Lagenaria siceraria ameliorates atheromatous lesions by modulating HMG-CoA reductase and lipoprotein lipase enzymes activity in hypercholesterolemic rats
2014-03-21MithunSinghRajputNeelamBalekarDineshKumarJain
Mithun Singh Rajput, Neelam Balekar, Dinesh Kumar Jain
Department of Pharmacology, College of Pharmacy, IPS Academy, Rajendra Nagar, A.B. Road, Indore - 452 012, M.P., India
Lagenaria siceraria ameliorates atheromatous lesions by modulating HMG-CoA reductase and lipoprotein lipase enzymes activity in hypercholesterolemic rats
Mithun Singh Rajput*, Neelam Balekar, Dinesh Kumar Jain
Department of Pharmacology, College of Pharmacy, IPS Academy, Rajendra Nagar, A.B. Road, Indore - 452 012, M.P., India
Objective: To investigate the antiatherosclerotic potential of Lagenaria siceraria (L. siceraria) by calculating percentage plaque area in aorta and grade of atheromatous lesions modulated by HMG-CoA reductase enzyme and lipoprotein lipase enzymes levels in hyperlipidemic rats.
Methods: Rats were divided into different groups, fed with high cholesterol atherogenic diet and in addition supplemented with ethanolic extract of fruits of L. siceraria and standard drug atorvastatin. At the end of the treatment schedule, the atherosclerotic lesion area was measured in cross-sections of the aortic root and grading of atherosclerotic lesions was done. The blood samples from animals of different groups were evaluated for serum lipid profile determination and plasma lipoprotein lipase activity and the hepatic HMG-CoA activity was also determined.
Results: Treatment of rats with ethanolic extract of fruits of L. siceraria significantly lowers the risk of atherosclerosis by lowering percentage plaque area in aorta and grade of atheromatous lesions in hypercholesterolemic rats and also serum cholesterol, triglyceride, LDL-c, VLDL-c and increased HDL-c levels as well. The extract also induced lipoprotein lipase activity and significantly decreased cholesterogenesis in liver by reducing HMG-CoA reductase activity in hypercholeaterolemic rats. Conclusion: It can be concluded that ethanolic extract of fruits of L. siceraria contains active components which ameliorates the atheromatous lesions in rat aorta and lowers the risk of atherosclerosis in hypercholesterolemic rats.
ARTICLE INFO
Article history:
Received 13 July 2013
Received in revised form 21 August 2013
Accepted 27 August 2013
Available online 20 March 2014
Atheromatous lesions
HMG-CoA reductase
Lagenaria siceraria
Lipid profile
Lipoprotein lipase
1. Introduction
Atherosclerosis is a disease of blood vessels and known colloquially as ‘hardening of the arteries’. It is characterized by the accumulation of fatty substance, cholesterol, cellular waste products, calcium and other substances in the inner lining of an artery. Major complications of atherosclerosis include angina pectoris, myocardial infarction and stroke, which are recognized as leading causes of morbidity and mortality in Western countries. The World Health Organization predicted that heart diseases and stroke are becoming more deadly, with a projected combined death toll of 24 million by 2030[1]. It is worth noting that the prevention of formation of atheromatous lesions has become one of the most important targets in the prophylaxis and therapy of cardiocirculatory disorders with atherothrombotic complications. The use of various drugs in treating such alignments is associated with high risk of myosistis, angiooedema, myoglobinuria, acute renal failure etc[2]. Because of the shortcomings of the available drugs, attempts are underway to explore natural sources with antiatherosclerotic activity.
A dietary product bottle gourd,Lagenaria siceraria(L. siceraria) (Molina) Standley, family Cucurbitaceae, is a climbing vine and possesses various medicinal properties.L. sicerariais official in Ayurvedic Pharmacopoeia of India. The fruits ofL. sicerariaare cooked as a vegetable part of food in India and many other countries. The fruits ofL. sicerariaare traditionally used as a nutritive agent having cardioprotective, cardiotonic, general tonic, diuretic, aphrodisiac, antidote to certain poisons and scorpion stings, alternative purgative and cooling effects. It cures pain, ulcers, and fever and also used for jaundice, pectoralcough, asthma and other bronchial disorders[3,4]. The edible portion of fruits is fair source of ascorbic acid, beta carotene and good source of vitamin B complex, pectin dietary soluble fibers and contains highest source of choline level-anisotropic factor[4]. Modern phytochemical screeningmethods showed the presence of triterpenoid cucurbitacins B, D, G, H and 22-deoxycucurbitacin “the bitter principle of cucurbitaceae”, fucosterol, campesterol[5,6], cytotoxic triterpenoids D:C-Friedooleanane[7], bryonolic acid an antiallergic compound[8]and flavone C-glycosides[9]. Lagenin, a novel ribosome inactivating protein has been isolated from the lyophilized water extract of seeds which is known to possess immunosuppressive, antitumour, antiviral, antiproliferative and anti-HIV activities[10]. A water soluble cytotoxic polysaccharide, isolated from fruits ofL. sicerariais composed of methyl-α-d-galactuoronate, 3-O-acetyle methyl-α-d-galacuoronate and β-dgalactose[11]. Pharmacological properties of the herb include hepatoprotective, antioxidant, antihyperglycemic, immunomodulatory, antihyperlipidemic, cardiotonic, diuretic, antibacterial, analgesic, anti-inflammatory, cytotoxic, cardioprotective, anti ulcer, anticestodal, urolithiatic, antihypertensive, fibrinolytic, antiplatelet, antithrombotic and antihelminthic activity[12,15] .
