Aldose reductase inhibitory potential of different fractions of Houttuynia cordata Thunb
2014-03-21ManishKumarDamikiLalooSatyendraPrasadSivaHemalatha
Manish Kumar, Damiki Laloo, Satyendra K. Prasad, Siva Hemalatha
Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi-221005, India
Aldose reductase inhibitory potential of different fractions of Houttuynia cordata Thunb
Manish Kumar, Damiki Laloo, Satyendra K. Prasad, Siva Hemalatha*
Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi-221005, India
Objective: To evaluate the aldose reductase (AR) inhibitory activity of different fractions from Houttuynia cordata (H. cordata) which used as a medicinal salad for lowering of blood sugar level.
Methods: AR inhibitory activity along with protein content was evaluated in vitro in rat lens. Total phenol and flavonoid contents were also determined in all the fractions. Results: All the four fractions were found to inhibit lens AR activity, but to different extent. From dose response curve (DRC), aqueous fraction (AQ) was found to be the most effective AR inhibitor followed by ethyl acetate (EA), chloroform (CL) and hexane fraction (HEX). The IC50values of AQ, EA, CL and HEX were calculated to be (64.62±3.90), (90.69±7.50), (134.59±4.90) and (151.58±3.30) μg/mL respectively. Quercetin was taken as positive control which exhibited AR inhibition with an IC50value of (3.21±0.60) μg/mL in a non-competitive manner. Conclusion: These findings indicated that, AQ fraction of H. cordata exhibited significant inhibitory effect on AR in a non-competitive manner, which may be attributed to the presence of high phenolic and flavonoid contents. Thus, the plant H. cordata may act as a promising source in the treatment of secondary complications like cataract associated with diabetes.
ARTICLE INFO
Article history:
Received 18 July 2013
Received in revised form 25 August 2013
Accepted 30 August 2013
Available online 20 March 2014
Aldose reductase
1. Introduction
Aldose reductase (AR) is a member of the aldo-keto reductase super family that reduces excess D-glucose into D-sorbitol with concomitant conversion of NADPH into NADP+[1]. This enzyme has been demonstrated to play important roles, not only in the cataract formation in the lens but also in the pathogenesis of diabetic complications that results in functional alterations of cornea, lens, retina, iris, peripheral nerve, and kidney[2]. In diabetic condition, AR increases the polyol pathway activity, which leads to an accumulation of polyol in lens fibers causing influx of water and generation of osmotic stress which finally leads to sugar cataracts[3]. The role of AR inhibitors has been extensively investigated in such complications. Literature have revealed that, cataract progression can be slowed or prevented by the use of natural therapies, particularly with plants having high flavonoid content and have shown considerable hypoglycemic andin vivoAR inhibitory activity[4]. Recently, plants such asOcimum sanctum(O. sanctum) Linn,Curcuma longa (C. longa)Linn,Azadirachta indica (A. indica) (A. Juss), Withania somnifera (W. somnifera)L. Duanl,Hybanthus enneaspermus (H. enneaspermus)Linn F. Muell,Ceasalpinia digyana (C. digyana)Rottlerand,Alagium lamarckii (A. lamarckii)Thwaits have been reported for their AR inhibitory and anticataract potential[5-7].
The herbHouttuynia cordata(H. cordata) Thunb is a single species of its genus and is native to Japan, South-East Asia, and Himalayas. In the Ri-Bhoi district of Meghalaya, India, whole plant ofH. cordatais eaten raw as a medicinal salad for lowering the blood sugar level and is commonly known by the name Jamyr-doh[8]. In southern China, green leaves and young roots are used as vegetable, while dried leaves are used to prepare drinks by boiling decoction. Major class of phytoconstituents reported inH. cordataare phenols, flavonoids and polysaccharides, whereas pharmacological activities of this plant includes hypoglycemic[9], antileukemic[10], anticancer[11],adjuvanticity[12], antioxidant[13]and inhibitory effects on anaphylactic reaction and mast cell activation[14]. However, there is no scientific data available for its AR inhibitory activity. Therefore, present study was aimed to evaluate the protective effects of different fractions ofH. cordataon diabetic complications such as aldose reductase inhibitory activity in rat lens.
