缺血性卒中:基于磁共振斑块成像的危险性评估和预防
2014-03-08孙杰
孙杰
位于颅内外动脉的粥样硬化斑块是缺血性卒中最常见的病因之一。近年来随着生活方式的变化,动脉粥样硬化危险因素的患病率明显升高[1-2]。一项在2010年进行的全国性流行病学调查中,满足糖尿病或糖尿病前期诊断标准的人群比率分别达到了11.6%和50.1%[3]。在这样的背景条件下,诊治动脉粥样硬化,预防初发和复发缺血性卒中成为了当前最为紧迫的临床和研究工作之一。
包括他汀和阿司匹林在内的药物治疗,血管成形及支架植入术(carotid artery stenting,CAS),以及颈动脉内膜剥脱术(carotid endarterectomy,CEA)等的发展为降低缺血性卒中的风险提供了有效的干预措施。然而,如何优化治疗方案,使患者最大程度地从干预中受益,同时避免不必要的治疗风险,对患者进行缺血性卒中风险的个体化评估成为临床决策过程的关键。血管造影显示的管腔狭窄程度曾经是临床医生认识粥样硬化斑块的唯一手段,并在一定程度上反映了斑块的临床风险[4]。例如,在北美症状性颈动脉内膜剥脱试验(North American Symptomatic Carotid Endarterectomy Trial,NASCET)中,具有轻度狭窄(<50%)的患者在术后随访期5年内的临床终点并不显著优于单纯药物治疗[4]。由于这些历史性的原因,颈动脉狭窄程度成为指导CEA的主要标准,并延用至今[5]。
然而,大规模的组织病理研究显示,斑块破裂并继发血栓是掩藏在管腔狭窄这一现象下,是引起动脉粥样硬化并发症的主要病理生理机制。Spagnoli等[6]对269例颈动脉斑块的病理分析显示,在管腔狭窄程度相同的情况下,74%和缺血性卒中相关的颈动脉斑块有明确的炎性纤维帽破裂和新鲜血栓附着,而这些特征仅出现在12%的无症状斑块中。一些在冠状动脉研究中发现和定义的高危斑块特征,如脂质坏死核心、纤维帽炎症细胞浸润、表面溃疡等,不但常见于颈动脉斑块,且在卒中发生后的数月内发生率逐渐减低,这和临床上卒中复发风险的变化一致[4,7]。纤维帽破裂诱导动脉血栓,可以逐渐进展造成原位栓塞,也有可能脱落引起远端栓塞,从而呈现出多样化的病程。
借助新兴的成像手段识别位于颅内外动脉的高危粥样硬化斑块将推动缺血性卒中临床干预策略的重大变革,从而成为近年来研究的重点。由于纤维帽破裂这一过程的生物学复杂性,准确评价斑块风险可能依赖于对包括脂质坏死核心、钙化、斑块内出血(intraplaque hemorrhage,IPH)、炎症反应、新生血管在内的诸多组分的有效分析。磁共振成像(magnetic resonance imaging,MRI)具有较好的空间分辨率和软组织分辨性。磁共振(magnetic resonance,MR)斑块成像技术的发展使临床医生能够无创地获取关于斑块形态和组分的丰富信息。越来越多的临床研究开始使用MR斑块成像来探讨粥样硬化斑块和缺血性卒中的关系。IPH和纤维帽破裂等一些MRI特征的临床意义逐渐明确。