Previous studies indicated that various extracts of fruits ofL. sicerariaand its isolated compounds inhibited the total cholesterol, triglycerides, low-density lipoproteins level and significantly increased the high density lipoproteins level in normal and hypercholesterolemic rats showing antihyperlipidemic and hypolipidemic effect[16,17]. However, the direct measurement of its ameliorating potential on the atheromatous lesions has not been investigated yet. The present study aims to investigate the antiatherosclerotic potential of a commonly edible vegetableL. siceraria, by investigating its protective effect on atheromatous lesions in hypercholesterolemic rats. Additionally, an attempt has been made to reveal out the possible mechanism of lipid lowering mechanism of ethanolic extract of fruits ofL. sicerariaby measuring HMG-CoA reductase enzyme and lipoprotein lipase enzymes levels in hyperlipidemic rats.
2. Materials and methods
2.1. Chemicals and instruments
Olive oil (Figaro, Spain) was purchased from the local pharmacy, Indore, India. Cholecalciferol (60 000 IU), Cadila Pharmaceuticals, Ankleshwar, India; Cholesterol, Alpha Chemika, Mumbai, India and Atorvastatin, Ranbaxy Lab. Sirmour, India, were procured. Total cholesterol, triglyceride and high density lipoprotein cholesterol diagnostic kids were purchased from Reckon Diagnostic Kits, Vadodara, India. All the other chemicals and reagents used were of analytical grade and obtained from Sigma-Aldrich Co., Mumbai, India. Spectra were determined on Shimadzu UV-1601 (Shimadzu, Kyoto, Japan) spectrophotometer over a scanning range of 200-800 nm. Colorimetric estimations were made using Spectronic-20 colorimeter (Lab Sales Corporation, India).
2.2. Plant material
Fresh fruits ofL. sicerariawere purchased in August 2009, from the local market of Indore city. Authentication of the plant on basis of pharmacognostic study and organoleptic characteristics was done by Mr T. Chakraborty, Scientist ‘D’for Joint Director, Botanical Survey of India, Pune, India. A voucher specimen (MITRALAS6) has been deposited in our college herbarium for future reference.
2.3. Preparation of L. siceraria ethanolic extract
Fruits ofL. sicerariawere sliced and shade dried. After complete removal of moisture, fruits were pulverized to obtain coarse powder and defatted by maceration with petroleum ether for 48 h. The defatted material was then subjected to Soxhlet extraction with 95% ethanol and concentratedin vacuoto obtainL. sicerariaethanolic extract (LSEE). LSEE was stored in tightly closed container and kept in refrigerator. The amount of extract calculated as per the dose was dissolved in distilled water for oral administration to experimental animals. Preliminary phytochemical screening of LSEE was done according to standard procedures[18] .
2.4. Animals
Wistar rats of either sex weighing (300 ± 15) g were procured from the animal house of College of Pharmacy, IPS Academy, Indore, India. Animals were placed randomly in polypropylene cages under standard conditions of humidity (55 ± 5)%, temperature (25 ± 2) ℃ and 12 h/12 h, light/dark cycles and free access standard pellet diet (Hindustan Lever Ltd., Mumbai, India) and filtered waterad libitum. Experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care as per the US guidelines (NIH publication #85-23, revised in 1985). Experiments were conducted after obtaining the approval from Institutional Animal Ethics Committee constituted as per Committee for the Purpose of Control and Supervision of Experiments on Animals.
2.5. Acute toxicity studies
Acute toxicity studies were performed as per OECD-423 guidelines on randomly selected rats of either sex (n=6). LSEE (5 mg/kg) was administered orally after overnight fasting. Since mortality was not observed, the procedure was repeated with higher doses viz. 50, 300 and 2 000 mg/kg body weight[19]. The animals were observed for toxic symptoms and mortality for 72 h.
2.6. Antiatherosclerotic activity in hypercholesterolemic rats
Albino wistar rats of either sex weighing (300 ± 15) g were divided into five different groups of six rats each. For the whole experimental period that is for five days, animals of first group received normal pellet diet (normal group), while second group received atherogenic diet (control group), third, fourth and fifth group in addition with atherogenic diet received 200, 400 mg/kg, p.o. of LSEE and atorvastatin (10 mg/kg, p.o., suspended in 1% gum acacia) respectively. Atherogenic diet contained per kg body weight and per day1.5 mL of an olive oil solution, containing per ml: vitamin D3(cholecalciferol) 320 000 IU and cholesterol 40 mg[20-22]. All the animals received commercial pellet diet and waterad libitumfor the period of study.
At the end of study period that is on sixth day the overnight fasted rats were sacrificed under ether anesthesia. Blood was collected directly from the heart and stored at -20 ℃for biochemical estimations. Liver was removed and stored in formaldehyde solution to determine HMG-CoA reductase activity. The aorta extending from the base of the heart to the iliac bifurcation was removed. The aorta was sliced to make strips of 2 × 1 cm and plaque formation on the internal surface was photographed by using a Sony digital still camera (DSC-W180). Planimetry studies of cross sections of aorta were made with the help of camera lucida drawings at 20×. The level of plaque formation on the internal aorta was calculated by using the equation: (area of plaque formation on internal aorta/total area) × 100. Grading of atherosclerosis was done on 0-4 scale as described by Duff and Mcmillan, 1949. The total number of lesions in each aorta was counted as follows. The total number of lesions in each group was then pooled and percentage of each grade of lesion in each group was calculated. Grade Zero: Gross inner surface of aorta was smooth and shining. Grade 1: Gross-small, pin head size elevations on intimal surface which could be easily separated from intimal surface. Grade 2: Fibrous plaques one or more often elongated in shape and their size varied from pin head to few millimeters elevated from surface and could not be detached easily. Grade 3: Irregular map like patches of 10-15 mm in size with yellow color. Grade 4: Big whitish yellow areas with marked deposition of calcium.
