甘油催化氢解制1,2-丙二醇的研究进展
2014-03-10童东绅赵立知任倩倩刘丰国俞卫华周春晖
肖 飒,童东绅,赵立知,任倩倩,刘丰国,俞卫华,周春晖
(浙江工业大学化学工程学院,浙江 杭州 310014)
甘油主要来自动植物和化工产业(如肥皂、生物柴油产业),具有可降解、可再生等优点。因其独特的性质,甘油成为近年来的研究热点。目前,甘油可转化为各种高附加值的精细化学品[1-2]。其中,从甘油催化氢解制1,2-丙二醇(1,2-PDO)的技术最受关注。1,2-PDO 的传统生产工艺包括环氧丙烷水解法、丙烯氧化法和酯交换法。上述工艺原料来自不可再生的石油资源,流程复杂,环境污染严重,经济效益低。而甘油氢解法使用绿色清洁的甘油作原料,产物种类少、收率高、易分离提纯,具有替代传统生产工艺的潜力。
甘油催化氢解反应成功的关键是选择合适的催化剂。目前研究较多的催化剂有两大类:贵金属催化剂(如Ru、Rh、Pt、Pd)和过渡金属催化剂(如Cu、Ni、Co),载体研究较多的是碳材料、金属氧化物和层状双氢氧化物。这些催化剂所采用的制备方法不同,工艺参数不同,催化性能也有差异。
1 催化剂研究开发
1.1 贵金属催化剂
单一贵金属催化剂的选择性一般较差,添加其他组分可提高选择性。例如Ru/C 催化剂中加入12-磷钨酸作共催化剂后,大幅度提高了1,2-PDO 的收率[3]。
此外,载体表面的酸碱度也对催化剂的性能有一定影响[4]。酸性位有利于C-O 键断裂,但酸性位过多会促进C-C 键断裂。对Ru 催化剂,TiO2载体的酸性位数量较合适[5],HZSM5 载体的酸性导致副产物CH4选择性提高,1,2-PDO 选择性降低[6]。碱性氧化物负载的Ru 催化剂中,因CeO2表面呈弱碱性,甘油转化率和1,2-PDO 选择性较高[7]。碱性的层状水滑石作载体对Pt 催化剂性能有促进作用[8]。
甘油氢解反应通常需在H2或合成气等还原性气氛下进行,存在一定的安全隐患。D’Hondt 等首次发现在不通氢气的条件下就能实现甘油氢解;采用Pt/NaY 催化剂,以20wt% 甘油水溶液为原料,在503 K 反应15 h 后,甘油转化率和1,2-PDO 选择性分别为85.4% 和64.0%[9]。同样在不加氢气条件下,Ru/Al2O3和Pt/Al2O3混合物[10]或Pd/Fe2O3催化剂[11]也能催化甘油氢解。
过去,Ag 基催化剂催化甘油转化制备1,2-PDO 的研究较少。最近研究表明,Ag 掺杂的分子筛催化剂[12]和Ag/γ-Al2O3催化剂[13]对于甘油催化氢解反应也具有活性和选择性。
1.2 Cu 催化剂
相对于贵金属催化剂,Cu 催化剂价格低廉,对C-O 键加氢活性较高,对C-C 键断裂活性较低,因此1,2-PDO 选择性较高。最近,开发高效Cu 催化剂成为研究热点。例如,在相对较低的压力下(1.4 MPa)使用Raney Cu 催化剂,甘油转化率可达100%,1,2-PDO 收率也高达94%[14]。
载体是影响催化性能的因素之一。对于氧化物改性的Raney Cu 催化剂,催化载体活性顺序是MgO>ZnO>SiO2>TiO2>ZrO2>Al2O3[15]。层状双氢氧化物作载体能提供碱性位[16],例如层状双氢氧化物Cu0.4Mg5.6Al2(OH)16CO3热解产物作催化剂,在453 K,30 bar H2压力下,反应20 h 后,1,2-PDO选择性98.2%,甘油转化率80%[17]。介孔分子筛SBA-15 作载体,1,2-PDO 选择性和甘油转化率最高分别为92.4%和96.0%[18]。1173 K 预处理SBA-15,可提高催化剂结构稳定性[19]。在463 K 和0.64 MPa H2压力下,使用Cu/ZnO/Al2O3双载体催化剂,1,2-PDO 选择性为92%[20]。
