Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
2014-03-25*
*
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
2Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization, CAS and Ecological Restoration and Biodiversity Conservation, Sichuan Province, Chengdu 610041, China
Group-spawning and Simultanous Polyandry of a Stream-dwelling Frog Feirana kangxianensis
Jie WANG1,2, Feng XIE1,2, Gang WANG1,2and Jianping JIANG1,2*
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
2Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization, CAS and Ecological Restoration and Biodiversity Conservation, Sichuan Province, Chengdu 610041, China
Here we provide an example of simultaneous polyandry based on genetic evidence in Feirana kangxianensis. This stream-dwelling species occurs only in Kangxian County, southern Gansu Province, where it is sympatric with its sibling species F. quadranus. During the breeding season the sex ratio of F. kangxianensis was marginally femalebiased (44♂:59♀) and the encounter rate in a relatively pristine habitat was signifi cantly higher than that in heavily quarried habitats (9.6 ± 4.8 indiv./km vs. 3.2 ± 2.5 indiv./km). Three egg masses containing an average of 698 eggs were deposited on the underside of one or two adjacent fl at rocks, 6.0–10.0 cm under the water surface and 1.0–3.5 cm above the streambed. Using Bayesian sibship clustering of nine polymorphic microsatellite genotypes, two females were detected as group-spawning in one oviposition site, with three males fertilizing each female’s eggs simultaneously. We also discuss the conservation requirements of this range-restricted species and the evolutionary implication of its unusual reproductive strategy.
group-spawning, oviposition site, sex ratio, simultaneous polyandry, habitat conservation
1. Introduction
Three species in the genus Feirana are known from central China and all occur in montane streams at 300–2000 m a.s.l. (Fei et al., 2012). Feirana quadranus occurs across the Qinling-Daba mountains with a total range of 290 000 km2(29–35°N, 104–112°E) whereas F. taihangnica has a smaller range of 30 000 km2at higher latitudes (33–36°N, 107–113°E). F. kangxianensis is distributed narrowly in Kangxian County (900–1700 m a.s.l.; 33.0692–33.2804° N, 105.3674–105.7756° E) (Yang et al., 2011), where it is sympatric with F. quadranus.
Feirana taihangnica is uniquely characterized by the swollen cloaca that occurs on both sexes. They are aggregate breeders and have no amplectant behaviors(Chen et al., 2011; Zhang et al., 2012). In contrast, F. kangxianensis has no swollen cloaca but spines on the cloaca, dorsal skin, thumbs, and index fingers in males (Yang et al., 2011). No data are available on the reproductive ecology of this geographically restricted species. Here we describe for the first time the habitat requirements and breeding biology of this poorly known species F. kangxianensis.
2. Study area and Methods
2.1 Study areaOur study area (33°11′55" N, 105°21′41" E) was the Liuba stream (5–15 m wide, 7 km long, 1600–1800 m a.s.l.) in Kangxian County, southern Gansu, which winds through a valley covered by croplands and mixed deciduous broad-leaved and coniferous trees. Five villages (about 500 families and 2000 villagers) distribute along both sides of the main stream. Nearly all large stones in the stream were quarried for construction purposes after the Wenchuan Earthquake in 2008, so that the runoff was altered and the water depth in the stream
varies from 0 to 80 cm. The air temperature and water temperature of the stream measured at 14:00 and 21:00 each day during 7–24th April were averaged 13.3 ± 3.5 °C (6.0–19.5) and 17.8 ± 5.2 °C (9.1–28.0), respectively.
2.2 Field surveyWe set fi ve transects, three in the main stream (A: 4.0 km, B: 0.6 km, C: 2.5 km) and two in tributaries (D: 1.0 km, E: 2.0 km; Figure 1). Transect D is located in a steep, pristine tributary with no adjacent villages. During the 7–24th April, two investigators walked along the stream and carefully inspected rocks larger than 10 cm in diameter during the daytime (08:30–18:00) or at night (20:00–22:00) with torches. Encounter rates were calculated for each census as number of encounters (adults only) divided by the total distance travelled (i.e. survey effort).
