钢液中镁合金复合脱氧热力学分析
2014-03-20张同生王德永刘承军张永启姜茂发
张同生,王德永,刘承军,张永启,姜茂发
(东北大学 材料与冶金学院,沈阳 110819)
钢液中镁合金复合脱氧热力学分析
张同生,王德永,刘承军,张永启,姜茂发
(东北大学 材料与冶金学院,沈阳 110819)
镁具有净化钢液、细化钢中非金属夹杂物的作用.针对钢液镁处理技术的发展需求,本文采用一阶和二阶相互作用系数,基于吉布斯自由能最小原理计算得到了Mg-Al-O、Mg-Si-O、Mg-Ti-O、Mg-Zr-O的热力学优势区域图,并探讨合金元素含量与脱氧平衡产物间的关系.通过热力学计算,揭示了钢液镁处理在不同脱氧制度下钢中脱氧产物的变化规律,可为镁处理在生产实践中的应用奠定理论基础.
镁合金;复合脱氧;热力学;非金属夹杂物
近年来,汽车工业、管道工程、大型桥梁、造船工业、工程机械的发展极大拉动了市场对洁净钢和低合金高强度钢的需求.随着用户对钢材性能要求日益严格,对钢铁的质量要求的不断提高,进一步减少钢中夹杂含量并有效控制夹杂物的形态,是很长时期钢铁行业的发展方向.
金属镁具有极强的脱氧、脱硫能力,且镁在钢液中溶解度较大[1~4].1 873 K时,镁在碳饱和铁液中的溶解度(质量分数)为0.053 2%,而钙仅为0.018%~0.031%.镁的脱氧、脱硫产物弥散性好,不易聚合,高温下稳定,且MgO、MgS与α-Fe之间具有较低的晶格错配度,有利于晶内铁素体附着形核[5~8].但金属镁相对密度小,熔点低,蒸气压高(1 873 K时,镁在碳饱和铁液中蒸气压高达2.02 MPa),直接加入钢液收得率极低.为提高金属镁的收得率,喷吹法或喂线法正引起广泛的关注.
针对不同钢液脱氧模式,有必要控制合理的镁加入量,以实现镁脱氧优势及对夹杂物的控制效果.为此,本文对Mg-Al-O、Mg-Si-O、Mg-Ti-O、Mg-Zr-O体系进行了脱氧平衡热力学计算,以评估二元合金脱氧时夹杂物成分控制区域,为工业应用提供理论基础.
1 脱氧热力学计算方法
镁脱氧热力学平衡及相平衡计算是基于吉布斯自由能变最小原理.由于研究的元素(如Mg、Ti、Al、Zr等)在钢液中浓度较低,将钢液假定为理想稀溶液,其中溶质遵循亨利定律.组元活度系数计算选用1%(质量)极稀溶液为标准态,组元活度系数与元素间相互作用系数采用瓦格纳提出的泰勒展开式表示,如式(1)所示.计算过程中涉及到钢液中元素相互作用系数见表1.
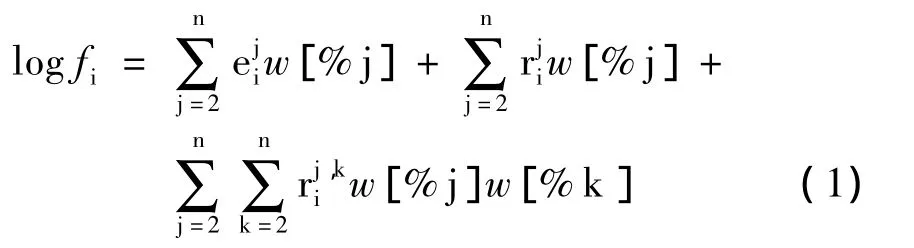
本文计算是利用文献中已知的反应式自由能变,通过线性组合的方法得到稳定区域转化的边界方程及其自由能变.然后根据其平衡常数,使用Matlab2012a计算并绘制出优势区域图.

表1 元素在铁液中1873K温度下的相互作用系数Table 1 Mass percent interaction parameters of the element in liquid iron at 1 873 K
2 计算结果及讨论
2.1 Mg-Al复合脱氧
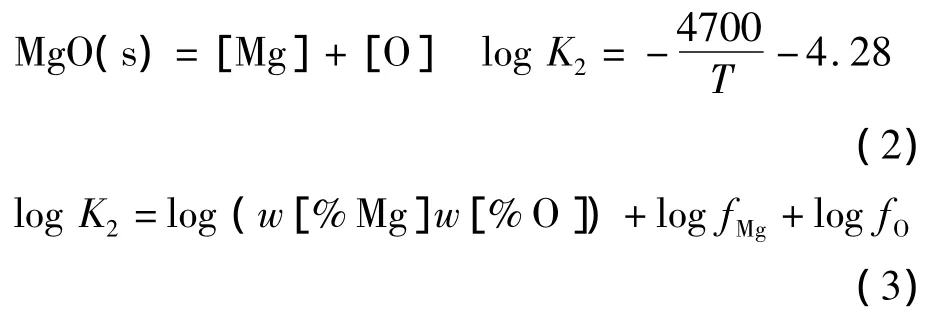
当活度系数使用瓦格纳关系式表达出来时,平衡常数可以利用 Lupis方程式表示成等式4[39].