Cu 还能与载体MxOy结合形成CuMxOy晶相。例如Cu 和CuCr2O4[21-22]之间有明显的相互作用,Cu/CuCr2O4活性比Cu/Cr2O3高。Cu-Fe 催化剂含有CuFe2O4晶相,在463 K,4.1 MPa H2压力下反应10 h,甘油转化率和1,2-PDO 选择性分别为47% 和92%[23]。纳米CuAl2O4催化剂只有CuAl2O4一个晶相,还原性高,对氢气吸附-脱附能力强,甘油转化率和1,2-PDO 选择性都大于90%[24]。
此外,制备方法不同,催化剂的催化性能也有差异。浸渍法制备的Cu/SiO2催化剂比离子交换法活性好,在1.5 MPa,528 K,300 mL/min H2反应条件下,甘油可完全转化,1,2-PDO 的选择性为87%[25]。共沉淀法制备的Cu/Al2O3催化剂,甘油转化率和1,2-PDO 选择性最高分别为63%和88%,而使用固态熔融法制备的Cu/Al2O3催化剂,甘油转化率最高仅39%[26]。溶胶凝胶法制备的Cu/ZnO催化剂表面积约为共沉淀法的两倍,因此活性更高[29]。
影响催化性能的因素还有负载量、甘油浓度、溶剂类型、氢气压力、pH 和反应温度等。Cu 负载量较低时,Cu/MgO 催化剂活性较高[27]。添加少量NaOH 进一步提高了Cu/MgO 的活性,可代替Ru/C (或Rh/SiO2)+Amberlyst、Pt/C (或Ru/C) +NaOH 催化剂体系[28]。反应中生成的水会造成Cu/ZnO 催化剂失活,改用1,2-丁二醇作溶剂,甘油转化率从5%增至55%[29]。Cu 催化剂添加助剂(如Ba[30]、Ga2O3[31])也可使催化剂抗失活。
1.3 Ni、Co 催化剂
除了Cu 催化剂,Raney Ni[32-33],Ni/AC[34],Ni/NaX[35],Ni/SiO2-Al2O3[36],Ni/Mg/Al 层状双氢氧化物[37]同样对甘油催化氢解具有很好的活性。Raney Ni 作催化剂时,可使用粗甘油作原料[38]。KBH4处理Ni/AC 催化剂,使得AC 表面的羰基还原为酚基,大大提高了催化剂酸性[34]。此外,Ni 还可以作为掺杂剂或者载体。例如,将金属Ni 掺在Cu-Cr催化剂中有助于提高1,2-PDO 的选择性[39]。若增加原料中水的含量,甘油的转化率和1,2-PDO 的选择性都会增加,这是因为低浓度甘油可减少脱水反应和加氢裂化[40]。
Co 催化剂的研究相对较少。在较高温度下处理Co/MgO 催化剂时,Co3O4和MgO 之间的相互作用得到了加强,形成了MgCo2O4晶体和Mg-Co-O 固溶体[41],减少了钴氧化物的还原性,同时阻止了Co 粒子的聚集,所得的Co/MgO 催化剂表现出良好的稳定性。Co/Zn/Al 催化剂在反应前后物理性质能保持几乎不变,可重复使用[42]。
1.4 双金属催化剂
使用双金属催化剂如Pt-Ru、Au-Ru 可提高1,2-PDO 的收率[43]。Re 的加入对抑制C-C 键断裂发挥了作用,Ru-Re[44-46]、Pd-Re[47]、Pt-Re[48]催化剂均比单一Ru、Pd、Pt 或Re 催化剂活性好。
此外,铜和贵金属结合的双金属催化剂的研究也有较多报道。例如,在453 K、2.0 MPa 氢气下,使用Pd0.04Cu0.4/Mg5.5Al2O8.56催化剂,反应10 h后,甘油的转化率为88.0%,1,2-PDO 选择性为99.6%[49]。使用Rh0.02Cu0.4/Mg5.6Al1.98O8.57催化剂,甘油转化率和1,2-PDO 的选择性最高分别为91.0%和98.7%[50]。此外,碳纳米管[51]、膨润土[52]、HMS[53]也可作为Cu-Ru 催化剂的载体。对于Cu-Ag/γ-Al2O3催化剂,Ag 的加入使CuO 原位还原为Cu,这有助于提高铜在载体表面上分散性[54],还能抑制甘油C-C 键断裂生成乙二醇,1,2-PDO 的选择性最高98.3%,此时甘油转化率为100%[55]。Ni-Cu/Al2O3催化剂能将溶剂中的氢转移到甘油之上[56-58]。当甲酸作氢源,45 bar N2,493 K,Ni-Cu/Al2O3催化剂,反应24 h,甘油转化率和1,2-PDO选择性分别为90% 和82%[59]。
