高分辨MRI技术对动脉粥样硬化斑块的评价
2014-03-08董莉于薇濮欣苑纯
董莉,于薇,濮欣,苑纯
心脑血管病已经逐渐成为威胁人类生命健康的“头号杀手”。在心脑血管临床事件中,动脉粥样硬化斑块破裂和血栓形成是主要的发病机制。研究发现,近70%的急性心血管事件是由斑块破裂造成的[1]。
传统的评价易损斑块的临床指标仍停留在对管腔狭窄程度的评估方面。但由于存在血管的正性重构(positive remodeling),即斑块占血管的横截面积小于40%时斑块所在处的动脉代偿性扩张[2],造成了管腔显影对斑块识别的局限性。Ballotta等[3]学者研究发现即使有症状的轻度颈动脉狭窄的患者,斑块内也存在着不同程度的溃疡、斑块破裂、出血及表面血栓形成。Dong等[4]对一组没有颈动脉狭窄但有高危因素的人群(n=46)进行磁共振(magnetic resonance,MR)血管壁成像检查,发现67.4%的患者存在脂质斑块,8.7%的患者斑块具有内出血,4.3%的患者斑块破裂。Libby[1]研究发现急性心血管事件的元凶超过2/3发生于非显著狭窄性病变。这些研究均表明单纯地只对管腔的狭窄程度进行评价有可能低估了病变自身的危险性。
既能够显示管腔狭窄,又能显示斑块形态及功能的成像技术,如MRI已成为当前的研究热点。MR血管壁成像结合黑血及亮血技术,可以提供血管组织结构、管壁厚度、斑块成分等信息。这将给临床提供除狭窄以外的一种检查手段和评判指标。同时对那些狭窄程度不是很重的高危病人提供更多的诊断信息。本文对MR血管壁成像即斑块负荷、斑块成分识别进行一简要综述。
1 斑块负荷定性、定量研究
动脉壁内皮损伤及脂质的沉积是目前公认的动脉粥样硬化始动因素。由于血管内皮细胞功能受损,血液中低密度脂蛋白颗粒进入血管壁,继而被巨噬细胞所吞噬形成脂质条纹(fatty streak),血管壁反应性地增厚[5]。因此,早期的动脉硬化表现为血管壁增厚。研究证实MR不仅可以清楚地探测血管壁,准确测量血管壁的厚度[6],而且不同的MR机型、不同扫描、不同阅片者之间都有很好的一致性(可重复性)[7-9]。另外,Underhill等[10]发现MRI测量颈动脉管壁与超声也有很好的一致性(r=0.93,P<0.001)。2000年,Fayad等[11]成功地在8位健康志愿者及5例冠状动脉粥样硬化性心脏病患者中得到了二维黑血冠状动脉管壁图像,并发现冠状动脉粥样硬化性心脏病患者的冠状动脉管壁较正常人增厚。2002年,Kim等[12]在12位健康志愿者及冠状动脉粥样硬化性心脏病患者中得到了三维的右冠状动脉近中段管壁图像。Desai等[13]对健康志愿者进行右冠状动脉近中段三维管壁成像,并在1个月后进行重复成像。对两次检查所显示的冠状动脉管壁长度、管壁厚度由不同的观察者进行观察,结果显示同一观察者前后两次判断及不同观察者间都具有良好的一致性。
2 斑块成分定性、定量研究
随着病程的发展,脂纹表层沉积大量胶原纤维,平滑肌细胞(smooth muscle cell)增生并分泌大量细胞外间质(extracellular matrix),构成薄厚不一的纤维帽(fibrous cap)。纤维帽下细胞外脂质、富含细胞内脂质的巨噬细胞和泡沫细胞以及脂纹则构成了脂核(lipid core)。脂核进一步发展可出现坏死(lipid-rich necrotic core)、斑块内微血管出血(intraplaque hemorrhage)、钙化(calcification)和斑块内微血管化(mircovessels)。MR多对比成像技术可以可靠地评价斑块内成分并进行定性、定量分析(信号特点见表1)。
2.1 脂核和纤维帽 多种MR加权成像[T1加权图像(T1weighted images,T1WI),T2加权图像(T2weighted images,T2WI)/质子密度加权图像(proton density weighted images,PDWI),时间飞跃(time of flight,TOF)]可以显示脂质核心(图1)。与胸锁乳突肌信号相比,脂核在T1WI和TOF上为等信号,T2WI为低信号。Fabiano等[14]用MR扫描离体斑块,发现其敏感性为92%,特异性为74%。在活体,与病理相对照,MR敏感性为92%,特异性为65%[15]。Cai等[16]对纤维帽进行了定量分析,发现MRI与病理有很好的相关性(最大纤维帽厚度:r=0.78,P<0.001;长度:r=0.73,P<0.001;面积:r=0.73,P<0.001)。如给予对比剂行增强扫描,可以显示脂核和纤维帽更多的信息[17-18]。对比剂可使纤维组织的信号提高79.5%,而脂核的信号下降28.6%[17]。强化的纤维帽和未强化的脂核形成了良好的对比,从而更容易勾勒出脂核的边界而得到准确的定量测量结果(图1)。Wasserman等[18]还发现与T2WI相比,对比剂增强后T1WI可提升脂核与纤维帽间的对比噪声比约2倍。Maintz等[19]尝试采用增强三维T1WI冠状动脉管壁成像(Navigator-gated Free-breathing and Cardiac-triggered T1-weighted Inversion-recovery and Fat-suppressed 3D black-blood Gradient-echo Sequence),探讨MR延迟强化评价冠状动脉斑块的价值。Yeon等[20]选择6位健康志愿者和14例冠状动脉粥样硬化性心脏病患者进行延迟强化MR冠状动脉管壁成像,发现具有动脉硬化斑块的冠状动脉节段延迟强化信号明显高于正常节段。
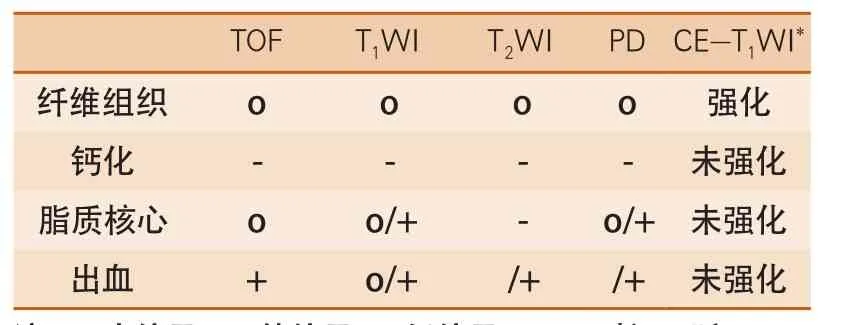
表1 MR多对比成像技术斑块内成分信号特点

图1 颈动脉脂质斑块
2.2 斑块内微血管出血 斑块内出血来自斑块内未成熟血管的红细胞渗漏[21]。尽管斑块内出血导致斑块破裂的机制还不十分清楚,但其可加速脂核的形成[22]。新近发表的研究表明,斑块内出血对斑块的发展进程以及转归也起着至关重要的作用。Sun等[23]对一组狭窄程度在50%~75%的无症状患者进行了54个月的随访,发现随访期间出现斑块内出血的患者,斑块生长速度明显高于斑块内未出血的患者[(34.2±9.0)mm3/year vs (5.3±7.2)mm3/year]。由于正铁血红蛋白可以不同程度地缩短T1弛豫时间从而在T1加权像上呈现高信号,表现为在T1WI和TOF上为高信号,早期出血在T2WI为低信号,晚期的则为等或高信号(图2)。此信号特点与病理相对照,敏感性为85%~95%,特异性为70%~77%[24]。Moody等[25]用三维重T1加权磁化强度预备梯度回波序列(magnetizationprepared rapid acquisition gradient-echo,MP-RAGE)观察出血,其敏感性为84%,特异性为84%。Ota等[26]在高场强(3.0T)MR设备进行探究,发现其敏感性为80%,特异性为97%。而利用MR血管壁成像对冠状动脉内血栓的显示敏感性为91%,特异性达到了88%[27]。新近研发的显示出血的序列(simultaneous noncontrast angiography and intraplaque hemorrhage,SNAP)不仅很好地显示出血病灶自身,并可以清晰地显示管腔情况,为临床提供了更加直观的信息[28]。
2.3 钙化 钙化在MR的T1WI、T2WI、TOF均表现为低信号(图3)。Fabiano等[14]报道MR探测钙化的准确性为98%,特异性为99%。Saam等[15]用MR测量了钙化的大小,与病理有很好的一致性(r=0.74,P<0.001)。

图2 颈动脉斑块内出血

图3 颈动脉斑块内钙化
尽管钙化经常在斑块内出现,但其是否导致斑块的不稳定性尚无定论。一些研究表明出现大量钙化与增加斑块破裂的危险性呈正相关[29-33],而另一些研究则提示钙化有助于斑块的稳定[34-36]。最近,研究者开始提出钙化出现的位置有可能影响斑块的稳定性。Li等[37-38]利用生物力学模型研究显示,如果钙化出现在薄纤维帽内,则纤维帽的最大剪切力相应增加47.5%。相反,如果钙化出现在脂核或远离纤维帽的位置,剪切力则没有增加。
2.4 斑块内微血管化形成 细胞内微血管化的形成一方面是供给斑块营养的来源,另一方面也是传导、运输炎性细胞、炎性因子的渠道。Moreno等[39]发现微血管的数目不仅和炎性细胞的数量相关,也和斑块破裂有关。Mofidi等[40]也发现微血管的数目和斑块内出血相关。目前有两种MR技术探测斑块内微血管化。一种是利用动态增强(dynamic contrast enhanced MRI,DCE-MRI)技术。这种技术最初被应用于肿瘤微血管化的研究。在斑块内部,利用这种技术同样可以观察微血管的数目和通透性[41-42]。有研究证实,血浆容量分数(fractional plasma volume,Vp)与微血管的面积相关[43],而对比剂的透过常数(transfer constant,Ktrans)与微血管的通透性相关[41]。另一种应用,超顺磁性氧化铁(ultrasmall super-paramagnetic iron oxide,USPIO)颗粒作为分子影像对比剂也用于斑块成分及稳定性的研究。USPIO颗粒可以通过受损的内皮细胞进入斑块内,并被巨噬细胞所吞噬,表现为信号缺失。Trivedi等[44]发现这种局部信号的缺失出现在75%的易损斑块中,而只有7%在稳定斑块中。
总之,在体MR多对比成像技术可以作为有效的方法定性、定量地诊断颈动脉斑块,识别易损斑块特征,并对斑块的转归或发展进行动态监测。这项技术已渐渐成熟走向临床,对卒中高危患者的早期识别,临床的早期干预治疗提供可靠的影像依据。
1 Libby P. Coronary artery injury and the biology of atherosclerosis inflammation, thrombosis, and stabilization[J]. Am J Cardiol, 2000, 86:3J-8J.
2 Glagov S, Weisenberg E, Zarins CK, et al.Compensatory enlargement of human atherosclerotic coronary arteries[J]. N Engl J Med, 1987, 316:1371-1375.
3 Ballotta E, Angelini A, Mazzalai F, et al. Carotid endarterectomy for symptomatic low-grade carotid stenosis[J]. J Vasc Surg, 2014, 59:25-31.
4 Dong L, Underhill HR, Yu W, et al. Geometric and compositional appearance of atheroma in an angiographically normal carotid artery in patients with atherosclerosis[J]. AJNR Am J Neuroradiol, 2010,31:311-316.
5 Hansson G. Inflammation, atherosclerosis, and coronary artery disease[J]. N Engl J Med, 2005,352:1685-1695.
6 Zhang S, Cai J, Luo Y, et al. Measurement of carotid wall volume and maximum area with contrast-enhanced 3D MR imaging:initial observations[J]. Radiology,2003, 228:200-205.
7 Kang X, Polissar NL, Han C, et al. Analysis of the measurement precision of arterial lumen and wall areas using high-resolution MRI[J]. Magn Reson Med, 2000,44:968-972.
8 Alizadeh Dehnavi R, Doornbos J, Tamsma JT, et al.Assessment of the carotid artery by MRI at 3T:a study on reproducibility[J]. J Magn Reson Imaging, 2007,25:1035-1043.
9 Vidal A, Bureau Y, Wade T, et al. Scan-rescan and intraobserver variability of magnetic resonance imaging of carotid atherosclerosis at 1.5 T and 3.0 T[J]. Phys Med Biol, 2008, 53:6821-6835.
10 Underhill H, Kerwin W, Hatsukami T, et al. Automated measurement of mean wall thickness in the common carotid artery by MRI:a comparison to intima-media thickness by B-mode ultrasound[J]. J Magn Reson Imaging, 2006, 24:379-387.
11 Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging[J].Circulation, 2000, 102:506-510.
12 Kim WY, Stuber M, Bornert P, et al. Threedimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease[J]. Circulation, 2002, 106:296-299.
13 Desai MY, Lai S, Barmet C, et al. Reproducibility of 3D free-breathing magnetic resonance coronary vessel wall imaging[J]. Eur Heart J, 2005, 26:2320-2324.
14 Fabiano S, Mancino S, Stefanini M, et al. Highresolution multicontrast-weighted MR imaging from human carotid endarterectomy specimens to assess carotid plaque components[J]. Eur Radiol, 2008,18:2912-2921.
15 Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI[J]. Arterioscler Thromb Vasc Biol, 2005, 25:234-239.
16 Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque:comparison of high-resolution, contrastenhanced magnetic resonance imaging and histology[J].Circulation, 2005, 112:3437-3444.
17 Yuan C, Kerwin WS, Ferguson MS, et al. Contrastenhanced high resolution MRI for atherosclerotic carotid artery tissue characterization[J]. J Magn Reson Imaging, 2002, 15:62-67.
18 Wasserman BA, Smith WI, Trout HH, et al.Carotid artery atherosclerosis:in vivo morphologic characterization with gadolinium-enhanced doubleoblique MR imaging initial results[J]. Radiology, 2002,223:566-573.
19 Maintz D, Ozgun M, Hoffmeier A, et al. Selective coronary artery plaque visualization and differentiation by contrast-enhanced inversion prepared MRI[J]. Eur Heart J, 2006, 27:1732-1736.
20 Yeon SB, Sabir A, Clouse M, et al. Delayedenhancement cardiovascular magnetic resonance coronary artery wall imaging comparison with multislice computed tomography and quantitative coronary angiography[J]. J Am Coll Cardiol, 2007,50:441-447.
21 Kockx M, Cromheeke K, Knaapen M, et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis[J]. Arterioscler Thromb Vasc Biol, 2003,23:440-446.
22 Virmani R, Kolodgie F, Burke A, et al. Atherosclerotic plaque progression and vulnerability to rupture:angiogenesis as a source of intraplaque hemorrhage[J]. Arterioscler Thromb Vasc Biol, 2005,25:2054-2061.
23 Sun J, Underhill HR, Hippe DS, et al. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage:a long-term time course study[J]. JACC Cardiovasc Img, 2012,5:798-804.
24 Chu B, Kampschulte A, Ferguson MS, et al.Hemorrhage in the atherosclerotic carotid plaque:a high-resolution MRI study[J]. Stroke, 2004, 35:1079-1084.
25 Moody A, Murphy R, Morgan P, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia[J]. Circulation, 2003, 107:3047-3052.
26 Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging:comparison of the diagnostic performance of three T1-weighted sequences[J]. Radiology, 2010,254:551-563.
27 Jansen CH, Perera D, Makowski MR, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction[J].Circulation, 2011, 124:416-424.
28 Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage(SNAP) imaging for carotid atherosclerotic disease evaluation[J]. Magn Reson Med, 2013, 69:337-345.
29 Stary H. Natural history of calcium deposits in atherosclerosis progression and regression[J]. Z Kardiol, 2000, 89:28-35.
30 Mintz G, Pichard A, Popma J, et al. Determinants and correlates of target lesion calcium in coronary artery disease:a clinical, angiographic and intravascular ultrasound study[J]. J Am Coll Cardiol, 1997, 29:268-274.
31 Raggi P, Callister T, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography[J]. Circulation, 2000, 101:850-855.
32 Taylor A, Burke A, O'Malley P, et al. A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death[J]. Circulation, 2000, 101:1243-1248.
33 Schmermund A, Erbel R. Unstable coronary plaque and its relation to coronary calcium[J]. Circulation, 2001,104:1682-1687.
34 Huang H, Virmani R, Younis H, et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques[J]. Circulation, 2001, 103:1051-1056.
35 Cheng G, Loree H, Kamm R, et al. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation[J]. Circulation, 1993,87:1179-1187.
36 Alderman E, Corley S, Fisher L, et al. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS). CASS Participating Investigators and Staff[J]. J Am Coll Cardiol, 1993,22:1141-1154.
37 Li Z, Howarth S, Tang T, et al. Does calcium deposition play a role in the stability of atheroma? Location may be the key[J]. Cerebrovasc Dis, 2007, 24:452-459.
38 Li Z, U-King-Im J, Tang T, et al. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm[J]. J Vasc Surg,2008, 47:928-935.
39 Moreno P, Purushothaman K, Fuster V, et al.Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta:implications for plaque vulnerability[J]. Circulation, 2004, 110:2032-2038.
40 Mofidi R, Crotty T, McCarthy P, et al. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease[J]. Br J Surg,2001, 88:945-950.
41 Kerwin W, O'Brien K, Ferguson M, et al. Inflammation in carotid atherosclerotic plaque:a dynamic contrastenhanced MR imaging study[J]. Radiology, 2006,241:459-468.
42 Kerwin W, Oikawa M, Yuan C, et al. MR imaging of adventitial vasa vasorum in carotid atherosclerosis[J].Magn Reson Med, 2008, 59:507-514.
43 Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque[J]. Circulation,2003, 107:851-856.
44 Trivedi R, U-King-Im J, Graves M, et al. In vivo detection of macrophages in human carotid atheroma:temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI[J]. Stroke, 2004, 35:1631-1635.