尽管IPH现象在早期文献中已有所描述,但由于病理研究的局限性,我们对IPH在斑块进展和临床事件中所起作用的认识一直以来进展缓慢[8]。得益于红细胞分解代谢的中间产物正铁血红蛋白所具有的顺磁效应,MRI很快成为研究IPH的最有力手段[9-10]。与传统的组织病理研究相比,MRI检测IPH避免了样本来源的局限性,在明确IPH的临床意义方面提供了令人信服的证据。多个研究报道,IPH相关的T1加权序列高信号在症状性斑块中的出现率显著高于无症状性斑块,尤其在血管造影仅提示轻度或中度狭窄的情况下[11-12]。这一发现不仅明确了IPH和缺血性卒中的联系,而且提示MRI检测IPH能够在一定程度上弥补临床单纯评价管腔狭窄的不足。在以MRI为手段随访斑块变化的研究中,IPH的出现能够显著地促进斑块负荷和脂质核心增长,支持其在改变斑块表型(亚临床→临床)上的重要意义[13-15]。
纤维帽破裂也许是提示斑块和下游卒中相关的最直接的影像学证据。隐源性卒中在临床并不少见,提示检测纤维帽完整性在明确病因和开展针对性二级预防上具有潜在意义[16]。结合多个序列,特别是时间飞跃(time of flight,TOF)、T2加权和增强T1加权序列对显示纤维帽的完整性具有良好的敏感性和特异性[17]。包含这些序列的多重对比MRI是目前显示纤维帽状态、诊断复杂斑块的标准MRI技术[18]。在多项回顾性研究中,近期发生过临床事件的斑块出现纤维帽破裂的比率显著高于无症状斑块[19-21]。Parmar等[22]在卒中急性期进行MR斑块成像研究,初步显示了MRI检测纤维帽状态或复杂斑块在病因诊断上的作用。
MRI显示的斑块易损性特征和缺血性卒中的关系已经十分明确,然而,用这些成像信息判断患者预后,包括新发脑缺血事件、新发脑梗死灶和大脑认知功能变化的临床研究尚处在早期(表1)。Takaya等[23]对154例颈动脉中度狭窄(50%~79%)的无症状患者进行随访,首次报道了IPH、纤维帽完整性及脂质核心大小等多个颈动脉斑块MRI测量指标和新发脑缺血事件风险的相关性。Kwee等[24]的研究进一步说明斑块特征和脑缺血事件的病理生理联系具有普遍适用性,在颈动脉30%~69%狭窄的症状性患者中同样能够预测新发脑缺血事件的风险。Altaf和Singh等[25-26]的研究证实了IPH在颈动脉中度狭窄的患者群体中预测初发或复发脑缺血事件的价值。这些单中心研究初步显示了MRI在评价斑块危险性方面的良好的前景,但由于样本量小和研究群体的差异,无法对某一斑块特征的临床预测价值给出较为准确的估计[23-32]。最近,Saam等[33]对以往研究IPH预测新发脑缺血事件的文献进行了及时的荟萃分析。IPH使患者发生脑缺血事件的风险增大约5倍,在症状性患者中预测复发脑缺血事件的风险比更是达到了11.7。因此,MRI检测IPH很有可能率先在指导临床决策方面起到重要作用。值得注意的是,关于MR斑块成像信息预测新发脑缺血事件的荟萃分析今年以来被多次报道,显示了临床研究者对这一主题的关注[27,33-34]。
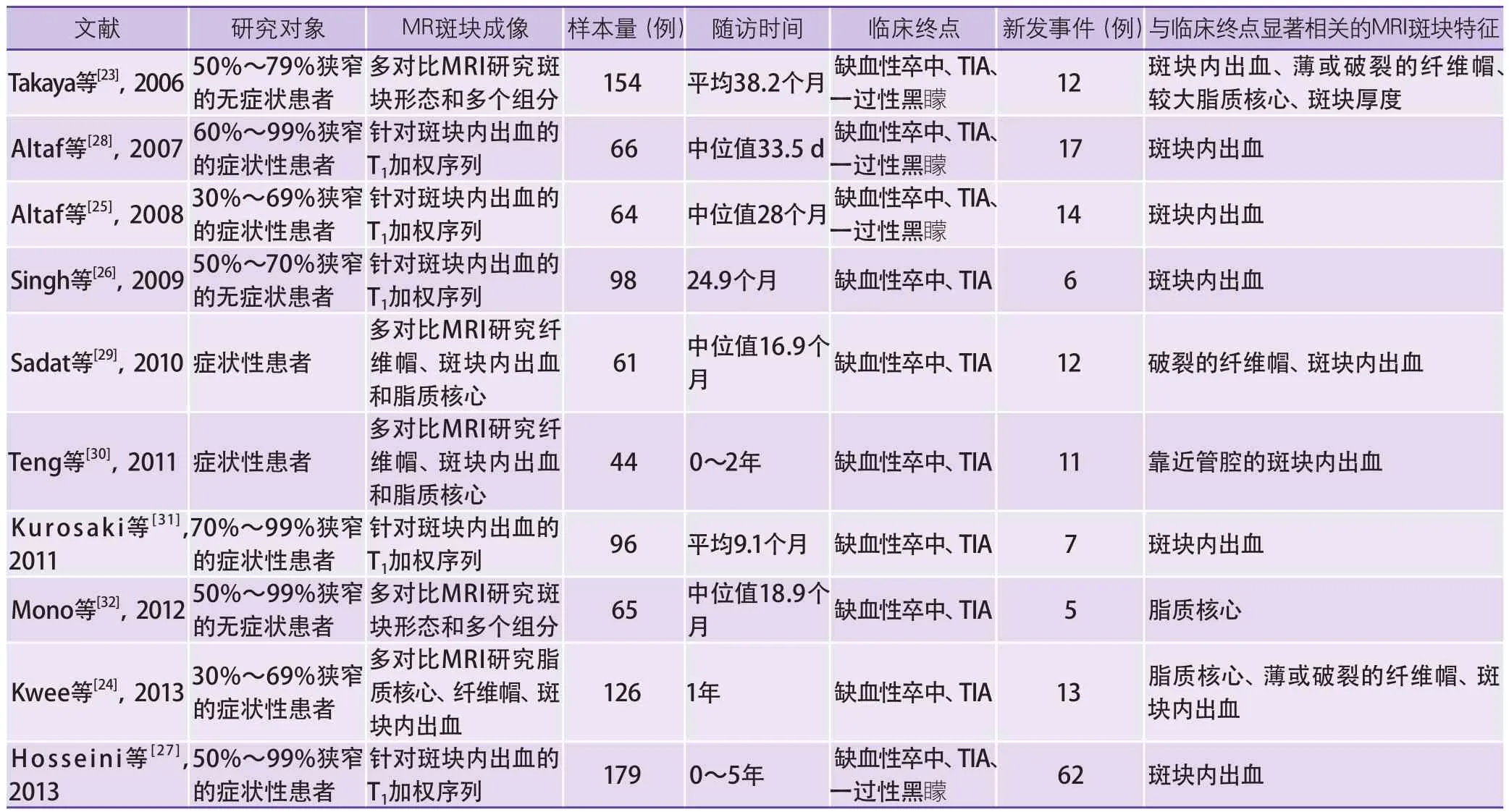
表1 利用MR斑块成像信息预测新发脑缺血事件的文献汇总
针对颅内动脉粥样硬化斑块的MRI研究仍然处在早期。由于缺少病理标本对比,MR颅内斑块成像的图像分析多借鉴颈动脉成像的经验。Li等[35]对比MR颅内斑块成像和磁共振血管造影(magnetic resonance angiography,MRA)的数据提示,作为目前临床常规检测手段的MRA并不能准确反映斑块负荷。Ma等[36]首先对基底动脉斑块的正性重构(positive remodeling)程度进行了定量的描述。斑块成分方面,Xu等[37]发现IPH现象也存在于大脑中动脉斑块,并和临床表现相关。