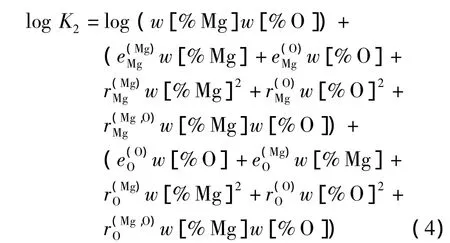
在钢铁生产过程中,由于铝有很强的脱氧能力,广泛作为终脱氧剂使用.并且,铝脱氧产物易与耐火材料及渣中的MgO反应生成夹杂物,故Mg-Al-O系夹杂物是生产中较为常见的一类夹杂物.从Al2O3-MgO相图中可以看到,在炼钢温度(1 873 K)下Al2O3和MgO中间产物只有镁铝尖晶石(MgO·Al2O3)一种,且生成区域相对较大.Al-Mg-O体系中除了存在反应(2)外,还存在如下反应[12,13]:
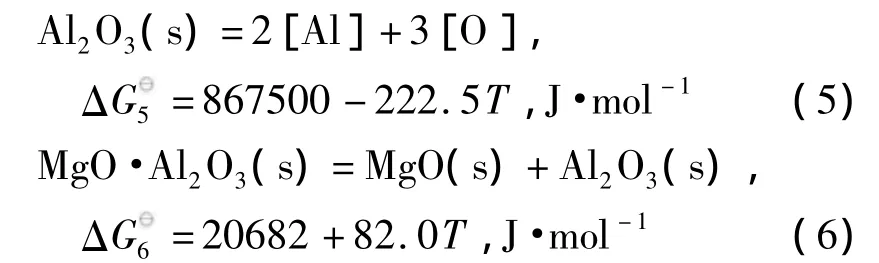
使用线性组合方法得到MgO·Al2O3与Al2O3和MgO与MgO·Al2O3的边界线方程:
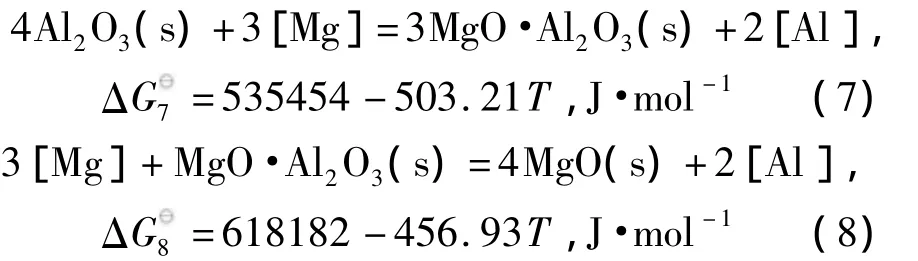
由边界线方程(7)和边界线方程(8),可以计算得到Mg-Al-O系脱氧产物的稳定区域图,如图1所示.随着钢中铝、镁含量的变化,脱氧平衡产物可以分为MgO、MgO·Al2O3和Al2O3三个区域.由图可知,对于钢中Al2O3夹杂物引起的水口结瘤问题,提高[Mg]和降低[Al]含量均可以得到有效的避免.
当钢中[Al]s质量分数在0.01%~0.1%范围内变化时,钢中微量的镁就能形成镁铝尖晶石产物.尖晶石夹杂物属脆性夹杂物,熔点高(2 135℃),不变形,属于硬质夹杂物,对钢的疲劳寿命有不利影响.但从另一个方面看,尖晶石夹杂物粒度小,分布弥散,相对于簇群状的Al2O3夹杂物,尖晶石夹杂物的危害程度明显减弱.文献[44]证实:若镁铝尖晶石形成细小、弥散型夹杂物存在于钢中不会对钢形成产生明显影响;但文献[45,46]则认为尖晶石夹杂物容易导致钢材制品缺陷,且浇注过程中,尖晶石夹杂物颗粒易沉积在侵入式水口内部造成水口堵塞.由于镁铝尖晶石相(MgO·Al2O3)稳定区域较宽,通过调整镁铝元素含量来避免尖晶石的生成会非常吃力.近年来,有研究[13]指出,采用钙处理的方法,可以将镁铝尖晶石夹杂物变质成CaO-Al2O3-MgO系中的液相区域,从而减少了镁铝尖晶石夹杂物对水口结瘤的危害.也有学者[17,38]通过提高钢液的[Ti]含量,从而对镁铝尖晶石进行有效的变质处理.