2. Material and methods
2.1. Chemicals used
DL-glyceraldehyde and quercetin used for AR activity determination were obtained from Sigma-Aldrich Chemical Co. (St. Louis, USA). Nicotinamide adenine dinucleotide phosphate (NADPH) was purchased from Hi-Media laboratory Pvt. Limited, India. Other reagents and solvents were of analytical grade, Double beam UV spectrophotometer (Shimadzu, Pharmaspec 1700) was used for determining the absorbance of the sample.
2.2. Animals
Albino rats of Charles foster strain with body weights of (160-200 g) were obtained from the Central Animal House (Reg. No. 542/02/ab/CPCSEA), Institute of Medical Science (IMS), Banaras Hindu University (BHU), Varanasi, India. Before and during the experiment, rats were fed with normal laboratory pellet diet (Hindustan lever Ltd., India) and waterad libitum. After randomization into various groups, the rats were allowed to acclimatize for a period of 2-3 day in the new environment before initiation of experiment. The experimental protocol has been approved by the institutional animal ethical committee (Reference no. Dean/10-11/58 dated 07.03.2011).
2.3. Plant material
H. cordataherb was collected from Jaintias Hills of Meghalaya, India. The plant was identified by Dr. B.K. Sinha, Botanical Survey of India. A voucher specimen (COG/ HC/011) of the plant has been deposited in the Department of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, Varanasi (U.P), India.
2.4. Preparation of extract and its fractions
Whole plant ofH. cordatawas washed with water, shade dried, ground in a mill and was passed through sieve #40 to obtain a homogenous plant powder. Dried powdered material (1 kg) of whole plant ofH. cordatawas extracted with 3 L ethanol by soxhletion until the whole plant material was completely exhausted. The resulting extract was concentrated under reduced pressure to obtain a dark crude residue (yield: 13.2% w/w). The extract was then mixed with silica gel (1:3) and was loaded in a column. Further, column was run with different solvents to obtain different fractions such as hexane: 5.3% w/w, chloroform: 3.9% w/w, ethyl acetate: 2.2% w/w and aqueous: 6.1% w/w respectively which were further dried. The fractions in a solid powdered form was then stored in a desiccator until use.
2.5. Determination of total phenolics and total flavonoid
Total phenolic compounds in the extracts were estimated by the Folin–Ciocalteu method using gallic acid as standard and expressed as mg/g of gallic acid equivalents (GAE)[15]. Total flavonoid content of fractions was measured as mg/g of rutin equivalents (RE) using a modified colorimetric method described previously by Kumaran and Karunakaran[16].
2.6. Preparation of lens homogenate
Non-cataractous transparent lenses were dissected through posterior approach from rat eye and 10% homogenate was prepared from rat lenses in 0.1 M phosphate buffer saline (pH 7.4). The homogenate was centrifuged at 5 000×g for 10 min in cooling centrifuge to obtain the supernatant. After centrifugation, the supernatant was collected and kept in ice for the determination of both AR activity and protein content. The protein content in the supernatant of the lens homogenate was determined as per the method of Lowryet al[17].