2 反应机理
要想设计出高效的催化剂,认识甘油氢解反应的机理至关重要,根据文献报道,可能的反应机理有5 种。其中脱氢-脱水-加氢机理[60]、脱水-加氢机理[61]和螯合氢解机理[62]已有较多综述提到,本文不予赘述,只对直接氢解机理和原位氢解机理进行解释说明。

图1 甘油氢解过渡态模型[63]
直接氢解机理由Shinmi 等提出,后来被该研究小组进一步完善[63-64]。首先,甘油的-CH2OH 基团吸附在ReOx表面上形成醇盐,然后活化氢攻击醇盐的3 号位,使C-O 键断裂,最后醇盐水解得到产物1,2-PDO(见图1)。
Chia 等提出了一种不同的直接氢解路径[65]。第一步甘油通过质子化-脱水反应形成碳正离子,-CH2OH 基团发生氢转移,碳正离子形成更稳定的含氧碳正离子。最后氢转移生成1,2-PDO 或1,3-PDO[65]。这与Qin 等的研究结果一致[66]。反应过程如下所示:
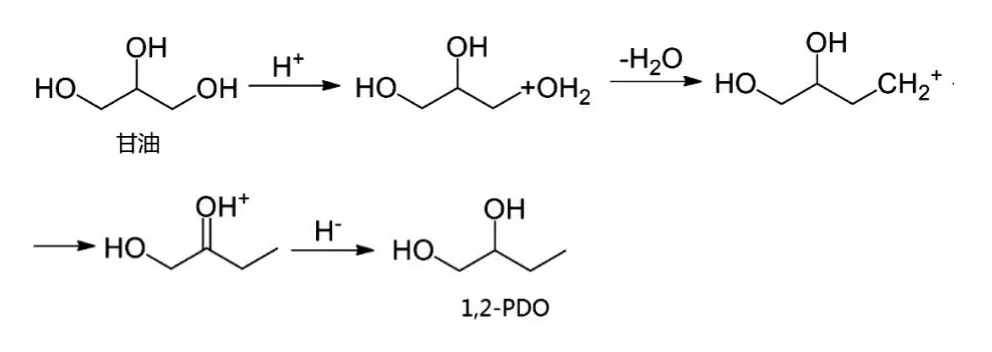
原位氢解机理是基于脱水-加氢机理发展而来的,理论上甘油自身可以通过水相重整(APR)产生氢气[67],将甘油制氢和甘油氢解结合起来,使生成的氢气被消耗,即APR 原位氢解机理(见图2)。除甘油本身作氢源,乙醇[11]、甲酸[56]、异丙醇[56]、甲醇[58]等也可作氢源,通过催化转移加氢(CTH)实现甘油催化氢解,即CTH 原位氢解机理。首先载体酸中心吸附甘油形成醇盐,金属活性位活化氢源分解产生的氢,然后醇盐和吸附的活性氢原子相互作用生成1,2-PDO。
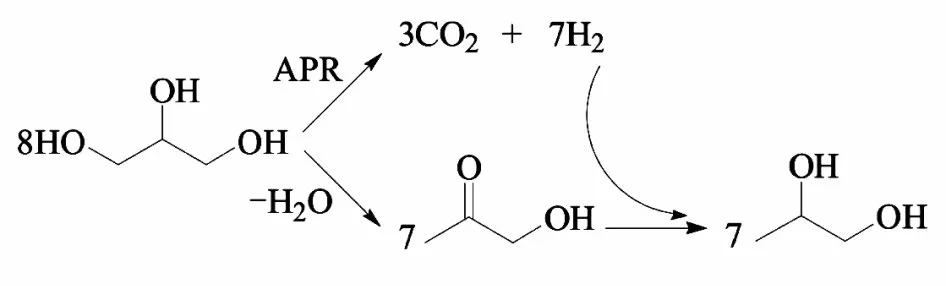
图2 甘油水相重整与氢解制备1,2-PDO[68]
3 结论
甘油催化氢解制1,2-PDO 已成为甘油转化的研究热点,近五年来有了新的进展。研究表明,金属催化剂性能顺序为Ru≈Cu≈Ni>Pt>Pd,其中Cu 催化剂价格相对低廉,1,2-PDO 选择性较高,具有工业化前景;双金属催化剂可提高金属分散性、减小金属粒径,不同金属针对不同反应步骤发挥作用,因此催化性能比单一金属好;载体、制备方法等也是影响催化剂性能的关键因素,特别是采用溶胶凝胶法制备的催化剂,甘油转化率和1,2-PDO 选择性较高;酸碱添加剂能提高催化剂的热稳定性,加快反应速率或减少副产物生成;金属氧化物类助剂可提高催化剂表面酸性,或使活性组分不易被氧化;此外,认识甘油氢解反应的各种机理,对设计高效催化剂具有重要意义。目前,甘油氢解制备1,2-PDO 的技术已趋于成熟,如何实现工业化应是未来探索的主要方向。
[1]Zhou C H,Zhao H,Tong D S,et al.Recent advances in catalytic conversion of glycerol[J].Catalysis Reviews,2013,55(4):369-453.
[2]Zhou C H C,Beltramini J N,Fan Y X,et al.Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals [J].Chemical Society Reviews,2008,37(3):527-549.
[3]BalaraJu M,Rekha V,Prasad P S,et al.Influence of solid acids as co-catalysts on glycerol hydrogenolysis to propylene glycol over Ru/C catalysts [J].Applied Catalysis A:General,2009,354(1):82-87.