2.2 Mg-Si复合脱氧
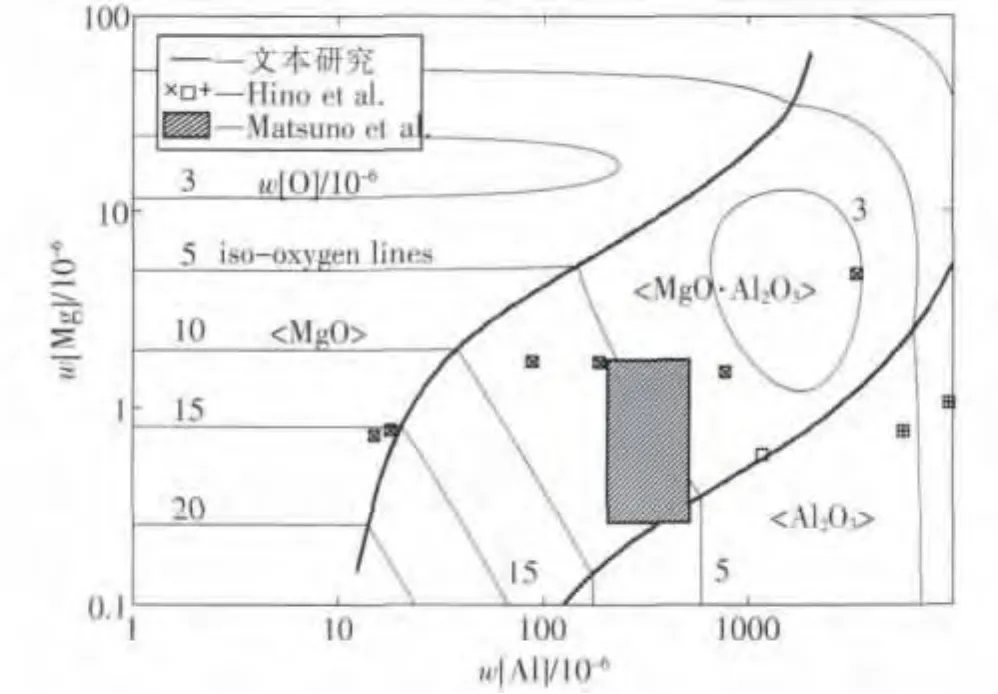
图1 1 873 K温度下MgO、MgO·Al2O3与Al2O3的稳定区域与等氧势线图Fig.1 Phase stability diagram of MgO,MgO·Al2O3,and Al2O3,and iso-oxygen contour lines calculated at 1 873 K
生产实践中,针对某些对B类、D类夹杂物敏感的钢种,如帘线钢、弹簧钢等,要尽量避免脱氧生成Al2O3夹杂物.为此,一般选用Si或Mn进行脱氧,Si-Mn复合脱氧钢中氧含量较高,但通过控制w[Mn]/w[Si]>2.5,可以实现夹杂物的塑性化,大幅提高钢的韧性和拉拔性能,并能有效防止浸入式水口结瘤发生[47].为了进一步降低Si-Mn脱氧钢中氧含量,充分发挥镁处理对钢液的净化作用,研究在Si-Mn脱氧的基础上进行镁处理是一种全新的尝试.为此,本文计算了Mg-Si复合脱氧体系,并绘制了Si-Mg-O复合脱氧产物稳定区域图.从SiO2-MgO相图中可知,1873 K下,SiO2和MgO中间产物只有镁橄榄石(2MgO·SiO2)一种.Si-Mg-O体系中存在如下反应[30,48]:

使用线性组合方法得到MgO、MgO·SiO2与SiO2的边界线方程:
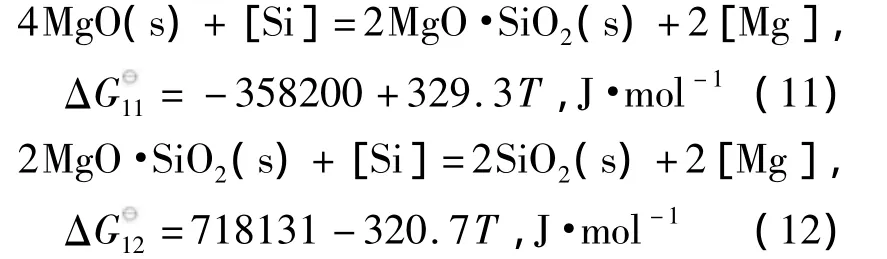
由边界线方程(11)和(12),可以得到MgO、2MgO·SiO2与SiO2的稳定区域图,如图2所示.由图可知,当钢中w[Si]≤0.1%时不能生成纯的SiO2,即使钢中微量的镁存在,平衡脱氧产物也是MgO,这是由于Si的脱氧产物被镁还原的结果.镁橄榄石(2MgO·SiO2)的存在稳定区域较大,几乎存在于钢中最常用的Si含量范围内.镁橄榄石(2MgO·SiO2)熔点较高,为1 890℃,因此,单独采用Si-Mg脱氧是不能实现夹杂物液相化的,但有关镁橄榄石夹杂物行为特征(如尺寸分布、聚合能力、晶内铁素体形核效果等)的研究尚未见报道,需在以后的研究中引起重视.