For biochemical estimations, serum samples were analyzed for total cholesterol (TC), triglyceride (TG) and high density lipoprotein cholesterol (HDL-c) using diagnostic kids (Reckon Diagnostic Kits, Vadodara, India). Low density lipoprotein cholesterol (LDL-c) and very low density lipoprotein cholesterol (VLDL-c) were determined using Fridwald’s formula. Atherogenic Index was also calculated to access the atherogenic risk[23].
Friedewald’s Formula: LDL = TC - [VLDL-c + HDL-c], where VLDL-c = TG/5.
Atherogenic Index (AI) = TG/HDL-c
For histopathological studies, aorta specimens were fixed in 10% buffered formaldehyde solution, sectioned and embedded in paraffin wax using conventional techniques. The aortas were cut transversely at the arch and mid thoracic level. They were fixed in bouin’s fluid for 24 h and process for routine histopathological examination by passing through graded alcohols. Tissue slide sections of 6-8 μm thickness were taken with the help of microtome, transferred on glass slides and stained with haematoxylin and eosin. The stained sections were examined under Olympus photomicroscope (Magnus MLX-B series, India Pvt. Ltd.) and photographed (40×).
2.7. Plasma lipoprotein lipase (LPL) assay
Lipoprotein lipase was assayed in plasma by the method of Korn (1962)[24]. The amount of glycerol liberated was determined calorimetrically.
Incubation: The incubation mixture consisted of 0.4 mL of 20% albumin (pH 8.5), 0.1 mL ammonium sulfate, 0.1 mL substrate, 0.1 mL enzyme source and sufficient water to make up a final volume of 1.0 mL. The plasma collected by centrifugation of blood from different group animals at 3 000 rpm for 5 min was used as the enzyme source. The human lipoid serum (TG < 400 mg/dL) was used as the substrate. Albumin was added to the incubation tube to bind the un-esterified fatty acids which otherwise will inhibit the reaction. Ammonium ions were also added to provide the necessary activating cation. The mixture was incubated at 37 ℃ for 1 h and the aliquots were taken into tubes containing 0.1 mL of 1 N H2SO4at intervals of 0, 30, 60, 90 min. The samples were transferred directly into a 10 mL conical tip centrifuge tube containing 0.1 mL of 1N H2SO4.
Glycerol determination: Sodium periodate (0.1 mL, 0.05 M) was added to the centrifuge tube, mixed well and allowed to stand at room temperature for 5 min. Then 0.1 mL sodium arsenate (0.05 M) was added, mixed well and again allowed to stand at room temperature for 10 min. This was followed by the addition of 9.0 mL chromotropic acid, mixed by inversion (covering the top of the tube by paraffin) and placed in a boiling water bath for 30 min, cooled and the volume adjusted to 10 mL with water. The optical density was read at 570 nm. The assay was standardized with glycerol solution of known molarity and the glycerol liberated was calculated. The activity of lipoprotein lipase is expressed as mg of glycerol liberated/h/dL plasma or mg of glycerol liberated/h/mg protein. The same procedure was repeated using plasma of animals treated with LSEE (200 and 400 mg/kg) and atorvastatin (10 mg/kg) for a period of 10 min. The glycerol liberated was calculated and compared with normal untreated group[24].
2.8. Hepatic hydroxy-methyl glutaryl-coenzyme a reductase (HMG-CoA reductase) assay
The ratio between HMG-CoA and mevalonate in tissues was taken as an index of the activity of HMG-CoA reductase as described by Raoet al[25]. Equal volumes of fresh 10% tissue homogenate and dilute perchloric acid were mixed, kept for 5 min and centrifuged at 2 000 × g for 10 min. To 1.0 mL of filtrate, 0.5 mL of freshly prepared alkaline hydroxylamine reagent was added, mixed and after 5 min 1.5 mL of FeCl3was added and shaken well. Readings were taken after 10 min at 540 nm against a similarly treated saline-arsenate blank. The ratio (absorbance of HMG-CoA/ absorbance of mevalonate) was determined and is taken as an index of the activity of HMG-CoA reductase required to convert HMG-CoA to mevalonate. Lower ratio indicates higher enzyme activity and vice-versa. The same procedure was repeated using tissue homogenates of animals treated with LSEE (200 and 400 mg/kg) and atorvastatin (10 mg/kg) [25].
2.9. Statistical analysis
Data was expressed as the mean ± standard error of mean(S.E.M) and statistical analysis was carried out employing‘unpaired two-tailed student t test, Welch corrected’ and one way analysis of variance (ANOVA) followed by ‘Dunnett’multiple comparisons test ofP<0.05 significance level using“Graphpad Instat” version 3.00 for windows 95, Graphpad software, San Diego, California, USA.
3. Results
The percentage yield of LSEE was found out to be 28.8%. Preliminary phytochemical screening of LSEE revealed the presence of polyphenolics as flavonoids (C-flavone glycosides, ellagitannins), triterpenoids (saponins, cucurbitacins), sterols, soluble diatery fibres, insoluble cellulose fibres, pectin, cardiotonic aglycones, proteins and carbohydrates.
