In-vivo and ex-vivo inhibition of intestinal glucose uptake: A scope for antihyperglycemia
2014-03-21ViviyanTherasaThirumalaiTamilselvanDavid
S Viviyan Therasa, T Thirumalai, N Tamilselvan, E David
1Department of Biotechnology, Thiruvalluvar University, Serkkadu, Vellore-632115, (T.N.), India
2P.G and Research Department of Zoology, Physiology Wing, Voorhees College, Vellore-632001, (T.N.), India
In-vivo and ex-vivo inhibition of intestinal glucose uptake: A scope for antihyperglycemia
S Viviyan Therasa1*, T Thirumalai2, N Tamilselvan2, E David1
1Department of Biotechnology, Thiruvalluvar University, Serkkadu, Vellore-632115, (T.N.), India
2P.G and Research Department of Zoology, Physiology Wing, Voorhees College, Vellore-632001, (T.N.), India
Objective: To study hypoglycemic effect of Phyllanthus amarus (P. amarus) leaf extract and its glucose uptake inhibition effect in rat small intestine ex-vivo and in vivo models. Methods: Hypoglycemic studies were carried out in glucose loaded and streptozotocin (STZ) induced diabetic albino rats. Blood glucose levels were estimated at I, III and IV hour time intervals after administration of aqueous leaf extract of P. amarus. The study on the effect of plant extract on intestinal glucose absorption in rat was carried out using everted gut sacs. Results: The blood glucose levels were significantly depleted in the animals administered with aqueous leaf extract of P. amarus (250 mg/kg body weight). Incubation of the rat everted intestinal sacs with the aqueous leaf extract of P. amarus resulted in the inhibition of glucose transport across the intestinal membrane. Conclusions: The kinetic studies on the glucose transport inhibition across the intestinal membrane by the plant extract was a non competitive type of inhibition of the intestinal glucose transporter protein (GLUT2 and SGLT1) revealing the probable mechanism of hypoglycaemic effect of the aqueous leaf extract of P. amarus.
ARTICLE INFO
Article history:
Received 2 July 2013
Received in revised form 29 July 2013
Accepted 20 August 2013
Available online 20 March 2014
Everted gut sac
1. Introduction
In drug discovery and development, medicinal herbs have consistently been considered the leading source of pharmaceuticals, employed in the treatment of various human diseases due to their high chemical diversity and broad biological functionality[1].
Diabetes mellitus and obesity remains the most common disorders of carbohydrate metabolism. Diabetes mellitus is a metabolic disorder of multiple etiologies characterized by chronic hyperglycaemia with disturbance of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both[2].
It affected about 171 million people worldwide in 2000 and the number is projected to increase to at least 366 million by 2030[3]. One therapeutic approach for treating diabetes is to decrease the post-prandial hyperglycaemia. This is done by inhibiting the glucose absorption at the small intestine[4-6].
Plants continue to play an important role in the treatment of diabetes. The increase in demand in industriallydeveloped countries to use alternative approaches to treat diabetes, such as plant-based medicines, is also due to the side effects associated with the use of insulin and oral hypoglycaemic agents[7]. More than 400 plants are being used in different forms to treat diabetes[8]. There are more than 200 pure compounds from plant sources that have been reported to show blood glucose lowering activity[7]. The wide variety of chemicals classes indicates that a variety of mechanisms of action are likely to be involved in lowering blood glucose levels. In this study,P. amarus Schumwas selected on the basis of their use in traditional medicines throughout Southeast Asia and experimentally determined its hypoglycaemic activityin-vivo.
Extracts of this species was tested for its inhibition effect on glucose transport across the rat gutin-vivoandex-vivo(everted gut sacs).P. amarusSchum(Family:Euphorbiaceae) is a widely distributed small erect, tropical annual herbal shrub whose stem has green capsule.
The seeds are longitudinally rogues[8]. It is found in parts of South India, Florida, Mexico and throughout Southern America[9].P. amaruslocally known as Keela Nelli.P.amarushas been reported to have several pharmacological activities.
Some of the other properties of this plant might exert beneficial effects during diabetes in addition to the glucose absorption inhibition reported in the present study. Antioxidant activity[10], lipid lowering activity[11] and antiinflammatory effect[12], may all play a part in the treatment of the various pathogenic effects of diabetes.