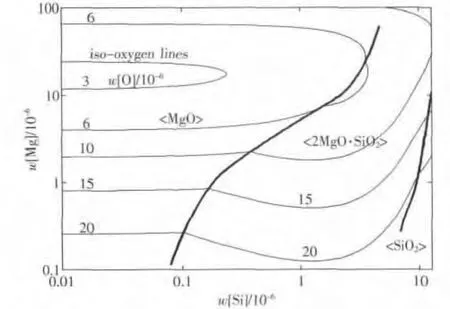
图2 1 873 K温度下MgO、2MgO·SiO2与SiO2的稳定区域图与等氧势线图Fig.2 Phase stability diagram of MgO,2MgO·SiO2and SiO2,and iso-oxygen contour lines calculated at 1 873 K
2.3 Mg-Ti复合脱氧
计算Mg-Ti复合脱氧是基于研究含钛钢种镁处理的可行性.钛系脱氧产物价态较多,如TixO2x-1(x≥3)型、TiO2及TiO等,但高价钛的氧化物一般只形成于钢液中氧活度较高的情况.文献[23]证明,在单独 Ti脱氧时,当 w[Ti]在0.25% ~4.75%范围内,脱氧产物以Ti2O3为主.利用热力学计算可以得出对应的溶解氧(质量分数)在3.5×10-3%以下.对于钛镁复合脱氧的情况,w[O]一般均在3.5×10-3%以下,故在此计算条件下,除Ti2O3之外,其他价态的钛氧化物的形成可以被忽略.Ti-Mg-O体系中存在Mg的脱氧反应产物MgO之外,同时也存在着Ti的脱氧产物Ti2O3,以及它们的中间产物Ti2O3·MgO.上述物质生成反应的平衡常数计算式如下[17,23]:
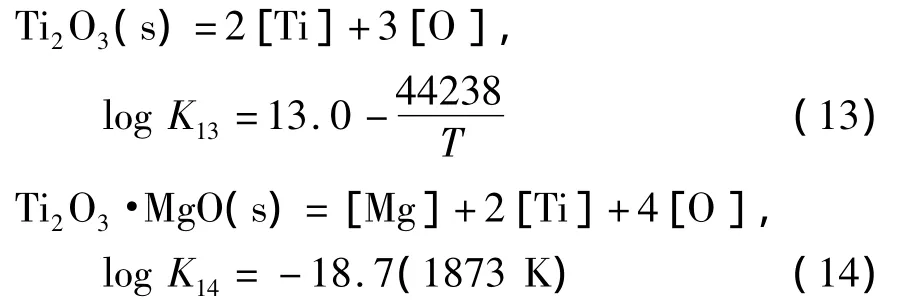
使用线性组合方法得到MgO、MgO·Ti2O3与Ti2O3的边界线方程:
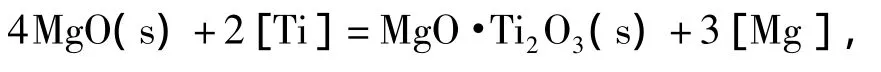
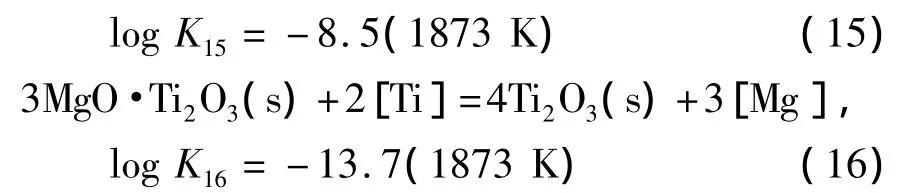
由边界线方程,可计算得到MgO、MgO·Ti2O3与Ti2O3的稳定区域图,如图3所示.由图可知,当钢中w[Ti]在0.01%~0.07%范围内,钢中微量镁存在即可形成镁钛尖晶石相MgO·Ti2O3稳定相,随着镁含量增加,镁钛尖晶石稳定区域逐渐减小,当w[Mg]大于0.001%时,平衡脱氧产物有转变成纯MgO的趋势.这是因为发生了反应(17):
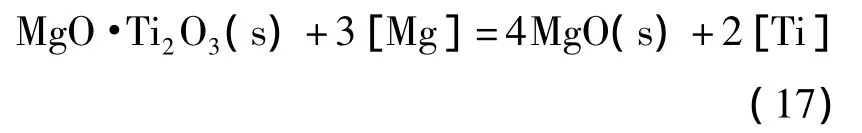

图3 1873K温度下MgO、MgO·Ti2O3与Ti2O3的稳定区域图与等氧势线图Fig.3 Phase stability diagram of MgO,MgO·Ti2O3,Ti2O3,and iso-oxygen contour lines calculated at 1873 K
关于Mg-Ti复合脱氧对钢水洁净度和夹杂物的细化效果,Kim[9]发现,Mn/Si/Ti脱氧钢中加入微量镁,随溶解镁含量增加,夹杂物成分按Ti2O3→Ti-Mg-O→MgTiO3→MgO转变,平均尺寸由2.1 μm减小至1.2 μm以下,且含镁夹杂物与钢液具有更好的润湿性.这与本计算脱氧平衡产物的演变规律是基本一致的.含钛脱氧钢中,有必要适当增加镁的加入量,以避开形成MgO·Ti2O3夹杂物,促进夹杂物转变成纯MgO,以实现夹杂物细化的目标.