2.7. Assay of reductase activity
AR activity was assayed as per the method described by Hayman and Kinoshita[18]. A sample cuvette containing 0.7 mL of phosphate buffer (0.067 M), 0.1 mL of NADPH (25 × 10-5M), 0.1 mL of lens supernatant, 0.1 mL of DL-glyceraldehyde (substrate) (5 × 10-4M) to a final volume of 1 mL was read against a reference cuvette containing all components except the substrate. The enzymatic reaction was initiated after the addition of substrate. Finally, the absorbance (OD) was recorded in a double beam UV-spectrophotometer at 340 nm for 3 min at 30 s interval. AR activity was expressed as OD/min/mg protein[19]. For determination of the AR inhibitory activity ofH. cordata, various concentrations of different fraction ofH. cordataranging from 25-300 μg/mL were prepared. The reaction was initiated by the addition of 0.1 mL DL-glyceraldehyde with 0.1 mL plant extract and rate of reaction was measured as described above. Different concentration of quercetin ranging from 1.25-10.00 μg/mL was prepared and was used as a standard. OD/ min/mg protein was calculated for each sample. Percent inhibition of AR activity was calculated with reference to the activity of normal rat lens as 100%. The concentration of inhibitors exhibiting 50% inhibition of enzyme activity (IC50) was calculated from the least-squares regression line of the logarithmic concentrations plotted against the residual activity.
2.8. Kinetics of AR inhibition
The inhibition was measured in presence of different fractions ofH. cordataand with varying concentrations of glyceraldehydes (5×10-4to 30×10-4M). Mode of inhibition was determined by following Lineweaver-Burk plot analysis of the data and calculated according to Michaelis-Menten kinetics in order to understand the probable mode of action. Kmand Vmaxwere also estimated. Kivalue in presence of fractions was determined by applying Cheg-Prusoff equation[20].
2.9. Statistical analysis
The data were analyzed with GraphPad Prism version 5 (San Diego, CA). Statistical analysis was done by One way ANOVA, followed by Tukey’s multiple comparison test. Data are expressed as mean ± S.E.M. A level ofP <0.05 was accepted as statistically significant.
3. Results
3.1. Total phenolic and total flavonoid content

Table 1. Phytochemical analysis and aldose reductase inhibitory activity of different fractions of H. cordata.
According to the data presented in Table 1, a significant difference (P<0.05) in the amount of the total phenolics and total flavonoids were observed among all these fractions, except total flavonoids in hexane and chloroform fraction. The richest amount of total phenolics was found to be in aqueous fraction (AQ, 36.79 mg/g GAE), followed by ethyl acetate fraction (EA, 24.41 mg/g GAE), chloroform fraction (CE, 12.02 mg/g GAE) and hexane fraction (HEX, 5.95 mg/ g GAE). Similarly, the highest flavonoid content (26.23 mg/g RE) was observed in AQ, whereas HEX showed the lowest content (5.19 mg/g RE).

Table 2. Enzyme kinetics of aldose reductase inhibition.
3.2. Aldose reductase inhibitory activity
The AR activity in normal rat lens was found to be (0.013 5 ± 0.005 0) μg/mL. Different fractions ofH. cordatawere found to inhibit lens AR to various extent with IC50values ranging from 50 μg/mL to >150 μg/mL (Table 1 & Figure 1). It is evident from the dose response curve (DRC) that AQ fraction possessed maximum AR inhibitory activity followed by EA, CH and HEX. At a concentration of 200 µg/mL, AQ fraction showed AR inhibition up to 99.97%. It is evident from the DRC that, the maximum inhibitory effects of AQ, EA, CL and HEX fractions were produced at the concentration of 200, 300, 300 and 300 μg/mL, respectively, and IC50values calculated was (64.62 ± 3.90), (90.69 ± 7.50), (134.59 ± 4.90) and (151.58 ± 3.30) μg/mL, respectively. Quercetin, which is well known for its potent AR inhibition, was used as a positive control which showed excellent activity with IC50value (3.21± 0.60) μg/mL.