[4]Gandarias I,Arias P L,Requies J,et al.Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst:The role of acid and metal sites on product selectivity and the reaction mechanism [J].Applied Catalysis B:Environmental,2010,97(1):248-256.
[5]BalaraJu M,Rekha V,Devi B L A,et al.Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol[J].Applied Catalysis A:General,2010,384(1):107-114.
[6]Li Y,Ma L,Liu H,et al.Influence of HZSM5 on the activity of Ru catalysts and product selectivity during the hydrogenolysis of glycerol [J].Applied Catalysis A:General,2014,469:45-51.
[7]Feng J,Xiong W,Xu B,et al.Basic oxide-supported Ru catalysts for liquid phase glycerol hydrogenolysis in an additive-free system[J].Catalysis Communications,2014,46:98-102.
[8]Yuan Z,Wu P,Gao J,et al.Pt/solid-base:A predominant catalyst for glycerol hydrogenolysis in a base-free aqueous solution[J].Catalysis letters,2009,130(1-2):261-265.
[9]D'Hondt E,Van de Vyver S,Sels B F,et al.Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen [J].Chemical Communications,2008(45):6011-6012.
[10]Roy D,Subramaniam B,Chaudhari R V.Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition[J].Catalysis Today,2010,156(1):31-37.
[11]Musolino M G,Scarpino L A,Mauriello F,et al.Selective transfer hydrogenolysis of glycerol promoted by palladium catalysts in absence of hydrogen [J].Green Chemistry,2009,11(10):1511-1513.
[12]Yadav G D,Chandan P A,Tekale D P.Hydrogenolysis of glycerol to 1,2-propanediol over nano-fibrous Ag-OMS-2 catalysts [J].Industrial &Engineering Chemistry Research,2011,51(4):1549-1562.