2.4 Mg-Zr复合脱氧
锆脱氧对钢中夹杂物的细化作用明显,近年来颇受研究者关注[49~54].钢中含痕量[Zr](质量分数)为3.0×10-3%时,夹杂物分布非常均匀,且脱氧产物ZrO2密度大(6.09 g/mm3),弥散、悬浮于钢中可促进MnS、TiN大量形核.迄今,有关金属锆与其他元素复合脱氧的研究不多,尤其是锆、镁、钛等复合脱氧对钢中夹杂物非自发形核的研究还不多见.Zr具有可以与Mg相媲美的脱氧能力,而且Zr脱氧也是目前氧化物冶金技术的研究热点,为此,计算Mg-Zr-O的优势区域图对于控制Mg-Zr复合脱氧产物具有重要的指导意义.从氧化物相图可知,MgO与ZrO2之间无中间产物.故在Mg-Zr-O体系中,只存在反应(2)和反应(21).将反应(18)、(19)、(20)通过线性组合推算得到式(21)[24].

所以,Zr在钢液中的脱氧反应及其自由能,如方程(21)所示.

再结合反应(2)推算得到边界方程(22),如下式所示.

利用边界方程(22),计算得到Mg-Zr-O的优势区域图,如图4所示.由图分析可知,对于Mg-Zr复合脱氧,当钢中镁含量较低时,脱氧平衡产物为纯的ZrO2,当镁含量较高时,脱氧平衡产物为纯的MgO,这是由于镁、锆竞争脱氧造成的.此外,对于Mg-Zr复合脱氧,当w[Mg]、w[Zr]分别大于0.001%,钢中w[O]可以降至极低,约为(3~4)×10-4%的水平,因此,Mg-Zr复合脱氧对提高钢水洁净度是非常有益的.
3 结语
近年来,钢液镁处理技术受到越来越广泛的关注.镁脱氧产物具有稳定不易分解及高度离散性等特征,在细化夹杂物尺寸方面具有良好的效果.因此,无论是对提高钢水洁净度还是发挥镁系脱氧产物在氧化物冶金技术中促进晶内铁素体形核方面均具有明显的优势.本文采用热力学原理计算了常用的几种含镁复合脱氧方式的脱氧特征,分析了脱氧产物的稳定区域与元素含量之间的关系,为进一步深入研究镁在不同钢液脱氧方式下夹杂物行为特征提供理论依据.