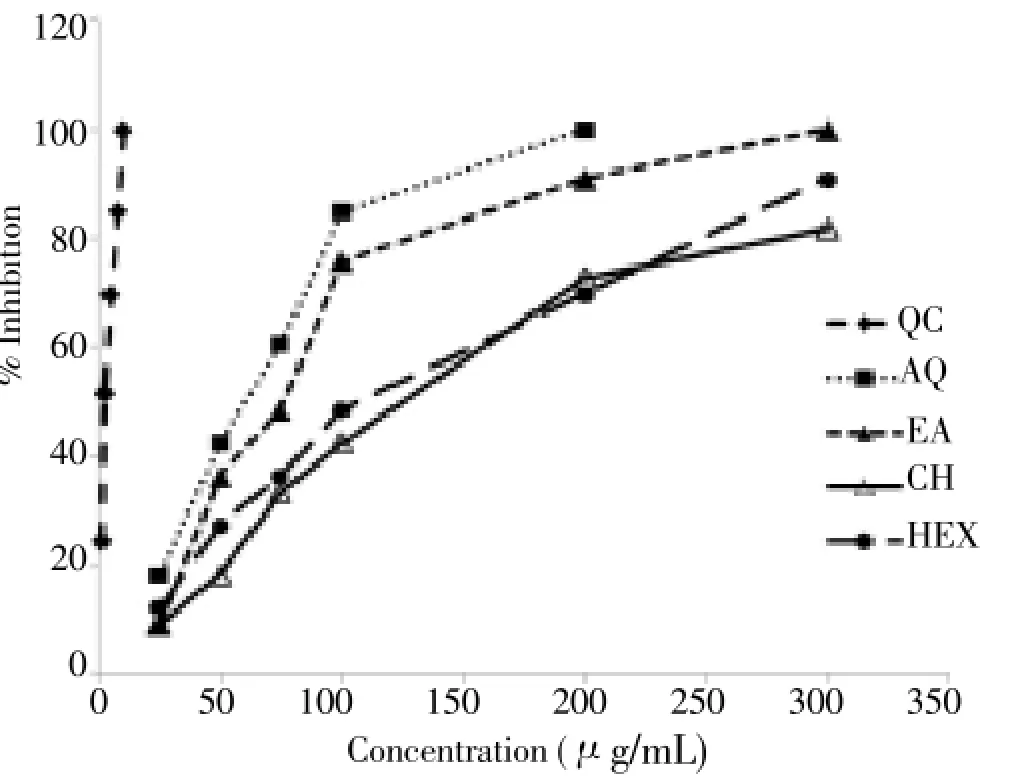
Figure 1. Effect of different fractions of H. cordata and quercetin on AR activity.
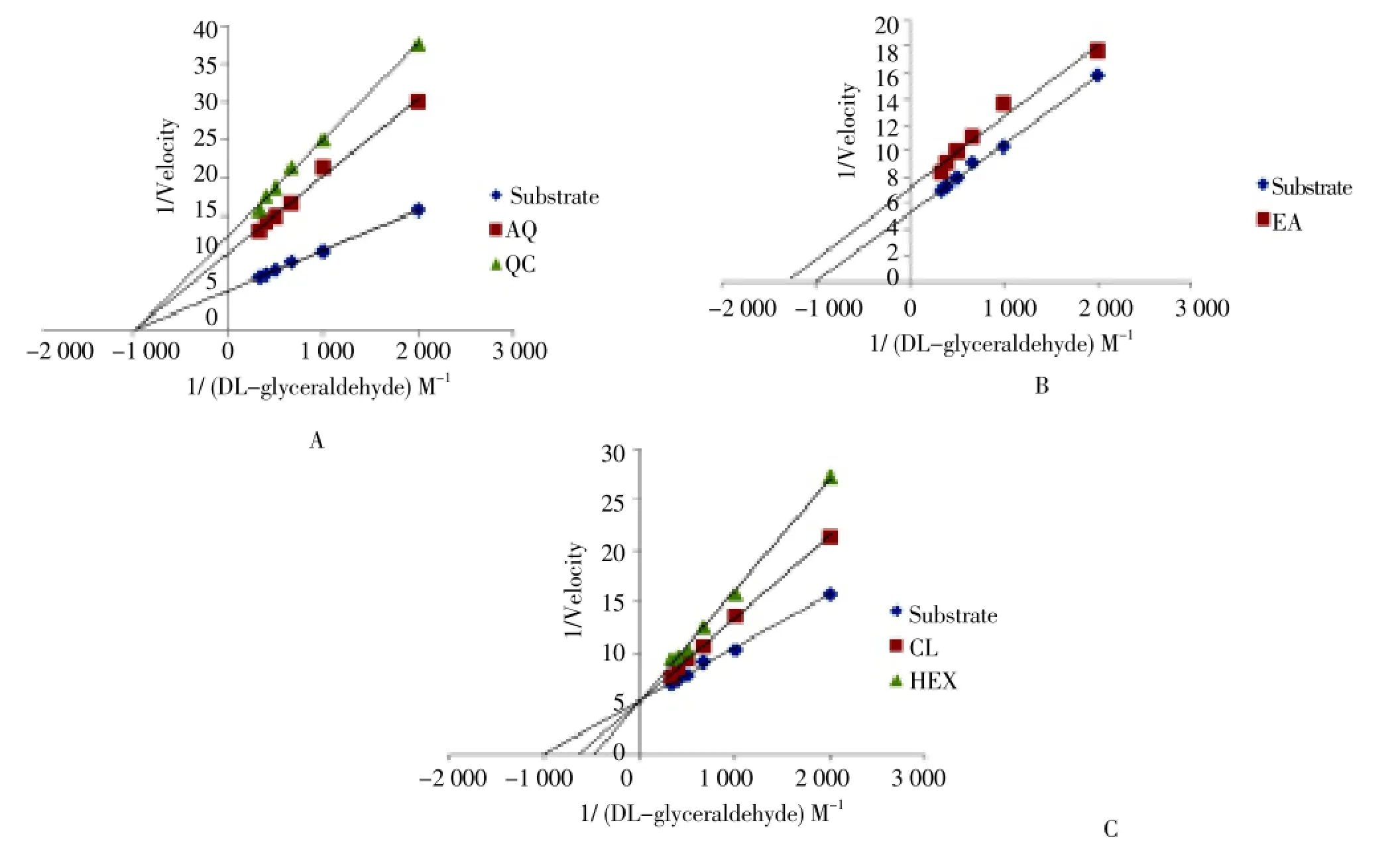
Figure 2. Effect of different fractions (A: aqueous fraction; B: ethyl acetate fraction; C: chloroform and hexane fraction) of H. cordata on the Lineweaver-Burk plot of AR activity with DL-glyceraldehyde as a substrate.
To determine the possible type of inhibition by different fractions ofH. cordataand quercetin, a kinetic study was conducted using dl-glyceraldehyde as a substrate (concentration: 5×10-4to 30×10-4M) at 5 different concentrations for each fraction. The Lineweaver-Burk plots of 1/velocity and 1/concentration for fractions and quercetin are shown in Figure 2a, 2b, 2c. The inhibitors concentration was always kept close to one which corresponds to 50% inhibition of the enzyme activity (IC50). Data obtained from LB plot equation clearly indicated a non-competitive inhibition of AR by quercetin and AQ fraction, since they did not alter the Kmbut altered the Vmax. Similarly, data showed a competitive inhibition of AR by CL and HEX fractions, since their Kmvalues were altered but the Vmaxremained constant. Moreover, the EA fraction showed a mixed type of inhibition (un-competitive).
Statistical analysis of the data obtained from the kinetic studies has showed significant difference between kinetic data obtained when DL-glyceraldehyde alone and DL-glyceraldehyde along with fractions ofH. cordata(P<0.001). A Low Kmvalue suggests that AR has higher affinity towards substrate (DL-glyceraldehyde). Statistical analysis by oneway ANOVA revealed that, there was a significant difference between the Vmaxand Kivalues which showed that, AQ fraction offers highest inhibition towards enzyme.