No mortality occurred in the group of rats receiving up to 2 000 mg/kg p.o. LSEE (LD50>2 000 mg/kg). Hence, in this study 1/10thand 1/5thof the dose that is, 200 and 400 mg/ kg has been used.L. sicerariais being used as a vegetable since ancient time, fresh juice obtained from 200-300 gL. sicerariafruit is recommended in ‘Dudhi Therapy’[26], indicating its well established safety.
The serum levels of cholesterol and triglycerides and atherogenic index were increased by 4.0, 5.6 and 12.60 fold respectively, five days after the administration of atherogenic diet in control group animals. LSEE (200 and 400 mg/kg, p.o.) administration effectively prevented this rise in cholesterol and triglycerides. The effect of LSEE is almost comparable to that of atorvastatin. Table 1 shows the values of serum lipid profile in normal, cholesterol fed (control) and various treatment groups. Serum total cholesterol, LDL-c, VLDL-c and triglycerides levels increased and HDL-c level decreased significantly (P<0.01) after 5 d of high atherogenic diet feeding. Concurrent administration of LSEE (200 and 400 mg/kg, p.o.) with atherogenic diet caused a significant decrease (P<0.01) in the levels of serum total cholesterol, LDL-c, VLDL-c and triglycerides and significant increase (P<0.01) in HDL-c when compared with cholesterol fed control rats. The atherogenic index was also declined significantly as compared to cholesterol fed control rats.
The aorta of atherogenic diet fed rats showed the presence of large atherogenic plaque protruding in the lumen covering 28.90% area. Administration of LSEE at dose of 200 and 400 mg/kg caused a significant amelioration in the atherosclerotic lesions as evidenced by significant (P<0.01) reduction in the plaque size up to 13.61% and 9.37%. Furthermore, atorvastatin (10 mg/kg) decreased the atherogenic plaque up to 2.82% (Table 2).
The liver HMG-CoA reductase and lipoprotein lipase enzymes activity are shown in Figure 1. LSEE at dose of 200 mg/kg did not exhibit significant change in hepatic HMGCoA reductase activity as compared with control group. However, the extract at dose of 400 mg/kg and atorvastatin (10 mg/kg) showed significant increase (P<0.05 andP<0.01 respectively) in the ratio of HMG-CoA to mevalonate, indicating decreased HMG-CoA reductase enzyme activity. For the measurement of lipoprotein lipase activity, LSEE treated animals for a period of 10 min showed increased production of glycerol as an index of the greater release. The glycerol liberated in the extract treated and atorvastatin treated animals was found to be increased significantly (P<0.01) as compared with control group.
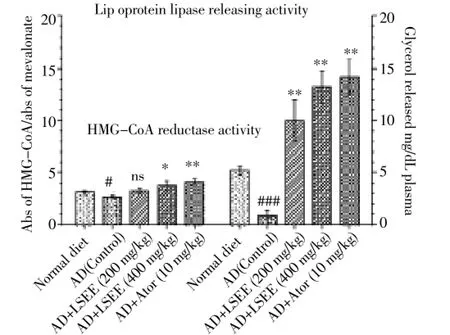
Figure 1. Modulatory effect of ethanolic extract of fruits of L. siceraria on plasma lipoprotein lipase releasing activity and HMGCoA reductase activity in hypercholesterolemic rats.AD: Atherogenic diet, it contained (kg-1, day-1) 1.5 mL of olive oil solution containing per ml: vitamin D3(cholecalciferol) 320 000 IU and cholesterol 40 mg. LSEE: L. siceraria ethanolic extract, LSEE: L. siceraria ethanolic extract, Ator: Atorvastatin.
The lesions in the rats receiving atherogenic diet only, weregenerally calcified and exhibited grade 4 atherosclerosis. Whereas the other groups treated additionally with LSEE (200 and 400 mg/kg) and atorvastatin (10 mg/kg) exhibited few lesions and largely atherosclerosis of grade 1 and 2. No calcified lesions were seen in these treated groups (Figure 2).
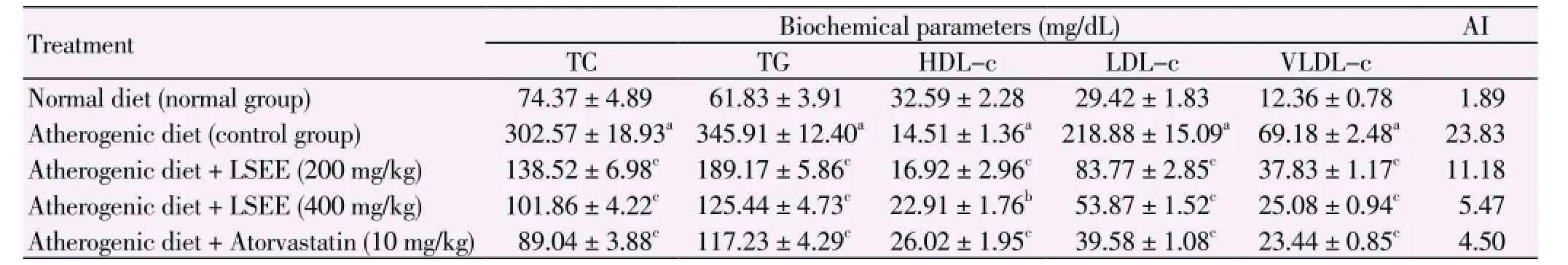
Table 1 Effects of ethanolic extract of fruits of L. siceraria and atorvastatin on serum biochemical parameters in hypercholesterolemic rats.