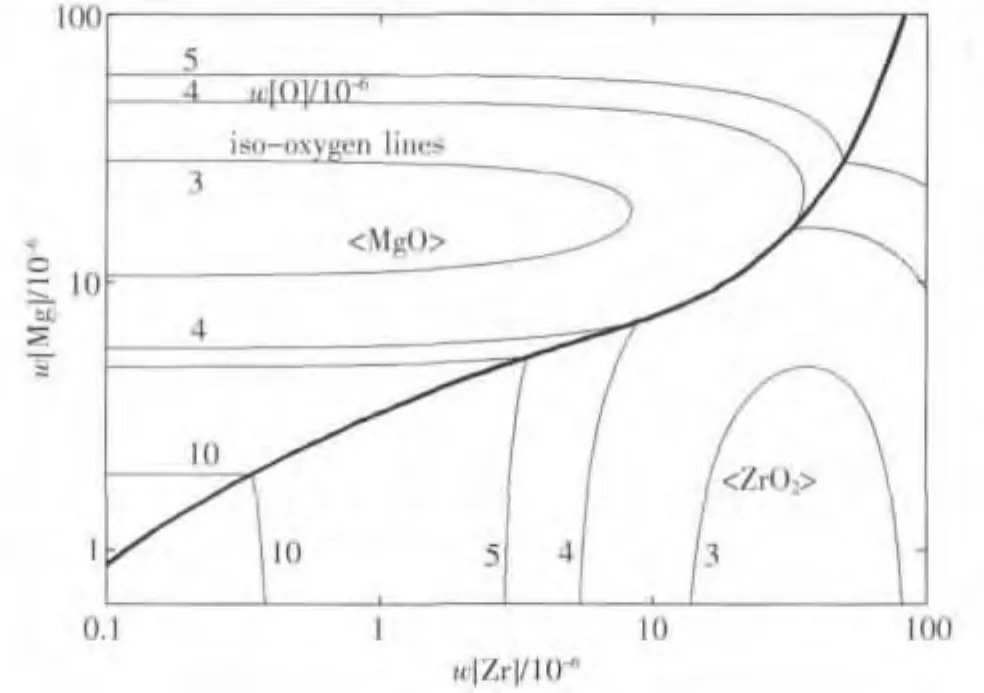
图4 1 873 K温度下MgO与ZrO2的稳定区域图与等氧势线图Fig.4 Phase stability diagram of MgO and ZrO2,and iso-oxygen contour lines calculated at 1873 K
[1]Yamamoto R,Fukaya H,Satoh N,et al.Magnesium deoxidation equilibrium of molten Fe-Cr-Ni alloy expressed by quadratic formalism and redlich-Kister type polynomial[J].ISIJ International,2011,51(6):895-900.
[2]Takata R,Yang J,Kuwabara M.Characteristics of inclusions generated during Al-Mg complex deoxidation of molten steel[J].ISIJ international,2007,47(10):1379-1386.
[3]Yonemoto L M,Miki T,Hino M.Magnesium deoxidation equilibrium of molten Fe-Ni alloy expressed by quadratic formalism and redlich-kister type polynomial[J].ISIJ International,2008,48(6):755-759.
[4]Haddock J T,Hussain I,Fox A G,et al.New MgO-CaO based reagent for ladle treatment of steel[J].Ironmaking and Steelmaking,1994,21(6):479.
[5]Saxena S K.Production of ultra-clean steels with better mechanical properties with magnesium treatment[C].1996 steelmaking conference proceeding,1996:89.
[6]Kimura S,Nakajima K,Mizoguchi S.In-situ observation of the precipitation ofmanganesesulfidein low -carbon magnesium-killed steel[J].Metallurgical and Materials Transactions A,2001,33(2):427-436.
[7]Kimura S,Nakajima K,Mizoguchi S.Behaviors of aluminamagnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steel[J].Metallurgical and Materials Transactions B,2001,32B(2):79-85.
[8]Fu J,Yu Y G,Wang A R,et al.Inclusion modification with Mg treatment for 35CrNi3MoV steel[J].Journal of Materials Science Technology,1998,14(1):53-56.
[9]Kim H S,Chang C H,Lee H G.Evolution of Inclusions and resultant microstructural change with Mg addition in Mn/Si/Ti deoxidized steel[J].ScriptaMaterialia,2005(53):1253-1258.
[10]Ono H,Nakajima K,Ibuta T,et al.Equilibrium relationship between the oxide compounds in MgO-Al2O3-Ti2O3and molten iron at 1873K[J].ISIJ International,2010,50(12): 1955-1958.
[11]Satoh N,Taniguchi T,Mishima S,et al.Prediction of nonmetallic inclusion formation in Fe-40mass%Ni-5mass% Cr alloy production process[J].Tetsu-to-Hagané,2009,95(12):827-836.
[12]Itoh H,Hino M,Ban-ya S.Deoxidation equilibrium of magnesium in liquid iron[J].Tetsu-to-Hagané,1997,83 (10):623-628.
[13]Itoh H,Hino M,Ban-ya S.Thermodynamics on the formation of non-metallic inclusion of spinel(MgO· Al2O3)in liquid steel[J].Tetsu-to-Hagané,1998,84 (2):85-90.
[14]Ohta H,Suito H.Calcium and magnesium deoxidation in Fe-Ni and Fe-Cr alloys equilibrated with CaO-Al2O3and CaO-Al2O3-MgO slags[J].ISIJ International,2003,43 (9):1293-1300.
[15]Itoh H,Hino M,Ban-ya S.Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel[J].Metallurgical and Materials Transactions B,1997,28B(10): 953-956.
[16]Todoroki H,Mizuno K.Effect of silica in slag on inclusion compositions in 304 stainless steel deoxidized with aluminum[J].ISIJ International,2004,44(8):1350-1357.