4. Discussion
Blindness in diabetics is largely due to retinopathy and/ or cataract. Cataract is the opacification of the lens, which interferes with the transmission of light on to the retina. Cataract alone is responsible for the cause of around 50% blindness worldwide. Activation of polyol pathway due to increased activity of aldose reductase has been implicated in diabetic cataract. This is a two step metabolic pathway in which glucose is reduced to sorbitol, which is then converted to fructose. AR (alditol: NADP+ 1-oxidoreductase, EC 1.1.1.21) has been implicated in the etiology of complications of diabetes such as neuropathy, nephropathy and retinopathy including catatractogenesis. It has been reported that, the cataract progression can be slowed or prevented by the use of natural therapies, particularly with plants having high flavonoid content mainly by AR inhibitory effect[21].
Quercetin, is a potent AR inhibitor for sorbitol accumulation in polyol pathway, therefore it was used as a standard in our study. The mode of inhibition of different fraction ofH. cordataand quercetin indicated a noncompetitive inhibition of AR by quercetin as well as AQ fraction, since they did not alter the Kmbut altered the Vmax. Low range of IC50value by AQ fraction to that of quercetin suggests the effectiveness AQ in inhibiting AR. The noncompetitive inhibitors, in general, are those substances that form strong covalent bonds with enzyme and consequently are not displaced by the addition of excess substrate and hence form irreversible reactions. Since, the richest amount of total flavonoids and phenols was found in the AQ fraction, they may render irreversible inhibition of AR by successfully blocking the polyol pathway that leads to catractogenesis.
The plantH. cordatahas been selected for the RLAR inhibitory activity as it was found to be an important source of natural polysaccharides, phenols and flavonoids[21-23]. Moreover, the hydroalcoholic extract has been shown to possess significant antioxidant and hypoglycaemic activity.
The results of our study suggested AR inhibitory activity of entire fractions with different magnitude. AQ fraction was found to be most potent inhibitor of AR followed by EA, CL and HEX fractions. Looking at the AR inhibitory potential, it is suggested that the above plant could be a potential tool in treatment of diabetic retinopathy which may circumvent the toxic effects of clinically tested AR inhibitors such as sorbinil, statil, epalrestat, tolrestat and alrestatin.
In the present study, AR inhibitory activity of different fractions ofH. cordatawas carried out on rat lens aldose reductase enzyme. Among the tested fractions, aqueous fraction showed potent AR inhibition activity, which may be attributed to the presence of high water soluble flavonoid contents. The mode of inhibition of AQ was similar as that of the positive control quercetin. These results suggest that,H. cordatacould potentially provide a new natural treatment for diabetic complications. However, further in vivo studies on the effects of these flavonoids on sorbitol accumulation is required to treat cataract.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
Financial assistance provided by Rajiv Gandhi National Fellowship Scheme (RGNFS) to Mr. Manish Kumar is greatly acknowledged. Authors also wished to acknowledge Dr. B.K. Sinha, Botanical Survey of India, Shillong, Meghalaya for the identification and authentication of the plant material.
[1] Jesus AF, Sonia M. Aldose reductase inhibitors from natural. Nat Prod Rep 2003; 20(2): 243-251.
[2] Haraguchi H, Ohmi I, Sakai S, Fukuda A. Effect of polygonum hydropiper sulfated flavonoids on lens aldose reductase and related enzymes. J Nat Prod 1996; 59(4): 443-445.
[3] Kubo E, Miyoshi N, Fukuda M, Akagi Y. Cataract formation through the polyol pathway is associated with free radical production. Exp Eye Res 1999; 68(4): 457-464.
[4] Lim SS, Jung SH, Ji J, Shin KH, Keum SR. Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. J Pharm Pharmacol 2001; 53(5): 653-668.
[5] Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol 2003; 86(1): 113-116.
[6] Patel DK, Kumar R, Kumar M, Sairam K, Hemalatha S. Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn F. Muell. Asian Pac J Trop Biomed 2012; 2(2): 134-139.