The present study is undertaken to estimate the glucose uptake inhibition activity ofP. amarusaqueous leaf extract across the rat gutin-vivoand everted rat gut sacsex-vivo.
2. Materials and methods
2.1. Animals
Malealbinorats of Wistar strain weighing around 160-180 g were procured from Tamilnadu Veterinary and Animal Sciences University, Chennai. The animals were kept in polypropylene cages (three in each cage) at an ambient temperature of (25±2)℃and 55%-65% relative humidity. A 12/12 h light and dark schedule was maintained in the animal house and animals were acclimatized to the laboratory conditions, fed with commercially available rat chow (Hindustan Lever Ltd., Bangalore, India) and had free access to water.
2.2. Plant material
TheP. amarus(Family:Euphorbiaceae) leaves were collected from Vellore District of Tamilnadu, India during August to October, 2009. The leaves were cleaned, shade dried, authenticated and a voucher specimen (No: VCV/43/2009) is kept at the Department of Botany, Voorhees College, Vellore - 632 001, Tamilnadu, India.
2.3. Plant extracts preparation
Fresh leaves were washed with distilled water and shade dried. The shade dried leaves were powdered in an electrical blender and stored at 5℃until further use. The powdered leaves were taken 100 g each and mixed with 200 mL of distilled water and were stirred magnetically at room temperature. The residue was removed by filtration and the aqueous extracts were used for experiments.
2.4. Hypoglycemic studies
Hypoglycemic studies were conducted in malealbinorats as described earlier[13,14]. The studies were conducted in two animal models. Glucose loaded rat model and diabetic rat models.
2.5. Glucose loaded model
Plant extracts were fed to the fasted rats. After half an hour interval 10% glucose solution (1.5 g/kg body weight) was fed by oral administration and blood glucose samples were collected for glucose estimation at I, III and IV hours of interval.
2.6. Diabetic model
The animals were subjected to fasting overnight and diabetes was induced by a single intra peritoneal injection of freshly prepared solution of streptozotocin (Sigma, USA) 35 mg/kg body weight in 0.1 M cold citrate buffer of pH 4.5. Streptozotocin (STZ) was used to induce diabetes in animals by various authors[15-17].
The animals were allowed to drink 5% glucose solution overnight to bring-on the drug induced hypoglycemia, control rats were administered with citrate buffer alone. The animals were considered diabetic if the blood glucose values were above 250 mg/dL on the third day after STZ injection.
2.7. Ex-vivo everted gut sac preparation to study glucose absorption inhibition
The everted rat gut sacs were prepared according to the method described by Wilson and Wiseman[18].
2.8. Experimental design and surgical procedure
Adult male Swissalbinorats weighing 160-180 g were housed at room temperature and were used in this experiment. Before each experiment, the animals were starved for twelve hours but allowed for tap water use. Rats were sacrificed by cervical dislocation. The abdomen was opened by a midline incision. The entire small intestine was removed quickly by cutting across the upper end of the duodenum and the lower end of the ileum, and by stripping the mesentery manually. The small intestine was then washed with normal saline solution (0.9% w/v NaCl) using a syringe equipped with blunt end.
2.9. Preparation of everted gut sacs
Intestinal segments (10±2) cm were everted and the sacs were filled with 0.5 mL of the incubation medium (serosal fluid) and were placed in 25 mL Erlenmeyer flasks with 5 mL of the same medium (mucosal fluid). After oxygenation of the flasks with 100% O2for 1 min, they were tightly stoppered and kept in a shaker (90-110 oscillations/min) for 1 h at room temperature. The incubation medium was Krebs-Henseleit Bicarbonate buffer (KHB). The composition of the buffer was (mM/L): NaHCO325; NaCl 118; KCl 4.7; MgSO41.2; CaCl21.2; and Na2EDTA 9.7 mg/L. Glucose (5.5-8.5 mM) was added to the medium just before the start of appropriate experiment.