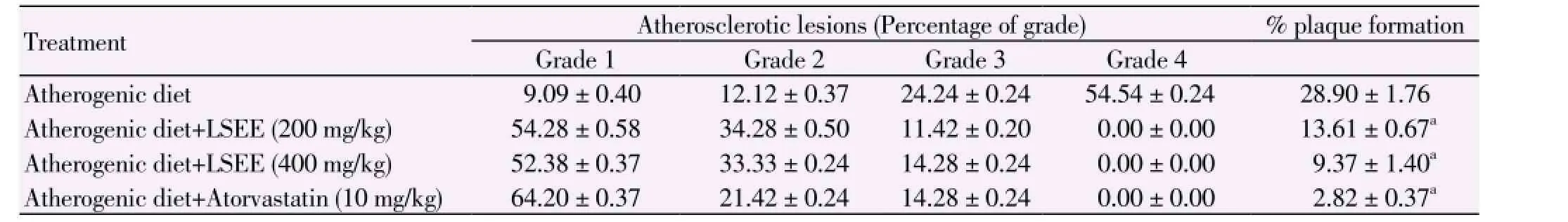
Table 2 Effects of ethanolic extract of fruits of L. siceraria and atorvastatin on atheromatous lesions in hypercholesterolemic rats.
4. Discussion
Recently there is an increased research in herbal medicines after observations that they are effective for conditions to which they have traditionally been applied. Herbal medicines are fast emerging as an alternative treatment to available synthetic drugs for treatment of various diseases possibly due to lower cost, availability, fewer adverse effects and perceive effectiveness. The present investigation has explored the use of one such plantL. siceraria, abundantly found in the Indian continent, for antiatherosclerotic activity.
Atherogenicity with subsequent cardiovascular manifestations is one of the important causes of high mortality and morbidity. It is well documented that elevated lipid levels and their oxidative modification appears to have an important role in initiation and progression of atherogenic changes in aorta. Cholesterol feeding in rats causes a significant increase in the circulating total cholesterol, LDL-c, VLDL-c, triglycerides and decrease in HDL-c levels indicating increased risk of atherosclerosis and coronary heart disease[27]. Previous studies indicate that various extracts of fruits ofL. sicerariaand their isolated compounds exhibit significant effects in lowering total cholesterol, triglyceride and LDL-c along with increase in HDL-c reporting marked antihyperlipidemic and hypolipidemic activity[16,17]. The effects of LSEE on serum TNF-α indicating lowering effect on fat amassment in obese rats has also been investigated[28]. Total cholesterol content of the aorta is a good indirect measure of atherosclerotic severity in cholesterol fed rats[29]. However, the direct measurement of its ameliorating potential on the atheromatous lesions has not been investigated yet. Hence, in this study an attempt has been made to access the antiatherosclerotic potential ofL. sicerariaby calculating percentage plaque area in aorta and grade of atheromatous lesions in hypercholesterolemic rats.
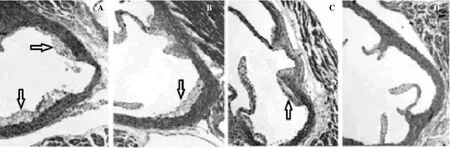
Figure 2. Histopathology of cross sections of aorta from atherogenic diet treated (A), atherogenic diet + LSEE (200 mg/kg) treated (B), atherogenic diet + LSEE (400 mg/kg) treated (C) and atherogenic diet + atorvastatin (10 mg/kg) treated (D) rats.Atherosclerotic lesions were detected as areas of light staining in the wall of the aortic sinus (arrows) (40×). Atherogenic diet: It contained (Kg-1, day-1) 1.5 mL of olive oil solution containing per ml: vitamin D3 (cholecalciferol) 320 000 IU and cholesterol 40 mg. LSEE: L. siceraria ethanolic extract.
The search for a laboratory model to study potential antiatherosclerotic compounds has been well documented[30]. Generally rabbit models are potential targets to study antihyperlipidemic and hypolipidemicstudiesin vivo. The rat has received little attention and is generally considered to be resistant to the induction of atheromatous lesions and required to more than one month to induce hyperlipidemia. However, following the reports of Altman (1972)[21] and Testaet al[31]a short-term induction of atherosclerosis in the rat seemed possible using large doses of vitamin D2and cholesterol. Parkeet al[22] have also undertaken a series of experiments to investigate the methodology to induce hyperlipidemia in rats in a period of five days and succeeded. Similarly, Lataet al[20]carried out a study to compare three herbal extracts for their beneficial effects against hyperlipidemia in rats in five days. In our study, successful attempts are made to induce hypercholesterolemia in five days in rats using cholesterol, cholecalciferol and olive oil and the results are found consistent with previous studies.
Various agents which affect hyperlipidemia are still not used for prevention of atherosclerosis because of their potential toxicity and intolerance.L. sicerariais reported to lower serum lipid levels and is well tolerated as it forms the constituent of customary Indian diet. In present study, LSEE (200 and 400 mg/kg) administration effectively prevented rise in the levels of serum total cholesterol, LDL-c, VLDL-c and triglycerides and significant increase in HDL-c when compared with cholesterol fed control rats, consistent with what have been reported previously[16,17,28].