[17]Ono H,Ibuta T.Equilibrium relationships between oxide compounds in MgO-Ti2O3-Al2O3with iron at 1873 K and variations in stable oxides with temperature[J].ISIJ International,2011,51(12):2012-2018.
[18]Nishi T,Shinme K.Formation of spinel inclusions in molten stainless steel under Al deoxidation with slags[J].Tetsu-to-Hagané,1998,84(12):837-843.
[19]Ohta H,Suito H.Deoxidation equilibria of calcium and magnesium in liquid iron[J].Metallurgical and Materials Transactions B,1997,28B(12):1131-1139.
[20]Cho S W,Suito H.Assessment of aluminum -oxygen equilibrium in liquid iron and activities in CaO-Al2O3-SiO2slags[J].ISIJ International,1994,34(2):177-185.
[21]Taguchi K,Ono -nakazato H,Nakazato D,etal.Deoxidation and desulfurization equilibria of liquid Iron by calcium[J].ISIJ International,2003,43(11):1705-1709.
[22]Ohta H,Suito H.Activities in CaO-MgO-Al2O3slags and deoxidation equilibria ofAl,Mg,and Ca[J].ISIJ International,1996,36(8):983-990.
[23]Pak J J,Jo J O,Kim W Y,et al.Thermodynamics of titanium and oxygen dissolved in liquid iron equilibrated with titanium oxides[J].ISIJ International,2007,47(1):16-24.
[24]Inoue R,Ariyama T,Suito H.Thermodynamics of zirconium deoxidation equilibrium in liquid iron by EMF measurements[J].ISIJ International,2008,48(9):1175-1181.
[25]Cha W Y,Nagasaka T,Miki T,et al.Equilibrium between titanium and oxygen in liquid Fe-Ti alloy coexisted with titanium oxides at 1873 K[J].ISIJ International,2006,46 (7):996-1005.
[26]Kishi M,Inoue R,Suito H.Thermodynamics of oxygen and nitrogen in liquid Fe-20mass%Cr alloy equilibrated with titania-based slags[J].ISIJ International,1994,34(11): 859-867.
[27]Ma Z,Janke D.Characteristics of oxide precipitation and growth during solidification of deoxidized steel[J].ISIJ International,1998,38(1):46-52.
[28]Karasev A,Suito H.Quantitative evaluation of inclusion in deoxidation of Fe-10 mass Pct Ni alloy with Si,Ti,Al,Zr and Ce[J].Metallurgical and Materials Transactions B,1999, 30B(4):149-257.
[29]Ohta M,Sano N,Morita K.Thermodynamic consideration on the oxide system containing MnO and TiO2as deoxidization productsin steels[J]. ISIJInternational,2000,40 (Supplement):S87-S91.
[30]Suzuki K,Ban-ya S,Hino M.Deoxidation equilibrium of Cr-Ni stainless steel with Si at the temperatures from 1823 to 1923 K[J].ISIJ International,2002,42(2):146-149.
[31]Higuchi Y,Numata M,Fukagawa S,et al.Effect of method of Ca treatment on composition and shape of non-metallic inclusions[J].Tetsu-to-Hagané,1996,82(8):671-676.
[32]Itoh H,Hino M,Ban-ya S.Assessment of Al deoxidation equilibrium in liquid Iron[J].Tetsu-to-Hagané,1997,83 (12):773-778.
[33]Ishii F,Ban-ya S,Hino M.Thermodynamics of the deoxidation equilibrium of aluminum in liquid nickel and nickel-lron alloys[J].ISIJ International,1996,36(1):25-31.
[34]Jo S K,Song B,Kim S H.Thermodynamics on the formation of spinel(MgO·Al2O3)inclusion in liquid iron containing chromium[J].Metallurgical and Materials Transactions B,2002,33B(10):703-709.
[35]Morioka Y,Morita K,Tsukihashi F,et al.Equilibria between molten steels and inclusions during deoxidation by titaniummanganese alloy[J].Tetsu-to-Hagané,1995,81(1):40-45.
[36]Sakao H.Equilibrium constants for the deoxidation of liquid Iron with silicon and aluminum[J].Tetsu-to-Hagané,1990,76(1):17-24.
[37]Suito H,Inoue R.Thermodynamics on control of inclusions composition in ultraclean steels[J].ISIJ International,1996,36(5):528-536.
[38]Park J H,Lee S B,Gaye H.Thermodynamics of the formation of MgO-Al2O3-TiOxinclusions in Ti-stabilized 11Cr ferritic stainless steel[J].Metallurgical and Materials Transactions B,2008,39(6):853-861.
[39]黄希祜.钢铁冶金原理(第三版)[M],北京:冶金工业出版社,2000.