2.10. Evaluation of intestinal glucose uptake under the influence of plant extracts.
For studying the effect of the plant extract on glucose (substrate) uptake, glucose was added into mucosal compartment fluid just before the start of the experiment. The aqueous leaf extract was also added in the same compartment (3.62 mg/mL). At the end of the incubation period (1 h), the sacs were removed from the flask and these sacs were emptied and the serosal fluid from the sacs was used for the estimation of glucose. Similar estimations were also performed on samples of mucosal fluid in the flasks. Glucose concentrations were measured using a commercially available glucose oxidase kit (Lifechem TM -Glucose-LR). The loss of glucose from the mucosal fluid assumed to represent the glucose taken up by the intestine, and the rise in glucose in serosal fluid, the glucose released. The difference is attributable to the glucose retained in the tissue. The amount of glucose transported from the mucosal compartment was characterized as“Uptake” while the serosal gain of the substances is treated as “release”. Uptake and release of glucose was expressed as PM/g tissue wet weight/h.
2.11. Kinetic study on ex-vivo glucose uptake
A kinetic study was conducted to understand the transport/inhibition of glucose across intestinal membrane. In terms of enzyme kinetics, the amount of glucose transported/hour was analogue to the velocity of transfer, in other words, to the concentration difference of the glucose between compartments at the beginning and end of an experiment[5].
The Michaelis-Menten constant (Km), which is the affinity of the transferring enzyme (glucose transporter) for the substrate (glucose). The maximal velocity (Vmax), which is the rate of transfer reaction in the presence as well as in the absence of the plant extracts were determined from the differences of the uptake and release values using Michelis-Menten and the Lineweaver-Burk Plots in Microsoft excel. Comparison of difference between the control and experimental groups were examined using one-way analysis of variance (ANOVA). In each series of experiments, control everted gut sacs derived from the same rat in a buffer containing no substrate(glucose) were run in parallel. The controls were run either with or without plant extract and results were corrected accordingly.
2.12. Statistical analysis
The results were expressed in mean + standard deviation. Statistical analysis was carried out by using one way ANOVA as in standard statistical software package of social science (SPSS) version 12.
3. Results

Table 1. Hypoglycemic effect of aqueous leaf extract of traditional medicinal plant P. amarus: a) Glucose loaded model; b) Streptozotocin (STZ) induced diabetic model-Levels of blood glucose at I, III and IV hour of intervals.

Table 2. Effects of P. amarus aqueous leaf extract on the uptake of the varying concentrations of glucose by everted gut sacs of rats.
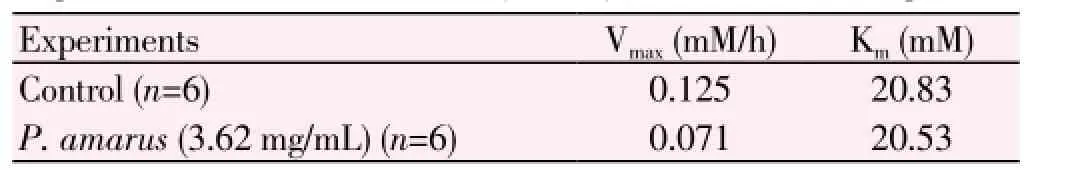
Table 3. Effect of P. amarus aqueous leaf extract: Kinetic parameters of the transport of D-glucose at different concentrations (5.5-8.5) across the rat everted gut sacs.
A significant elevation of blood glucose level was recorded in the glucose loaded group when compared to that of normal. The results revealed a significant blood glucose lowering effect in theP. amarusleaf extract (250 mg/kg body weight) administered group at I hour, III hour and IV hour intervals when compared with that of the control (Table 1a). After 48 h of the injection of STZ to the normal rats diabetes was evidenced.
The blood glucose level was significantly elevated in the STZ injected rats when compared to that of normal (placebo) and therefore considered as diabetic animals (Control). The plant extract administered diabetic animals recorded a significant blood glucose lowering effect at I h, III and IV h intervals (Table 1b). Incubation of the rat everted gut sacs with the aqueous extracts ofP. amarusat concentration 3.62 mg/mL resulted in the inhibition of transport of glucose. The glucose transport inhibition was significant at varied concentrations i.e. 5.5, 6.5, 7.5 and 8.5 mM/L in the incubation medium (Table 2). The pattern of glucose uptakeex-vivoin various experimental protocols was analyzed using Michelis-Menten and Lineweaver-Burk Plots.
The Km (Michaelis-Menton constant, which is the affinity of the transferring enzyme for the substrate). Vmax(Maximal velocity, which is the rate of transfer reaction, in the presence as well as in the absence of aqueous leaf extract was determined from the differences of uptake and release values using Michaelis-Menten and Lineweaver-Burk Plots in Microsoft Excel) as single entities.