For each captured frog, we measured its snout-vent length (SVL) to the nearest 0.1 mm and mass to the nearest 0.1 g, clipped the fi rst segment of one or two toes for DNA sample (Gonser and Collura, 1996; preserved in 95% ethanol), and then released at the site of capture. For each egg mass found, we identifi ed the stages of embryo development and estimated the laying date following the timing of developmental stages in Tao et al. (2010). We randomly sampled 56 embryos from different positions in one egg mass (503 eggs in total) to determine the number of mothers and the paternity of offspring.
2.3 Genetic classification of sympatric speciesTotal genomic DNA was extracted from toe tissues or embryos using the EasyPure Genomic DNA Extraction Kit (Beijing TransGen Biotech Co., Ltd.). To differentiate females of F. kangxianensis and F. quadranus, a portion (701 bp) of the mitochondrial NADH dehydrogenase subunit 2 gene (ND2) was amplified and sequenced following the procedure of Wang et al. (2009) using the pair of primers: Pmet (Wang et al., 2009) and ND2mid (5′-GGTCATGATGAGGAGAT-3′, designed in this work). All ND2 sequences were aligned with the CLUSTRAL W option and default parameters in MEGA 6.0.6 (Tamura et al., 2013). Bayesian inference was used for species assignment and were performed using MRBAYES version 3.2 (Huelsenbeck and Ronquist, 2001; Ronquist et al., 2012).
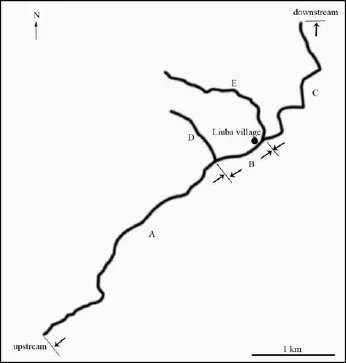
Figure 1 Five transects were set at the Liuba stream, Kangxian County: A (4.0 km), B (0.6 km), C (2.5 km), D (1.0 km), E (2.0 km). D is a pristine tributary without adjacent villages.
2.4 Maternity and paternity of embryosNine microsatellite loci (CIBfq01, 03–08, 17 and 21) that developed on F. quadranus were selected for amplifi cation in all samples according to the procedure of Wang et al. (2014). Basic descriptive statistics for each microsatellite locus and the sensibility of these microsatellites to resolve paternity or maternity was determined by using Cervus 3.0 (Marshall et al., 1998; Kalinowski et al., 2007). Sibships of embryos were reconstructed by using COLONY program (Jones and Wang, 2010), with settings of full-pedigree likelihood method, polygamous mating system, sibship complexity prior, allelic dropout rate of 0.01 and false allele rate of 0.01 for each locus. The same dataset was processed in at least eight runs with different random seeds to confirm the reliability of results. The inferred paternal genotypes were compared with those of candidate parents (i.e. adults captured 0–1000 m from the breeding site) based on the inheritance rule of codominant markers in diploid species. If mismatches were found at two or more loci, an adult was excluded as a potential genetic parent.
3. Results
3.1 Population composition and habitat selectionA total of 44 males and 59 females of F. kangxianensis were captured between 7th and 24th April. The sex ratio of this sample was female-biased (1:1.3), although the bias was not significant (χ2= 2.18, P = 0.14). Ignoring the ages of frogs sampled, males were significantly smaller than females (SVL: 9.58 ± 1.07 vs. 8.04 ± 0.85 cm; t = 7.43, df = 89, P < 0.001).
The encounter rate of F. kangxianensis varied among transects A, B, C, D, and E, and averaged 1.9, 2.8, 6.4, 9.6, and 2.0 indiv./km, respectively (Figure 2).
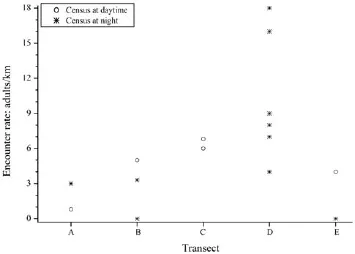
Figure 2 Encounter rate of F. kangxianensis in the fi ve transects in the Liuba stream, Kangxian County, Gansu Province. Detailed information of transects is provided in Figure 1.
3.2 Aggregate breedingFrogs may aggregate in the breeding season as we captured seven and nine adults respectively in two pools with diameters of 3–5 m and a depth of 1.8–2.3 m. Four females and fi ve males were found in one bank hole (30 cm × 13 cm × 13 cm) when eggs being laid inside.
Three oviposition sites, with the inter-distance of 6–12 m, were found where the stream was 7.0–8.0 m wide and 9.0–14.0 cm deep at 15:00–16:00 on 17th April. Eggs were arranged in a single horizontal layer beneath one or two adjacent rocks (50–76 cm × 26–36 cm) that were attached to the riverbank (n = 2) or in the middle of the stream (Figure 3). The egg masses (20–50 cm × 15–25 cm) were located 6.0–10.0 cm beneath the water surface and 1.0–3.5 cm above the streambed.
Embryo development stage of the three egg masses (522, 1069, and 503 eggs) were identified as 4-cell to early blastula stage, neural tube stage, and muscular response stage, and thus egg laying was backdated on April 17, 12, and 9th, respectively.
3.3 Mating systemBased on the genotypes of nine polymorphic microsatellite loci in 37 females and 35 males, the combined probabilities of non-exclusion for the first and second parent were 1.9×10-3and 6.0×10-5respectively (Table 1), indicating a sufficient power to resolve paternity. Furthermore, these markers were able to discriminate between full siblings with a power of 6.3×10-12.
Using Colony, the 56 sampled embryos were inferred to come from two mothers with 41 and 15 offspring respectively, and from three fathers with 35, 5, and 1 offspring, respectively (Table 2). The dominant male fertilized 45 of 56 sampled eggs (80%).
4. Discussion
4.1 Habitat selectionThe water flow through oviposition sites is heavily slowed down by tree roots and stones, forming small cavities (Figure 3 A, C). Eggs were scattered in a monolayer close to the water surface (6–10 cm), which may maximize oxygen exchange for embryo development in the stream (Seymour and Bradford, 1995; Wells, 2007). Some females may deposit eggs beneath huge rocks (> 1 m in diameter) or rock walls in pristine habitat like transect D. Unfortunately, we could not adequately survey these places for the presence of eggs without overturning such rocks.
Nearly all adults sheltered beneath big rocks in the main stream during the daytime, indicating that this species inhabits a very specialized habitat. This may be the reason why the encounter rate in the main stream and another tributary E, which have been heavily quarried, is lower than in the undisturbed tributary D (Figure 2) and why a very small number of egg masses (n =3) were found in the long transect (7.1 km) in the main stream.
4.2 Mating systemAmong anuran amphibians, two forms of polyandry have been identifi ed according to the time of eggs being fertilized: simultaneous and sequential (Roberts and Byrne, 2011; Byrne and Roberts, 2012). The former arises when sperm ejaculated by more than one male are present at the same time to fertilize eggs, and the latter occurs when a female mates with a series of males in relatively long intervals of time with no risk of sperm competition (Zhang et al., 2012). In this study, we provided the genetic evidence that two females were group-spawning, with three males fertilizing each female’s eggs, indicating that this species exhibits simultaneous polyandry, which is similar to the behavior of its sister species F. taihangnica (Zhang et al., 2012).
Polyandrous mating systems are commonly associated with high male bias at breeding sites, suggesting polyandry may be a facultative response to male–male competition (Roberts and Byrne, 2011). In contrast to the many examples of facultative polyandry, the operational sex ratio of F. kangxianensis is slightly biased to females (59♀:44♂) in the breeding season, which is different with its sister species F. taihangnica (190♀:86♂, Chen et al., 2011). The dominant male F. kangxianensis fertilized about 85% eggs of Female 1 but only 67% eggs of Female 2, which may support the hypothesis that polyandry may be obligate to increase the fertilization rate of large aquatic egg clutches for females, and (or) to reduce the risk of missing annual reproductive opportunities for

Figure 3 Ovipositon sites, indicated by red arrows, were beneath the bottom of fl at stones, in the middle of stream (A, B) or attached to the bank (C). Photo by Jie WANG.
