HPTLC evaluation of oleanolic acid and ursolic acid from the methanol extract of Wattakaka volubilis
2014-03-21VelmaniGopalVivekanandaMandalSubhashMandal
Velmani Gopal, Vivekananda Mandal, Subhash C. Mandal*
1Pharmacognosy and Phytotherapy Research Laboratory, Division of Pharmacognosy, Department of Pharmaceutical Technology, Jadavpur University, Kolkata-700032, India
2Institute of Pharmacy, Guru Ghasidas Central University, Bilaspur 495009, India
HPTLC evaluation of oleanolic acid and ursolic acid from the methanol extract of Wattakaka volubilis
Velmani Gopal1, Vivekananda Mandal2, Subhash C. Mandal1*
1Pharmacognosy and Phytotherapy Research Laboratory, Division of Pharmacognosy, Department of Pharmaceutical Technology, Jadavpur University, Kolkata-700032, India
2Institute of Pharmacy, Guru Ghasidas Central University, Bilaspur 495009, India
Objective: To find out the secondary metabolites present in the methanol extract of Wattakaka volubilis (W. volubilis). Methods: High performance thin layer chromatography method for the quantification of triterpenoids in soxhlet methanol extract of W. volubilis is described by densitometric scanning. The linear regression data from the calibration curve was plotted over the range of 10-25 μg/mL, r2= 0.992 46, 0.950 42 respectively. A mixture of toluene: methanol (9:1) was used as mobile phase for oleanolic acid were petroleum ether: chloroform: ethyl acetate: methanol (4:1:0.1:0.1) were used for ursolic acid. Results: The results showed that the presence of oleanolic acid and ursolic acid in methanol extract. The content found to be 218.30 ng and 509.99 ng/10 mg of extract. Conclusion: For conclusion, above study scientifically validated as a useful traditional medicine with the identification of bioactive secondary metabolites.
ARTICLE INFO
Article history:
Received 24 July 2013
Received in revised form 29 August 2013
Accepted 31 August 2013
Available online 20 March 2014
Wattakaka volubilis
1. Introduction
There has been an increasing interest in the study of medicinal plants as natural products to different parts around the world[1]. The medicinal value of plants depends on bioactive phytochemical constituents who produce definite physiological action in the human body. Based on World Health Organization recommendations, plant origin is important for use in traditional medicine[2].
Since ancient times, the medicinal properties of plants have been investigated in the recent scientific developments throughout the world. Although the original hopes regarding their therapeutic usefulness were not immediately realized, recent researchers have demonstrated their involvement in the medicinal action of a number of plant drugs. It is very probable that a number of herbal remedies, the constituents of which are still unknown. Quantitative estimation of these compounds is important for current research, and a variety of methods are required for this.Wattakaka volubilis(W. volubilis) is a medicinal member of the family Asclepiadaceae well-known for their ethnobotanical importance and widely used in Indian traditional medicines.
In detailed chemical investigation of theW. volubiliswere reported that phenolic acids (chlorogenic acid and hydroxycinnamic acids), flavonol glucosides (rutin, kaempferol3-rutinoside, isorhamnetin 3-rutinoside) and triterpenoids[3-5]. GC–MS analysis of ethanol extracts ofW. volubilisidentified octadecatrienoic acid,n-hexadecanoiacid tetracosahexaene, diiooctylester, phytol, benzenedicarboxylic acid, 3-0-methyl–d-glucose, and octadecatrienic acid[6], multiflor-7-en-12, 13-ether and multiflor-7-en-12α-ol[7] were reported as the major components.
Consequently, present study was focused on the quantitative estimation in the leave methanol extract ofW. volubilisfor the identification of ursolic acid and oleanolic acid by high performance thin layer chromatography.
2. Materials and methods
2.1. Chemicals and reagents
Toluene, methanol, petroleum ether, chloroform, ethyl acetate. The TLC plates 60F254, E. Merck were used without any pretreatment. All the chemicals and reagents procured were of A.R. grade.
2.2. Plant material and extraction
Leaves ofW. volubiliswere collected from Ooty, Tamil Nadu, India. It was identified by the Dr. Rajan, field botanist, Ooty. The leaves was dried and powered for soxhlet method of extraction (72 h) using methanol. The extract was then filtered and concentrated under vacuum to obtain crude form of methanol extract with this extract was taken for HPTLC analysis.