[13]Zhou J,Zhang J,Guo X,et al.Ag/Al2O3for glycerol hydrogenolysis to 1,2-propanediol:activity,selectivity and deactivation[J].Green Chemistry,2012,14(1):156-163.
[14]Schmidt S R,Tanielyan S K,Marin N,et al.Selective conversion of glycerol to propylene glycol over fixed bed raneyCu Catalysts[J].Topics in Catalysis,2010,53(15-18):1214-1216.
[15]Yue C J,Zhang Q Y,Gu L P,et al.Oxides-modified Raney copper as catalysts for selective hydrogenolysis of glycerol [J].Asia-Pacific Journal of Chemical Engineering,2014,doi:10.1002/apj.1787.
[16]Sánchez T,Salagre P,Cesteros Y,et al.Use of delaminated hectorites as supports of copper catalysts for the hydrogenolysis of glycerol to 1,2-propanediol [J].Chemical Engineering Journal,2012,179:302-311.
[17]Yuan Z,Wang L,Wang J,et al.Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts[J].Applied Catalysis B:Environmental,2011,101 (3):431-440.
[18]Zheng J,Zhu W,Ma C,et al.Hydrogenolysis of glycerol to 1,2-propanediol on the high dispersed SBA-15 supported copper catalyst prepared by the ion-exchange method[J].Reaction Kinetics,Mechanisms and Catalysis,2010,99(2):455-462.
[19]Vasiliadou E S,Eggenhuisen T M,Munnik P,et al.Synthesis and performance of highly dispersed Cu/SiO2catalysts for the hydrogenolysis of glycerol[J].Applied Catalysis B:Environmental,2014,145:108-119.
[20]Huang L,Zhu Y L,Zheng H Y,et al.Continuous production of 1,2-propanediol by the selective hydrogenolysis of solvent-free glycerol under mild conditions [J].Journal of chemical technology and biotechnology,2008,83 (12):1670-1675.
[21]Kim N D,Oh S,Joo J B,et al.The Promotion effect of cr on copper catalyst in hydrogenolysis of glycerol to propylene glycol [J].Topics in Catalysis,2010,53 (7-10):517-522.
[22]Ma Z,Xiao Z,van Bokhoven J A,et al.A non-alkoxide sol-gel route to highly active and selective Cu-Cr catalysts for glycerol conversion [J].Journal of Materials Chemistry,2010,20(4):755-760.
[23]Xiao Z,Jin S,Wang X,et al.Preparation,structure and catalytic properties of magnetically separable Cu-Fe catalysts for glycerol hydrogenolysis [J].Journal of Materials Chemistry,2012,22(32):16598-16605.
[24]Kwak B K,Park D S,Yun Y S,et al.Preparation and characterization of nanocrystalline CuAl2O4spinel catalysts by sol-gel method for the hydrogenolysis of glycerol[J].Catalysis Communications,2012,24:90-95.
[25]Bienholz A,Hofmann H,Claus P.Selective hydrogenolysis of glycerol over copper catalysts both in liquid and vapour phase:Correlation between the copper surface area and the catalyst's activity [J].Applied Catalysis A:General,2011,391(1):153-157.
[26]Mane R B,Kondawar S E,Niphadkar P S,et al.Effect of preparation parameters of Cu catalysts on their physicochemical properties and activities for glycerol hydrogenolysis[J].Catalysis Today,2012,198(1):321-329.
[27]BalaraJu M,Jagadeeswaraiah K,Prasad P S S,et al.Catalytic hydrogenolysis of biodiesel derived glycerol to 1,2-propanediol over Cu-MgO catalysts[J].Catalysis Science&Technology,2012,2(9):1967-1976.
[28]Yuan Z,Wang J,Wang L,et al.Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts[J].Bioresource technology,2010,101(18):7088-7092.
[29]Bienholz A,Schwab F,Claus P.Hydrogenolysis of glycerol over a highly active CuO/ZnO catalyst prepared by an oxalate gel method:influence of solvent and reaction temperature on catalyst deactivation [J].Green Chemistry,2010,12(2):290-295.
[30]Mane R B,Ghalwadkar A A,Hengne A M,et al.Role of promoters in copper chromite catalysts for hydrogenolysis of glycerol[J].Catalysis Today,2011,164(1):447-450.