(Huang Xihu.The principle of iron and steel metallurgy(the third edition) [M].Beijing:MetallurgicalIndustry Press,2000.)
[40]Seo W G,Han W H,Kim J S,et al.Deoxidation equilibria among Mg,Al and O in liquid Iron in the presence of MgO· Al2O3spinel[J].ISIJ International,2003,43(2):201-208.
[41]Han Q,Zhou D,Xiang C.Determination of dissolved sulfur and Mg-S,Mg-O equilibria in molten iron[J].Steel Research,1997,68(1):9-14.
[42]SeoJ,Kim SH.ThermodynamicassessmentofMg deoxidation reaction of liquid iron and equilibria of[Mg]-[Al]-[O]and[Mg]-[S]-[O][J].Steel Research,2000,71(4):101-106.
[43]Kawashita Y,Suito H.Precipitation behavior of Fe-Al-O inclusions under unidirectional solidification of Fe-30mass% Ni alloys saturated with CaO -Al2O3slags[J].ISIJ International,1995,35(12):1459-1467.
[44]Saxena S K,Using magnesium treatment to produce super clean steel with excellent mechanical property[J].SEAISI,1997,(7):42-52.
[45]Mizuno K,Todoroki H,Noda M,et al.Effects of Al and Ca in ferrosilicon alloys for deoxidation on inclusion composition in type 304 stainless steel[J].Iron and Steel-maker,2001,28 (8):93-101.
[46]Todoroki H,Inada S.Recent innovation and prospect in production technology of specialty steels with high cleanliness[J].Bull.Iron Steel Inst.,2003,8(2):575.
[47]陈家祥.钢铁冶金学(炼钢部分)[M].北京:冶金工业出版社,2009:14-15.
(Chen Jiaxiang.The ferrous metallurgy(steelmaking)[M].Beijing:Metallurgical Industry Press,2009:14-15.)
[48]Jiang Z H,Li S J,Li Y.Thermodynamic calculation of inclusion formation in Mg-Al-Si-O system of 430 stainless steel melts[J].JournalofIron and SteelResearch International,2011,18(2):14-17.
[49]Sawai T,Wakoh M,Ueshima Y,et al.Analysis of oxide dispersion during solidification in Ti,Zr-deoxidized steel[J].ISIJ International,1992,32(1):169-173.
[50]Isselin J,Kasada R,Kimura A,et al.Effects of Zr addition on the microstructure of 14%Cr4%Al ODS ferritic steel[J].Materials Transactions,2010,51(5):1011-1015.
[51]Guo A M,Li S R,Guo J,et al.Effect of zirconium addition on the impact toughness of the heat affected zone in a high strength low alloy pipeline steel [J]. Materials Characterization,2008,59(2):134-139.
[52]Trindade V B,Mello R S T,Payao J C,et al.Influence of zirconium on microstructure and toughness of low-alloy steel weld metals[J].Journal of Materials Engineeringand Performance,2006,15(3):284-289.
[53]Karasev A V,Suito H.Characteristics of fine oxide particles produced by Ti/M(M=Mg and Zr)complex deoxidation in Fe-10 mass%Ni alloy[J].ISIJ International,2008,48 (11):1507-1516.
[54]Ohta H,Suito H.Characteristics of particle size distribution of deoxidation products with Mg,Zr,Al,Ca,Si/Mn and Mg/ Al in Fe-10mass%Ni alloy[J].ISIJ International,2006,46 (1):14-21.
Thermodynamic analysis of complex deoxidization by magnesium-alloy in the liquid steel
Zhang Tongsheng,Wang Deyong,Liu Chengjun,Zhang Yongqi,Jiang Maofa
(School of Materials and Metallurgy,Northeastern University,Shenyang 110819,China)
Magnesium has a role to clean the steel and to refine the inclusions.In order to meet development of magnesium-treat technology,in this paper,the phase stability diagrams of Mg-Al-O、Mg-Si-O、Mg-Ti-O、Mg-Zr-O were drawn through first-and second-order interaction parameters,based on principle of Gibbs free energy minimization.And the relationship between the element content and the deoxidation products was discussed simultaneously.Through thermodynamic calculation,an evolution regulation for the deoxidation products was revealed,under different magnesium-treat conditions.The authors hoped that our work may present a theoretical basis for the practice.
magnesium-alloy;complex deoxidation;thermodynamic;inclusions
TF 704.1
A
1671-6620(2014)02-0079-06
2013-11-11.
国家自然科学基金资助项目 (50904017);中央高校基本科研业务费项目 (N120602005)和 (N120502004).
张同生 (1984—),男,博士研究生,E-mail:neu_zts@163.com;姜茂发(1955—),男,东北大学教授,博士生导师.
文献中[12]给出的镁在钢液中脱氧反应式及其平衡常数为:
