R.2013. Genus-level phylogeny of snakes reveals the origins of species richness in Sri Lanka. Mol Phylogenet Evol, 66(3): 969–78
Sambrook J., Fritsch E. F., Maniatis T.1989. Molecular cloning:
A laboratory manual, seconded. New York: Cold Spring Harbor Laboratory Press
Sun X. Y., Liu S. Y., Huang S.2011. Tibetan Plateau Relict Snakes of the Genus Thermophis and Their relationship to New World Relict Snakes. Asian Herpetol Res, 2(3): 161–168
Swofford D. L.2002. PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer Associates, Sunderland, Massachusetts
Tamura K., Dudley J., Nei M., Kumar S.2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol, 24: 1596–1599
Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G.1997. The CLUSTAL-X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res, 25: 4876–4882
Tong W., Liao Z. J., Liu S. B., Zhang Z. F., You M. Z., Zhang M. T.2000. Thermal Springs in Tibet. Beijing: Science Press, 1–300 (In Chinese)
Wall F.1907. Some new Asian snakes. J Bombay Nat Hist Soc, 17: 612–618
Yang Z. H., Rannala B.2010. Bayesian species delimitation using multilocus sequence data. Proc Nat Acad Sci USA, 107: 9264–9269
Zhao E. M.2006. Snakes of China. Hefei, China: Anhui Sciences and Technology Publishing House, 1–365 (In Chinese)
Zheng D., Li B. Y.1990. Evolution and differentiation of the physico-geographical environment of Qinghai-Xizang Plateau. Geogr Res, 9: 1–10 (In Chinese)
BOOK REVIEW
Salamanders of the Old World, Max Sparreboom. KNNV Publishing (2014). ISBN: 9789050114851
Currently nine families of tailed amphibians, including 682 species, are recognised in the world. Five of these families, including some 166 species, occur in the Old World (Europe, Asia, and Northern Africa). The family of hynobiid salamanders (hynobiidae) is endemic to this region, whereas the other families also are represented in the New World (America). Salamanders of the Old World is a comprehensive monograph authored by Max Sparreboom. With this book, which aims to be an update of Robert Thorn’s book of 1969, the author presents updated information on salamander species diversity in this region. The book is bound to become a classic in its fi eld: it contains descriptions of all fi ve families and 166 salamander species, a taxonomical diagnosis, distribution maps, and a wealth of information on habitat preferences, behaviour, threats, conservation, etc. Every species chapter is followed by a comment by the author and references to technical literature. For many species the biological information comes from the author himself, who made observations of behaviour in aquariums and in the fi eld. This knowledge is supplemented with information from the scientific literature, which is synthesized in a systematic way. What will especially appeal to the reader is the great number of beautiful pictures in the text, showing details of morphological characters, habitat and behaviour in the different life history stages. These features add to the value of the book, both in scientifi c and artistic aspects.
There is an increasing interest in the phenomenon of amphibian population declines in many parts of the world. Indeed, the decline of salamanders may be more serious than that of other amphibians. This book therefore comes at the right time. As a text book and reference, it is not only invaluable for the herpetologist, naturalist and conservationist, but it is also very useful for amateurs, who wish to identify and enjoy these fascinating creatures. The important effect of this book is that it sends a convincing message that salamanders deserve further study and conservation.
Feng XIE
Chengdu Institute of Biology,
Chinese Academy of Sciences,
No. 9, Section 4, Renminnanlu street,
Chengdu 610041, China
E-mail: xiefeng@cib.ac.cn
#Both authors contributed equally to this work.
*Corresponding author: Prof. Song HUANG, from Huangshan University, with his research focusing on ophiology, molecular ecology and biogeography.
E-mail: snakeman666@sina.com
Received: 13 November 2013 Accepted: 17 December 2014
杂志排行
Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
- Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Body Size and Reproductive Tactics in Varanid lizards
- Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