[31]Bienholz A,Blume R,Knop-Gericke A,et al.Prevention of catalyst deactivation in the hydrogenolysis of glycerol by Ga2O3-modified copper/zinc oxide catalysts[J].The Journal of Physical Chemistry C,2010,115(4):999-1005.
[32]Maglinao R L,He B B.Catalytic thermochemical conversion of glycerol to simple and polyhydric alcohols using Raney nickel catalyst [J].Industrial &Engineering Chemistry Research,2011,50(10):6028-6033.
[33]Yin A Y,Guo X Y,Dai W L,et al.The synthesis of propylene glycol and ethylene glycol from glycerol using Raney Ni as a versatile catalyst [J].Green Chemistry,2009,11(10):1514-1516.
[34]Yu W,Xu J,Ma H,et al.A remarkable enhancement of catalytic activity for KBH4treating the carbothermal reduced Ni/AC catalyst in glycerol hydrogenolysis[J].Catalysis Communications,2010,11(5):493-497.
[35]Zhao J,Yu W,Chen C,et al.Ni/NaX:a bifunctional efficient catalyst for selective hydrogenolysis of glycerol[J].Catalysis letters,2010,134(1-2):184-189.
[36]Marinoiu A,Ionita G,Gáspár C L,et al.Glycerol hydrogenolysis to propylene glycol [J].Reaction Kinetics and Catalysis Letters,2009,97(2):315-320.
[37]Meher L C,Gopinath R,Naik S N,et al.Catalytic hydrogenolysis of glycerol to propylene glycol over mixed oxides derived from a hydrotalcite-type precursor [J].Industrial &Engineering Chemistry Research,2009,48 (4):1840-1846.
[38]Hosgün H L,Y1ld1z M,Ger觭el H F.Hydrogenolysis of aqueous glycerol over Raney nickel catalyst:Comparison of pure and biodiesel by-product[J].Industrial &Engineering Chemistry Research,2012,51(10):3863-3869.
[39]Marinoiu A,Ionita G,Gáspár C L,et al.Selective hydrogenolysis of glycerol to propylene glycol using heterogeneous catalysts [J].Reaction Kinetics,Mechanisms and Catalysis,2010,99(1):111-118.
[40]Chiu C W,Tekeei A,Ronco J M,et al.Reducing byproduct formation during conversion of glycerol to propylene glycol [J].Industrial &Engineering Chemistry Research,2008,47(18):6878-6884.
[41]Guo X,Li Y,Shi R,et al.Co/MgO catalysts for hydrogenolysis of glycerol to 1,2-propanediol [J].Applied Catalysis A:General,2009,371(1):108-113.
[42]Guo X,Li Y,Song W,et al.Glycerol hydrogenolysis over Co catalysts derived from a layered double hydroxide precursor[J].Catalysis letters,2011,141(10):1458-1463.
[43]Maris E P,Ketchie W C,Murayama M,et al.Glycerol hydrogenolysis on carbon -supported PtRu and AuRu bimetallic catalysts [J].Journal of Catalysis,2007,251(2):281-294.
[44]Ma L,He D.Hydrogenolysis of glycerol to propanediols over highly active Ru-Re bimetallic catalysts [J].Topics in Catalysis,2009,52(6-7):834-844.
[45]Ma L,He D.Influence of catalyst pretreatment on catalytic properties and performances of Ru-Re/SiO2in glycerol hydrogenolysis to propanediols[J].Catalysis Today,2010,149(1):148-156.
[46]Shinmi Y,Koso S,Kubota T,et al.Modification of Rh/SiO2catalyst for the hydrogenolysis of glycerol in water [J].Applied Catalysis B:Environmental,2010,94(3):318-326.
[47]Li Y,Liu H,Ma L,et al.Glycerol hydrogenolysis to propanediols over supported Pd-Re catalysts [J].RSC Advances,2014,4(11):5503-5512.
[48]Daniel O M,DeLaRiva A,Kunkes E L,et al.X-ray absorption spectroscopy of bimetallic Pt-Re catalysts for hydrogenolysis of glycerol to propanediols[J].ChemCatChem,2010,2(9):1107-1114.
[49]Xia S,Yuan Z,Wang L,et al.Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors[J].Applied Catalysis A:General,2011,403(1):173-182.
[50]Xia S,Yuan Z,Wang L,et al.Catalytic production of 1,2-propanediol from glycerol in bio-ethanol solvent [J].Bioresource technology,2012,104:814-817.
[51]Wu Z,Mao Y,Wang X,et al.Preparation of a Cu-Ru/carbon nanotube catalyst for hydrogenolysis of glycerol to 1,2-propanediol via hydrogen spillover [J].Green Chemistry,2011,13(5):1311-1316.
[52]Jiang T,Zhou Y,Liang S,et al.Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids[J].Green Chemistry,2009,11(7):1000-1006.
[53]Vasiliadou E S,Lemonidou A A.Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis[J].Applied Catalysis A:General,2011,396(1):177-185.
[54]Zhou J,Guo L,Guo X,et al.Selective hydrogenolysis of glycerol to propanediols on supported Cu -containing bimetallic catalysts [J].Green Chemistry,2010,12(10):1835-1843.
[55]Sun D,Yamada Y,Sato S.Effect of Ag loading on Cu/Al2O3catalyst in the production of 1,2-propanediol from glycerol [J].Applied Catalysis A:General,2014,475,63-68.
[56]Gandarias I,Arias P L,Requies J,et al.Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source [J].Journal of Catalysis,2011,282(1):237-247.
[57]Gandarias I,Requies J,Arias P L,et al.Liquid-phase glycerol hydrogenolysis by formic acid over Ni-Cu/Al2O3catalysts[J].Journal of Catalysis,2012,290:79-89.
[58]Gandarias I,Arias P L,Fernández S G,et al.Hydrogenolysis through catalytic transfer hydrogenation:Glycerol conversion to 1,2-propanediol [J].Catalysis Today,2012,195(1):22-31.
[59]Gandarias I,Fernández S G,El Doukkali M,et al.Physicochemical study of glycerol hydrogenolysis over a Ni-Cu/Al2O3catalyst using formic acid as the hydrogen source[J].Topics in Catalysis,2013,56(11):995-1007.
[60]Montassier C,Giraud D,Barbier J.Polyol conversion by liquid phase heterogeneous catalysis over metals[J].Studies in Surface Science and Catalysis,1988,41:165-170.
[61]Dasari M A,Kiatsimkul P P,Sutterlin W R,et al.Lowpressure hydrogenolysis of glycerol to propylene glycol[J].Applied Catalysis A:General,2005,281(1):225-231.
[62]Chaminand J,aurent DJakovitch L,Gallezot P,et al.Glycerol hydrogenolysis on heterogeneous catalysts [J].Green Chemistry,2004,6(8):359-361.
[63]Amada Y,Shinmi Y,Koso S,et al.Reaction mechanism of the glycerol hydrogenolysis to 1,3-propanediol over Ir-ReOx/SiO2catalyst [J].Applied Catalysis B:Environmental,2011,105(1):117-127.
[64]Nakagawa Y,Shinmi Y,Koso S,et al.Direct hydrogenolysis of glycerol into 1,3-propanediol over rhenium-modified iridium catalyst[J].Journal of Catalysis,2010,272(2):191-194.
[65]Chia M,Pagán-Torres Y J,Hibbitts D,et al.Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts [J].Journal of the American Chemical Society,2011,133 (32):12675-12689.
[66]Qin L Z,Song M J,Chen C L.Aqueous-phase deoxygenation of glycerol to 1,3-propanediol over Pt/WO3/ZrO2catalysts in a fixed-bed reactor [J].Green chemistry,2010,12(8):1466-1472.
[67]杨光星,赖超凤,李爽,等.甘油制氢研究进展[J].工业催化,2010,18(1):1-6.
[68]Hu J,Liu X,Fan Y,et al.Physically mixed ZnO and skeletal NiMo for one-pot reforming-hydrogenolysis of glycerol to 1,2-propanediol[J].Chinese Journal of Catalysis,2013,34(5):1020-1026.