Administration of LSEE (200 and 400 mg/kg) caused a significant amelioration in the atherosclerotic lesions (28.90%) as evidenced by significant reduction in the plaque size up to 13.61 and 9.37%. It has been reported that changes in membrane cholesterol content affects its fluidity, permeability to ions, activities of membrane bound enzymes and increased degradation of phospholipids[32]. Hypercholesterolemia has been reported to cause endothelial cell dysfunction, as evidenced by an increase in endothelial cell turnover in cholesterol fed rabbits and swine and increased permeability of the endothelium in cholesterol fed rabbits[33]. In our study, aortic cross sections from rats fed with atherogenic diet exhibited well defied calcification, which is an indication of advanced complicated plaques. With extract treatment aortic cross sections were found to be normal and atherosclerosis was reversed.
Alteration of lipid profile is controlled by those enzymes that are responsible for lipid metabolism. The current study has demonstrated the effect of LSEE on HMG-CoA reductase enzyme and lipoprotein lipase enzymes activity for cholesterogenesis and plasma lipids regulation respectively revealing possible mechanism of antihyperlipidemic action of the extract. In this study cholesterol fed rats showed a significant decrease in plasma lipoprotein lipase activity. Lipoprotein lipase is the atheroprotective enzyme expressed by parenchymal cells, involved in the metabolism of triglycerides rich lipoproteins. Elevated triglyceride rich lipoprotein levels may not only promote a more rapid progression of atherosclerosis but also lead to myocardial ischemia[32]. The lower activity of this enzyme indicates decreased uptake of circulating triglyceride rich lipoproteins. LSEE treated group showed a significant increase in lipoprotein lipase activity indicating its protective effects towards atherogenesis in hypercholesteroemic rats due to elevation of HDL-c. The HDL-c has a dual anti-atherogenic effect: it functions as a removal vehicle for cholesterol, transporting it away from areas of production to the liver where it can be excreted as bile salts and, in addition, it has been shown to prevent the oxidation of LDL-c by metal ions[34] .
The liver occupies a key position in cholesterol metabolism. Hepatocytes derive cholesterol from circulating lipoproteins or byde novosynthesis and use it for membrane synthesis, bile acid synthesis and secretion, secretion of free sterol into the bile, lipoprotein formation, and storage of excess sterol as cholesterol ester[34]. The hepatic enzyme HMG-CoA catalysis the rate limiting step in cholesterol biosynthesis in the tissues and its activity closely correlates with cholesterogenesis in the tissues. Regulation appears to involve changes in the rate of reductase synthesis, modulation of catalytic activity and the action of hormones. Defective regulation of HMG-CoA reductase has been demonstrated previously in hepatic tumor cells and has been implicated in familial hypercholesterolemia. The increased activity of the enzyme in the liver of rats fed with high fat diet corresponds with increased cholesterogenesis, as indicated by the higher incorporation of blood plasma total cholesterol and LDL-c cholesterol was observed in our current study.
The animals treated with LSEE (400 mg/kg) and atorvastatin (10 mg/kg) had shown decreased activity of enzyme as evidenced by the reduction in plasma total cholesterol and LDL-c. By inhibiting HMG-CoA reductase, statins reduce the hepatocyte cholesterol content and increase expression of LDL-c receptors, responsible for LDL-c cholesterol uptake via receptor-mediated endocytosis[35]. Additionally, a second mechanism of LDL-c reduction may relate to LDL-c and VLDL-c interactions. However, increases in HMGCoA reductase synthesis shortly after statin therapy restore cellular VLDL-c levels, and the ultimate effect of reductase inhibition is enhanced LDL-c receptor expression and lower plasma LDL-c in the setting of normal cellular cholesterol content[36] .
Kaempferol and isoquercetrin flavonoids have been isolated from the fruits ofLagenaria siceraria[37,13]. Flavonoids may augment the activity of lecithin aryl transferase (LAT) which regulates blood lipids by incorporation of free cholesterol into HDL-c and transferring it back to VLDL-c and LDL-c which are taken back later in lever cells. Polyphenolic compounds also possess a variety of biological activities, such as reduction of plasma lipids, which might be due to the up-regulation of LDL receptor expression[38], inhibition of hepatic lipid synthesis[39] and lipoprotein secretion[40]. It is possible that the activityin lowering lipid levels and aortic plaque formation of theL. sicerariafruit extract may result from the phenolic compounds present in the extract.
It has also been ascertained that parts of soluble dietary fibers such as pectin from the fruits ofL. sicerariamay have cholesterol lowering effect. Soluble dietary fiber contents are having the beneficial effect in the promotion of bile acid formation and their excretion in the stool or in the blockade of cholesterol absorption[41].
The lipid lowering effects of the extract may be due to its content of plant sterols and fixed oils which are considered as good source of mono and poly unsaturated fatty acids and cardiac aglycones. The white sterol crystals or phytosterols from the methanol extract ofL. sicerariawere isolated and identified as a mixture of four sterols, including fucosterol, racemosol, stigmasterol and stigmasta-7,22-dien-3β, 4β-diol[42]. Plant sterol reduces intestinal absorption of cholesterol resulting in an increase in fecal excretion of neutral lipids and steroids which results in decrease of body lipids[43].