[7] Kumar R, Patel DK, Laloo D, Sairam K, Hemalatha S. Inhibitory effect of two Indian medicinal plants on aldose reductase of rat lens in vitro. Asian Pac J Trop Biomed 2011; 4(9): 694-697.
[8] Medicinal Plants Conservation and Sustainable Utilisation-Meghalaya (MPCSU). Medicinal Plants used in Ri-Bhoi District, Meghalaya, India; 2003, p. 72-75.
[9] WHO. Medicinal plants in the Republic of Korea. Manila: WHO Regional Publications for Western Pacific; 1998.
[10] Chang JS, Chiang LC, Chen CC, Liu LT, Wang KC, Lin CC. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am J Chin Med 2001; 29(2): 303-312.
[11] Kim SK, Ryu SY, No J, Choi SU, Kim YS. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharmacal Res 2001; 24(6): 518-521.
[12] Wang D, Yu QH, Eikstadt P, Hammond D, Feng Y, Chen N. Studies on adjuvanticity of sodium houttuyfonate and its mechanism. Int Immunopharmacol 2002; 2(10): 1411-1418.
[13] Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine 2003; 10: 544-551.
[14] Li GZ, Chai OH, Lee MS, Han EH, Kim HT, Song CH. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol Pharm Bull 2005; 28(10): 1864-1868.
[15] Jung HA, Islam MD, Kwon YS, Jin SE, Son YK, Park JJ, et al. Extraction and identification of three major aldose reductase inhibitors from Artemisia Montana. Food Chem Toxicol 2011; 49(2): 376-384.
[16] Kumaran A, Karunakaran J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWTFood Sci Technol 2006; 40: 344-352.
[17] Lowry OH, Rosenborough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 1951; 193(1): 265-275.
[18] Hayman S, Kinoshita JH. Isolation and properties of lens aldose. J Biol Chem 1965; 240: 877-882.
[19] Patel MB, Mishra SM. Aldose reductase inhibitory activity of C-glycoside flavonoids derived from Enicostemma hyssopifolium. J Altern Complement Med 2009; 6(1): 1-17.
[20] Daniellou R, Zheng H, Palmer DRJ. Kinetics of the reaction catalysed by inositol dehydrogenase from Bacillus subtilis and inhibition by fluorinated substrate analogs. Can J Chem 2006; 84(4): 522-527.
[21] Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, et al. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assayguided comparison. Plant Sci 2002; 163(6): 1161-1168.
[22] Meng J, Leung KSY, Jiang Z, Dong X, Zhao Z, Xu LJ. Establishment of HPLC-DAD-MS fingerprint of fresh Houttuynia cordata. Chem Pharm Bull 2005; 53(12): 1604-1609.
[23] Nuengchamnong N, Krittasilp K, Ingkaninan K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC-ESI-MS coupled with DPPH assay. Food Chem 2009; 117(4): 750-756.
ment heading
10.1016/S2221-6189(14)60015-9
*Corresponding author: Dr. (Mrs) S. Hemalatha, Department of Pharmaceutics, Indian Institute of Technology, (Banaras Hindu University), Varanasi, (U.P.) 221005, India.
Tel: +919415256481
E-mail: shemalatha.phe@itbhu.ac.in
Cataract
Houttuynia cordata
Rat lens
杂志排行
Journal of Acute Disease的其它文章
- Gender-Differences in aortic dissection
- Lagenaria siceraria ameliorates atheromatous lesions by modulating HMG-CoA reductase and lipoprotein lipase enzymes activity in hypercholesterolemic rats
- Effect of the methanol leaves extract of Clinacanthus nutans on the activity of acetylcholinesterase in male mice
- Animal bite incidence in the County of Shush, Iran
- In-vivo and ex-vivo inhibition of intestinal glucose uptake: A scope for antihyperglycemia
- Surgical treatment of hydrocephalus and spinal dysraphism