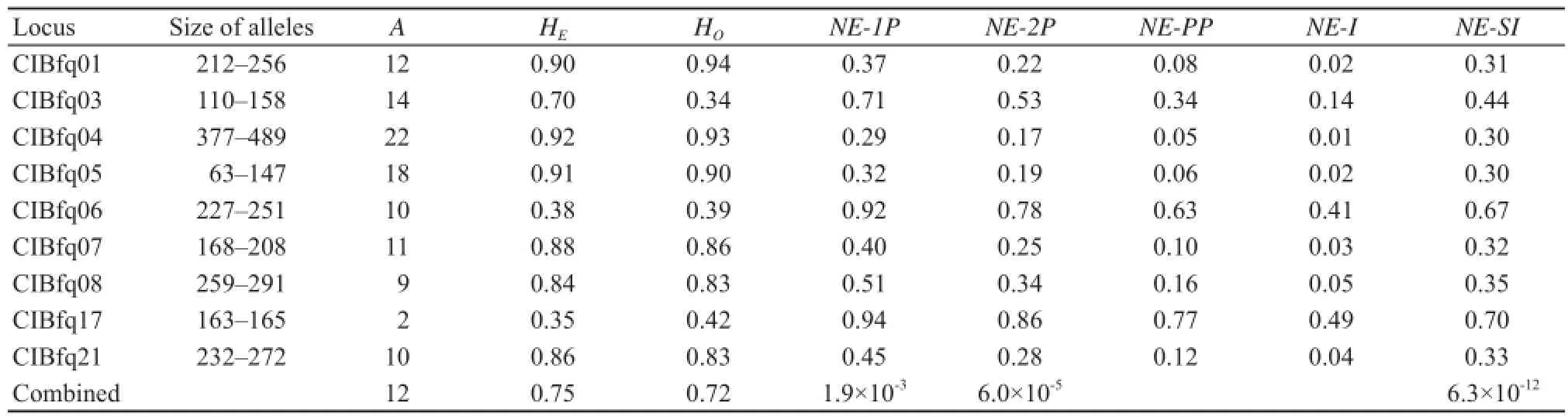
Table 1 Characteristics of the genotypes of nine microsatellite loci that screened in 37 females and 35 males of F. kangxianensis.
Note: Number of alleles (A), expected (HE) and observed (HO) heterozygosity, non-exclusion probability of one candidate parent (NE-1P) or one candidate parent given the genotype of a known parent of the opposite sex (NE-2P) or a candidate parent pair (NE-PP), non-exclusion probability for identity of two unrelated individuals (NE-I) or of two siblings (NE-SI).males in explosive breeding species (Zhang et al., 2012).

Table 2 Sibship clustering results using COLONY program based on the genotypes of nine polymorphic microsatellite loci in 56 embryos that collected from one egg mass (containing 503 eggs).
More detailed studies are needed to understand the evolution of the mating model displayed by F. kangxianensis and F. quandranus both in sympatry and allopatry. In future investigations, several interesting questions should be addressed in F. kangxianensis: How does the dominance in fertilization success link with phenotypic traits and sperm competition? What mode of communication is applied by either sex to achieve aggregation for breeding?
4.3 Species conservationFeirana kangxianenesis is recommended to be listed as vulnerable for the IUCN Red List because this species is in significant decline due to habitat degradation and over harvesting (Fei et al., 2012). In this study, we found a high dependence of this species on big stones for shelter and oviposition. However, rural
infrastructure construction is one of the needs of China’s modernization and lots of townships and high-class roads are being constructed. More than half of the available stones had been moved away from our study stream to fill the ground base of roads or houses. Meanwhile, sand had been quarried for the stone or brick concretion. A large proportion of the stream is being concreted to reduce bank erosion or to facilitate irrigation. Moreover, the normal runoff decreased or altered after the original riverbeds were destroyed. These activities obviously reduced the specialized habitats and breeding sites of F. kangxianensis, thus, habitat conservation, especially putting an end to quarrying, is needed for the persistence of this range-restricted species.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (31471964, 31200411) and the Chinese Academy of Sciences (Y3C3011100). We are grateful for Xulin LI and Jing ZHANG for laboratory assistance and anonymous reviewers for constructive comments.