2.3. Standard stock solutions and sample preparation
Stock solutions of all standards (1 mg/mL) were prepared by dilution in ethanol, with the obtained working standard solutions were then applied in volumes of 10, 15, 20 and 25 μg/mL to RP-TLC plates to prepare linear calibration curves.
2.4. HPTLC analysis
Chromatography was performed on pre-activated (100℃) silica gel 60F254HPTLC plates (10 cm × 10 cm; 0.25 mm layer thickness; Merck). The CAMAG densitometry (Camag Model-3 TLC scanner equipped with Camag CATS 4 software), a reflectance pectrometer of monitoring range 190–700 nm was employed for the analysis. The slit was set to 8 mm × 0.4 mm and data acquisition and processing were performed using the software win CATS.
Samples (10 μL) were applied to the layers at 8 mm wide bands, positioned 10 mm from the bottom of the plate, using a Camag (Mutten, Swizterland) Linomat IV automated TLC applicator with nitrogen flow providing delivery from the stringe at a speed of 10 mL/s was maintained for all analyses. TLC plate development was performed using a Camag twintrough glass tank, which had been pre-saturated with mobile phase for 2 h. Solvent was allowed to run up the plate to a height of 8 cm.
TLC analyses were made under room temperature. A mixture of toluene: methanol (9:1) was used as mobile phase for oleanolic acid were petroleum ether: chloroform: ethyl acetate: methanol (4:1:0.1:0.1) were used for ursolic acid.
After development, the layers were dried and the components were visualized by UV light at 525 and 366 nm. The quantitative determination was performed by win CATS software program using the external calibration method[8].
3. Results

Figure 1. HPTLC profile: (1) UV-Visible spectrum of standard oleanolic acid (A) and W. volubilis (B); (2) chromatogram; (3) standard calibration curve of oleanolic aicd. Eluent: Toluene: Methanol (9:1 v/v); Detection: 525 nm.
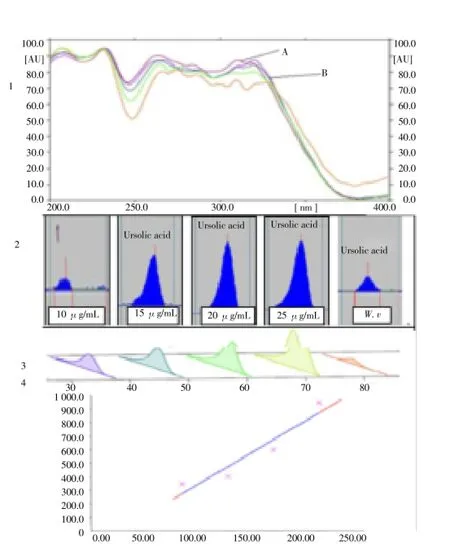
Figure 2. HPTLC profile: (1) UV-Visible spectrum of standard ursolic acid (A) and W. volubilis (B); (2) chromatogram; (3) standard calibration curve of ursolic acid. Eluent: petroleum ether: chloroform: ethyl acetate: methanol (4:1:0.1:0.1); Detection: 366 nm.
The identity of the bands of triterpenoids in the methanol extract was confirmed by comparing the UV-Vis absorption spectra with those of standards using a CAMAG HPTLCscanner. Comparison of the spectral characteristic of the peaks for standards and sample revealed the identity of standards in the methanol extract, qualitatively similar HPTLC fingerprints were obtained for the methanol extract from different locations giving reliable indication of the same identity Figure 1 & 2, is the illustration of the HPTLC scanned chromatogram of standard markers. The methanol extract was able to resolve 2 compounds in the developing solvent system by using mobile phase, which produced good separation withRfvalues of methanol extract is 0.31 and 0.49. The desired resolution of two terpenoids, which showed the presence of oleanolic acid and ursolic acid peak were detected. The standard oleanolic acid hasRfvalue of 0.31, a good linear relationship (r2= 0.992 46 and 0.996 18 with respective to peak height and area respectively) was observed in 10-25 μg/mL, Linear regression equation,Rf, and standard deviation are given in Table 1.

Table 1 System precision, linear regression equation and Rfvalues of the developed method.
The regression equation was found to be Y= 5.012+0.086 74X with respective to height and Y=186.1+2.004X with respect to area, where Y is the peak height/area and X is concentration of oleanolic acid, the amount in methanol extract was 218.30 ng/mL. System precision was performed by spotting four samples each from the standard stock solutions, the results of areas were given. A triterpene aglycone, ursolic acid used as reference substanceRf0.49 have been identified in these TLC condition, after spraying with anisaldehyde, glacial acetic acid, sulphuric acid and ethanol (Figure 1).