Saponins and fatty oil are the major phytoconstituents present in fruits ofL. siceraria. Bitter fruits yield 0.013% of a solid foam containing cucurbitacin B, D, G and H, mainly cucurbitacin B. These bitter principles are present in the fruit as aglycones[3]. The presence of large amount of saponins in fruits ofL. sicerariamay be the phytoconstituent responsible for lipid lowering property. Saponins also act as antihyperlipidemics by one of the following mechanisms as by binding with cholesterol in intestinal lumen, so that cholesterol is less readily absorbed or bile acids causing reductions in its extra hepatic circulation and increasing metabolism of cholesterol to sterols through their fecal secretion. Increase in bile acid excretions offset by enhanced synthesis from cholesterol in the lever consequently lowers the plasma cholesterol. Saponins are also reported to increase the lipoprotein lipase activity, which is helpful in faster removal of free fatty acid from circulation that causes in turn a decrease in total cholesterol[44,45]. Thus on the basis of results of the present study, it can be concluded that ethanolic extract of fruits ofL. sicerariahave a definite potential as reported for several traditional antihyperlipidemic drugs. Further research on the fractionation of extracts, isolation, purification and characterization of active constituents responsible for the lipid lowering activity and their mechanisms are in progress.
From these result it can be concluded that ethanolic extract of fruits ofL. sicerariacontains active components which decreases serum lipid profile and lowers the risk of atherosclerosis by lowering percentage plaque area in aorta and grade of atheromatous lesions in hypercholesterolemic rats.
Ethanolic extract of fruits ofL. sicerariahas been shown to induce lipoprotein lipase activity and significantly decreased cholesterogenesis in liver by reducing HMG-CoA reductase activity in hypercholeaterolemic rats. However, further studies are required to gain more insight in revealing out the precise mechanisms underlying these effects.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgement
This investigation is financially supported by the Department of Pharmacology, College of Pharmacy, IPS Academy, Indore, M.P., India.
[1] Reinhardt E. Health Watch: The Atlas of Heart Disease and Stroke. 1st ed. New York: UN Chronicle online; 2005.
[2] Rang HP, Dale MM, Ritter JM, Flower RJ. Pharmacology. London: Churchill Livingstone; 2007.
[3] Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun: Oriental Enterprises; 2001.
[4] Nadakarni KM, Nadakarni AK. Indian Materia Medica. Mumbai: Popular Prakashan; 1992.
[5] Enslin PR, Holzapfel CW, Norton KB. Bitter principles of the cucurbitaceae. Part XV, Cucurbitacins from a hybrid of Lagenaria siceraria. J Chem Soc (C) 1967; 10: 964-965.
[6] Shirwaikar A, Sreenivasan KK. Chemical investigation and antihepatotoxic activity of the fruits of Lagenaria siceraria. Ind J Pharm Sci 1996; 58(5): 197-202.
[7] Chen CR, Chen HW, Chang CI. D:C-Friedooleanane-type triterpenoids from Lagenaria siceraria and their cytotoxic activity. Chem Pharm Bul 2008; 56(3): 385-388.
[8] Tabata M, Taluka S, Shimakura J, Ito M. Production of an antiallergic triterene bryonolic acid by plant tissue cultures. J Nat Prod 1993; 56(2): 165-175.
[9] Baoranoswka KM, Cisowski W. HPLC determination of flavone-C glycosides in some species of Cucurbitaceae family. J Chromatogram-A 1994; 675(1-2): 240-243.
[10] Wang HX, Ng TB. Lagenin, a novel ribosome-inactivating protein with ribonucleolytic activity from bottle gourd (Lagenaria siceraria) seeds. Life Sci 2000; 67(21): 2631-2638.
[11] Ghosh K, Chandra K, Arnab K, Ojha SS, Islam SS. Structural identification and cytotoxic activity of a polysaccharide from the fruits of Lagenaria siceraria (Lau). Carbohydrate Res 2009; 344(5): 693-698.
[12] Upaganlawar A, Balaraman R. Bottle gourd (Lagenaria siceraria) a vegetable food for human health - a comprehensive review. Pharmacol Online 2009; 1: 209-226.
[13] Rajput MS, Mathur V, Agrawal P, Chandrawanshi HK, Pilaniya U. Fibrinolytic activity of kaempferol isolated from fruits of Lagenaria siceraria (Molina) Standley. Nat Prod Res 2011; 25(19): 1870-1874.
[14] Takawale RV, Mali VR, Kapase CV, Bodhankar SL. Effect of Lagenaria siceraria fruit powder on sodium oxalate inducedurolithiasis in wistar rats. J Ayur Integret Med 2012; 3(2): 75-79.
[15] Rajput MS, Balekar N, Jain DK. Inhibition of ADP-induced platelet aggregation and involvement of non-cellular blood chemical mediators are responsible for the antithrombotic potential of fruits of Lagenaria siceraria. Chinese J Nat Med 2013. [Accepted] [Elsevier].
[16] Ghule BV, Ghante MH, Saoji AN, Yeole PG. Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian J Exp Biol 2006; 44(11): 905-909.
[17] Mohale DS, Dewani AP, Saoji AN, Khadse CD. Antihyperlipidemic activity of isolated constituents from the fruits of Lagenaria siceraria in albino rats. Int J Green Pharm 2008; 2(2): 104-107.
[18] Harborne JB. Phytochemical Methods-A Guide to Modern Techniques of Plant Analysis. 2nd ed. New York: Chapman and Hall; 1984.
[19] Ecobichon DJ. The Basis of Toxicology Testing. 2nd ed. New York, CRC Press, 1997.
[20] Lata S, Saxena KK, Bhasin V, Saxena RS, Kumar A, Srivastava VK. Beneficial effects of Allium sativum, allium cepa and Commiphora mukul on experimental hyperlipidemia and atherosclerosis a comparative evaluation. J Postgrad Med 1991; 37(1): 132-135.
[21] Altman RF. A simple method for the rapid production of atherosclerosis in rats. Experientia 1973; 29(2): 256-257.