The value of Vmaxwas significantly depleted in the entire experimental samples incubated along with the plant extract when compared with the control. However the Km remained constant throughout the study (Table 3).
4. Discussion
The most challenging goal in the management of diabetes mellitus is to achieve blood glucose level as close to normal as possible. The importance of postprandial glucose control in the development of diabetic complications is widely recognized based on the direct stimulation of endothelial cells by glucose, supports the hypothesis that postprandial glucose spikes are important in the early development of both microvascular and macrovascular diseases[19].
The hypoglycemic studies on the aqueous leaf extract of traditional medicinal plantP. amarusrevealed significant hypoglycemic effect in the glucose loaded as well as STZ induced diabetic rat models. The glucose lowering effect of the plant extract in the present study might be attributed to delayed intestinal glucose absorption and/or increased glucose utilization by the intestine with reference to anaerobic glucose metabolism thereby creating decreased passage of glucose from mucosal side to serosal side of the intestine.
Further studies were designed to understand the intestinal glucose uptake both in the presence and absence of the plant extract. The results obtained in the presentex vivostudy envisaged that the aqueous leaf extract ofP. amarussignificantly inhibits glucose absorption/transport in the everted rat gut and significantly reduced when compared to control.
The Michelis-Menten constant (Km) of the glucose uptake was calculated for all the experiments. Km is the affinity of the transferring enzyme for the substrate. In the present study it is simulated that Km is the affinity of glucose transporters i.e. GLUT2 and SGLT1 for glucose.
The maximal velocity (Vmax) is regarded as the glucose uptake rate in the presence as well as in the absence of plant extract. The decrease in the Vmaxin the presence of the aqueous extract of the studied plant extract indicated that the transmembranal glucose transport was significantly decreased. However the Km remained unaltered in the presence as well as in the absence of the extract. This indicates that the aqueous plant extract ofP. amarusact by bringing a non-competitive type of inhibition of transport of glucose at the level of small intestine. This might be due to the inhibition of glucose transporter proteins (GLUT2 and SGLT1) activity. Previous studies in our lab[6], recorded that the aqueous seed extract ofTrigonella foenum-graecum(T. foenum graecum) inhibited the glucose absorption in rat everted gut sacs and studies elsewhere recorded that the aqueous extracts ofT. foenum graecum(seed) andEugenia jambolana(seed) decreases the glucose absorption in rat everted gut sacs[20].
Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. The aqueous extract ofMomordica charantiainhibited the glucose transported across the rat everted gut sacsin-vitro[4].Nigella sativainhibits intestinal glucose absorption and improve glucose tolerance in rats[21]. Oral administration of aporphines and secoaporphines had an inhibitory effect on intestinal glucose uptake[22]. The above studies in our lab revealed that the aqueous extract of traditional medicinal plant,P. amaruscontain phytochemical constituents that are potent to inhibit the glucose uptake across intestinal membrane which might be due to decreased activity of glucose transporter transmembranal proteins in the small intestine ofrat and thereby opening new scope for postprandial glycemic control.
Based on the data obtained in the present study we propose thatP. amarusaqueous leaf extract possesses hypoglycemic property that inhibits the glucose transport at the site of intestinal brush border membrane in rats.
Conflict of interest statement
The authors report no conflict of interest.
[1] Jaroszewski JW. HPLC-SPE-NMR in natural products research. [Online]. Available from: http://www.farmacia. unifi.it/gacongress2005.html. [Accessed on August 21st 2005].
[2] Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539-553.
[3] Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes Estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-1053.
[4] Mahomoodally MF, Fakim AG, Subratty AH. Momordica charantia extracts inhibit uptake of monosaccharide and amino acid across rat everted gut sacs in-vitro. Biol Pharm Bull 2004; 27: 216-218.
[5] Patel M, Mishra S. A kinetic study for in-vitro intestinal uptake of monosaccharide across rat everted gut sacs in the presence of some antidiabetic medicinal plants. Int J Altern Med 2009; 7: 1.
[6] Viviyan S, Sivaraj A, Sivakumar C, Elumalai EK, Thirumalai T, David E. Trigonella foenum gracum seed extract inhibit uptake of glucose across rat everted gut sacs in-vitro. Int J Pharmtech 2010; 2: 359-362.