The detection of ursolic acid was observed in the linear calibration curves of standard compounds were found to be linear over the range 100-250 ng/mL (Figure 2). When the content of the ursolic acid in the methanol extract were analyzed by linear regression by HPTLC method was observed height and area isr=0.952 99, 0.950 42, the amount of ursolic acid was found to be 509.99 ng/mL. Using the techniques of the HPTLC and UV Vis spectra the amount of standards in the methanol extract were found to 218.30 and 509.99 ng/mL after compare nearest matching peaks of standards (Table 1).
This indicates that the HPTLC method is reliable for good estimation of the unknown compounds in methanol extract. Therefore the method could be used for initial screening or semiquantitative analyses.
4. Discussion
In conclusion, the method used in this work resulted in good peak shape and enabled good resolution of terpenoids from other constituents of the methanol extract. Using HPTLC analytical method, oleanolic acid and ursolic acid could be determined simultaneously, and the validity of the method was also verified.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgement
The authors wish to thank UGC, New Delhi for providing the financial support as UGC major research project for conducting the experiments (file no. F. No. 37-211/2009 (SR). The authors are thankful to the authority of Jadavpur University for providing necessary facilities.
[1] Gazzaneo LR, Paiva de lucena RF, Paulino de Albu querque U. Knowledge and use of medicinal plants by local specialists in an region of Atlantic forest in the state of Pernambuco (Northeastern Brazil). J Ethnobiol Ethnomed 2005: 1-9.
[2] World Health Organization. World Health Organization Expert Committee on Diabetes Mellitus. Second Report: Technical Report Series. Geneva: WHO; 1980, p. 646.
[3] Niranjan Sahu P, Panda N. Polyoxypregnane glycosides from the flowers of Dregea volubilis. Phytochem 2002; 61: 383-388.
[4] Sanyacharernkul S, Itghiarbha A, Kongtawelert P, Meepowpan P, Nuntasaen N, Pompimon W. A new polyoxypregnane glycoside from the roots of Dregea volubilis (L.f) Benth. ex Hook. f and its chondroprotective effect. Am J Biochem Biotech 2009; 5: 202-209.
[5] Ravikanth V, Reddy VLN, Prabhakarrao T, Diwan PV, Ramakrishna S, Venkateswarlu Y. Macrocyclic diterpenes from Euphorbia nivulia. Phytochem 2002; 59: 331-335.
[6] Lalitharani S, Mohan VR, Regini GS, Kalidass C. GC-MS analysis of ethanolic extracts of Pothos scandens L. leaf. J Herb Med Tox 2009; 3: 159-160.
[7] Niranjan Reddy VL, Ravikanth V, Vijender Reddy A, Prabhakar Rao T, Venkateswarlu Y. An unusual novel triterpenoid ether, multiflor-7-en-12, 13-ether and a new multiflor-7-en-12-ol from Wattakaka volubilis. Tetrahed Lett 2002; 43: 1307-1311.
[8] Akowuah GA, Zhari I, Norhayati I, Mariam A. HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J Food Compo Anal 2006; 19: 118–126.
ment heading
10.1016/S2221-6189(14)60013-5
*Corresponding author: Dr. Subhash C Mandal, Associate Professor, Pharmacognosy and Phytotherapy Research Laboratory, Division of Pharmacognosy, Department of Pharmaceutical Technology, Jadavpur University, Kolkata-700032, India.
Tel: (0) 9433098372
Fax: (033) 2837-1078
E-mail: subhashmandal@yahoo.com
HPTLC
Oleanolic acid
Ursolic acid
杂志排行
Journal of Acute Disease的其它文章
- Gender-Differences in aortic dissection
- Lagenaria siceraria ameliorates atheromatous lesions by modulating HMG-CoA reductase and lipoprotein lipase enzymes activity in hypercholesterolemic rats
- Effect of the methanol leaves extract of Clinacanthus nutans on the activity of acetylcholinesterase in male mice
- Animal bite incidence in the County of Shush, Iran
- In-vivo and ex-vivo inhibition of intestinal glucose uptake: A scope for antihyperglycemia
- Surgical treatment of hydrocephalus and spinal dysraphism