[22] Parke DV, Sacra PJ, Thornton PC. Experimental atherosclerosis in the Wistar rat. British J Pharmacol 1978; 63(2): 346-347.
[23] Freidewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6): 499-502.
[24] Korn ED. Lipoprotein lipase (clearing factor). In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. 5th ed. New York: Academic Press; 1962.
[25] Rao AV, Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl CoA reductase (NAOPH) activities in liver tissue. Clin Chem 1975; 21(10): 1523-1525.
[26] Kothari M. Hridaya Rakshak Lauki Ras. 1st ed. Nagpur: Hridaya Mitra Mandal; 2005.
[27] Ram A. Effect of Plumbago zeylanica in hyperlipidaemic rabbits and its modification by vitamin E. Ind J Pharmacol 1996; 28(3): 161-166.
[28] Nadeem S, Dhore P, Quazi M, Pawar S, Raj N. Lagenaria siceraria fruit extract ameliorate fat amassment and serum TNF-in high-fat diet-induced obese rats. Asian Pac J Trop Med 2012; 5(9): 698-672.
[29] Nielson LB, Novdenstgard BG, Stender S, Kieldness K. Aortic esterified cholesterol is not superior to total cholesterol as a measure of atherosclerosis severity in cholesterol fed rabbits. Atherosclerosis 1993; 99(1): 133-136.
[30] Constantinides P. Experimental Atherosclerosis. 1st ed. London: Elsevier; 1965.
[31] Testa R, Canestrini C, Oldani C. Experimental atherosclerosis in the rat: Biochemical evaluation. J Pharm Pharmacol 1975; 27(9): 699-700.
[32] Upaganlawar A, Balaraman R. Effect of Lagenaria siceraria fruit juice on lipid profile and glycoprotein contents in cardiotoxicity induced by isoproterinol in rats. Toxicol Int 2012; 19(1): 15-19.
[33] Siriniwas M, Annapurna A, Reddy YN. Anti-atherosclerotic effect of atorvastatin and clopidogrel alone and in combination in rats. J Exp Biol 2008; 46(10): 698-703.
[34] Prasanth KG, Kalpana E, Dineshkumar B, Monogaran E, Geetha G, Venkatanarayanan R. Tetrahydrocurcumin: Beneficial effects on HMG-CoA reductase enzyme and lipoprotein lipase enzymes in high-fat diet-induced hypercholesteremia in rabbits. Pharmacog Commun 2012; 2(2): 50-60.
[35] Brown MS, Goldstein JL. Multivalent feedback regulation of HMG-CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res 1980; 21(5): 505-517.
[36] Kostner GM. Gavish D. Leopold B. HMG-CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation 1989; 80: 1313-1319.
[37] Gangwal A, Parmar SK, Sheth NR. Triterpenoid, flavonoids and sterols from Lagenaria siceraria fruits. Der Pharmacia Lettre 2010; 2(1): 307-317.
[38] Kuhn DJ, Bums AC, Kazi A, Dou QP. Direct inhibition of the ubiquitin-proteosome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochimica Biophysica Acta 2004; 1682(1-3): 1-10.
[39] Theriault AG, Wang Q, Van Iderstine SC, Chen B, Franke AA, Adeli K. Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid. J Lipid Res 2000; 41(12): 1969-1979.
[40] Borradaile NM, de Dreu LE, Barrett PHR, Behrsin CD, Huff MW. Hepatocyte apoB containing lipoprotein secretion is decreased by grapefruit flavonoid, naringenin, via inhibition of MTP mediated microsomal triglyceride accumulation. Biochemistry 2003; 2(5): 1283-1291.
[41] Sannoumaru YJ, Shimizu M. Effects of semi-purified dietary fibers isolated from Lagenaria siceraria, Raphanus sativus and Lentinus edobus on fecal steroid excretion in rats. J Nutritional Sci Vit 1996; 42(2): 97-110.
[42] Kalsait RP, Khedekar PB, Saoji AN, Bhusari KP. Isolation of phytosterols and antihyperlipidemic activity of Lagenaria siceraria. Archieves Pharm Res 2011; 34(10): 1599-1604.
[43] Purohit A, Vyas KB. Antiatherosclerotic effect of Caparis decidua fruit extract in cholesterol-fed rabbits. Pharm Biol 2006; 43(10): 172-177.
[44] Sidhu GS, Oakenful DG. A mechanism for the hypercholesterolemic activity of saponins. Br J Nutr 1986; 55: 643-649.
[45] Sidhu GS, Oakenful DG. Could saponins be a useful treatment for hypercholesteroaemia? Eur J Clin Nutr 1990; 44(1): 79-88.
ment heading
10.1016/S2221-6189(14)60004-4
*Corresponding author: Mithun Singh Rajput, Department of Pharmacology, College of Pharmacy, IPS Academy, Rajendra Nagar, A.B. Road, Indore - 452 012, India.
Tel: +917314041627
E-mail: mithun.sgsits@gmail.com
杂志排行
Journal of Acute Disease的其它文章
- Acute reaction to erroneous injection of adrenaline to the patients with hyperthyroidism
- Acute hepatitis with observed increased blood phenytoin level: a case study
- The value of C-reactive protein in emergency medicine
- Attitude and perception of junior resident doctors’ regarding antibiotic resistance - A pilot study
- MRSA toxic shock syndrome associated with surgery for left leg fracture and co-morbid compartment syndrome
- Stromal opacity secondary to preservative in dilating drops - A case report and review of literature