Byrne P. G., Roberts J. D.2012. Evolutionary causes and consequences of sequential polyandry in anuran amphibians. Biol Rev, 87: 209–228
Chen X. H., Yang J., Qiao L., Zhang L. X., Lu X.2011. Reproductive ecology of the stream-dwelling frog Feirana taihangnicus in central China. Herpetol J, 21: 135–140
Fei L., Ye C. Y., Jiang J. P. 2012.Colored Atlas of Chinese Amphibians and Their Distributions. Chengdu: Sichuan Publishing House of Science and Technology
Gonser R. A., Collura R. V.1996. Waste not, want not: Toe-clips as a source of DNA. J Herpetol, 30: 445–447
Huelsenbeck J. P., Ronquist F.2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17: 754–755
Jones O. R., Wang J.2010. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour, 10: 551–555
Kalinowski S. T., Taper M. L., Marshall T. C.2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol, 16: 1099–1106
Marshall, T. C., Slate J., Kruuk L. E. B., Pemberton J. M.1998. Statistical confi dence for likelihood-based paternity inference in natural populations. Mol Ecol, 7: 639–655
Roberts J. D., Byrne P. G.2011. Polyandry sperm competition, and the evolution of anuran amphibians. Adv Stud Behav, 43: 1–53
Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P.2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol, 61: 539–542
Seymour R. S., Bradford D. F.1995. Respiration of amphibian eggs. Physiol Zool, 68: 1–25
Tamura K., Stecher G., Peterson D., Filipski A., Kumar S.2013. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol, 30: 2725–2729
Tao J., Yang J., Chen X. H.2010. The early embryonic development and ecological adaptation of Feirana taihangnicus. Chin J Zool, 45: 39–46
Wang B., Jiang J. P., Xie F., Chen X. H., Dubois A., Liang G., Wagner S.2009. Molecular phylogeny and genetic identifi cation of populations of two species of Feirana Frogs (Amphibia: Anura, Ranidae, Dicroglossinae, Paini) endemic to China. Zool Sci, 26: 500–509
Wang J., Zhang J., Yang X., Li X. L., Jiang J. P.2014. Isolation and characterization of 26 microsatellite loci for the frog Feirana quadranus. Conserv Genet Resour, 6: 57–58
Wells K. D.2007. The Ecology and Behavior of Amphibians. Chicago: University of Chicago Press
Yang X., Wang B., Hu J. H., Jiang J. P.2011. A new species of the Genus Feirana (Amphibia: Anura: Dicroglossidae) from the western Qinling Mountains of China. Asian Herpetol Res, 2: 72–86
Zhang L. X., Yang J., Lu Y., Lu X., Cheng X. H.2012. Aquatic eggs are fertilized by multiple males not engaged in amplexus in a stream-breeding frog. Behav Process, 91: 304–307
*Corresponding author: Prof. Jianping JIANG, from the Chengdu Institute of Biology, Chinese Academy of Sciences, with his research focusing on systematics, evolution and conservation biology of amphibians.
E-mail: jiangjp@cib.ac.cn
Received: 20 October 2014 Accepted: 9 December 2014
杂志排行
Asian Herpetological Research的其它文章
- Population Dynamics Following the Last Glacial Maximum in Two Sympatric Lizards in Northern China
- A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation
- Can an Invasive Prey Species Induce Morphological and Behavioral Changes in an Endemic Predator? Evidence from a South Korean Snake (Oocatochus rufodorsatus)
- Food Habits and Distribution of the Lake Taal Sea Snake (Hydrophis semperi Garman 1881) and the Sympatric Little File Snake (Acrochordus granulatus Schneider 1799) in Lake Taal, Philippines
- Body Size and Reproductive Tactics in Varanid lizards
- Sexual Dimorphism in Mass of the Hindlimb Muscles of the Piebald Odorous Frog (Odorrana schmackeri)