[7] Geethalakshmi R, Sarada DVL, Marimuthu P, Ramasamy K. α-amylase inhibitory activity of Trianthema decandra L. Int J Biotechnol Biochem 2010; 6: 369-376.
[8] Wadkar KA, Magdum CS, Patil SS, Naikwade NS. Antidiabetic potential and Indian medicinal plants. J Herbal Med Toxicol 2008; 2: 45-50.
[9] Adeneye AA, Benebo AS, Agbaje EO. Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on alcohol - induced hepatotoxity in rats. West Afr J Pharmacol Drug Res 2006; 22: 42-50.
[10] Raphael KR, Sabu MC, Khuttan R. Hypoglycaemic effect of methanol extract of Phyllanthus amarus Schum & Thonn on alloxan induced Diabetes Mellitus in rats and its relaxation with antioxidant potential. Ind J Exper Biol 2002; 40: 905-909.
[11] Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipidemic rats. J Ethnopharmacol 2002; 82: 19-22.
[12] Kassuya CA, Silvestre A, Menezes-de-Lima OJR, Marotta DM, Rehder VL, Calixto JB. Antiinflammatory and antiallodynic actions of the lignan niranthin isolated from Phyllanthus amarus. Evidence for interaction with platelet activating factor receptor. Eur J Pharmacol 2006; 546: 182-188.
[13] Nithyanand S. Screening of compounds/Natural products for hypolipidemic and hypoglycemic activity. In: Dhawan BN, Srimal RC.(eds). The use of pharmacological techniques for the evaluation of natural products. Lucknow: The Lucknow publishing House; 1984, p. 65-68.
[14] Mukherjee B, Mukherjee SK. Blood sugar lowering activity of Swertia chirata (Buch-Ham). Inter J Crude Res 1987; 25: 97-102.
[15] Bwititi P, Musabayane CT, Nihachi CF. Effects of Opuntia mwgacantha on blood glucose and kidney function in STZ diabetic rats. J Ethnopharmocol 2001; 69: 247-252.
[16] Hemalatha S, Wahi AK, Singh PN, Chansouria JPN. Hypoglycemic activity of Withania Coagulans dunal in streptozotocin induced diabetic rats. J Ethnopharmacol 2004; 93: 261-264.
[17] Ojewole JA. Laboratory evaluation of hypoglycemic effect of Anacardium occidentale Linn (Anacardiaceae) stembark extracts in rats. Methods Find Exp Clin Pharmacol 2003; 25: 199-204.
[18] Wilson TH, Wiseman G. The use of sacs of everted small intestine for the study of transference of substances from the serosal surface. J Physiol 1954; 123: 116-125.
[19] Haller H. Postprandial glucose and vascular disease. Diabetic Med 1997; 14: 50-56.
[20] Mayurkumar BP, Shrihari MM. Aldose reductase inhibitory activity of a C-glycosidic flavonoid derived from Enicostemma hyssopifolium. J Comp Integ Medie 2009; 6: 1.
[21] Meddah B, Ducroc R, Faouzi MEA, Eto B, Mahraoui L, Andaloussi AB, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharm 2009; 121: 419-424.
[22] Lin CJ, Chen CH, Liu FW, Kang JJ, Chen CK, Lee SL, et al. Inhibition of intestinal glucose uptake by aporphines and secoaporphines. Life Sci 2006; 79: 144-153.
ment heading
10.1016/S2221-6189(14)60008-1
*Corresponding author: Dr. S. Viviyan Therasa, Guest Lecturer, Department of Biotechnology, Thiruvalluvar Univeristy, Vellore-632115 (T.N.) India.
Tel: +91-8122609688
E-mail: viviyansathia@gmail.com
Hypoglycaemic study
GLUT2
Phyllanthus amarus
杂志排行
Journal of Acute Disease的其它文章
- Acute reaction to erroneous injection of adrenaline to the patients with hyperthyroidism
- Acute hepatitis with observed increased blood phenytoin level: a case study
- The value of C-reactive protein in emergency medicine
- Attitude and perception of junior resident doctors’ regarding antibiotic resistance - A pilot study
- MRSA toxic shock syndrome associated with surgery for left leg fracture and co-morbid compartment syndrome
- Stromal opacity secondary to preservative in dilating drops - A case report and review of literature