目前来看,MR斑块成像在临床研究中的应用丰富了我们对粥样硬化性缺血性卒中的病理生理机制的认识,也在小样本的前瞻性研究中初步显现了它在临床诊断和预后判断上的潜在价值。基于MRI的多中心大样本的斑块自然史研究,以及用斑块信息(而非管腔狭窄程度)指导干预的临床试验,仍然是MR斑块成像技术最终转化到临床实践中的关键步骤。与此同时,新的MRI技术仍然不断涌现和完善,一方面使得具有针对性的斑块成分成像更简单快捷并易于临床应用[38],另一方面在持续探索斑块功能层面提供有益的信息[39]。基于MR斑块成像的个体化危险性评估将在预防缺血性卒中上发挥重要的作用。
1 Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China:twenty-one-year observational study from the Sino-MONICA-Beijing Project[J]. Stroke, 2008, 39:1668-1674.
2 Liu L, Wang D, Wong KS, et al. Stroke and stroke care in China:huge burden, significant workload, and a national priority[J]. Stroke, 2011, 42:3651-3654.
3 Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults[J]. JAMA, 2013, 310:948-959.
4 Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators[J]. N Engl J Med, 1998, 339:1415-1425.
5 Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack:A guideline for healthcare professionals from the American Heart Association/American Stroke Association[J]. Stroke, 2011, 42:227-276.
6 Spagnoli LG, Mauriello A, Sangiorgi G, et al.Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke[J]. JAMA, 2004,292:1845-1852.
7 Redgrave J, Lovett JK, Gallagher PJ, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms- The Oxford plaque study[J]. Circulation, 2006,113:2320-2328.
8 Gao P, Chen ZQ, Bao YH, et al. Correlation between carotid intraplaque hemorrhage and clinical symptoms:systematic review of observational studies[J].Stroke, 2007, 38:2382-2390.
9 Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques[J].Circulation, 2001, 104:2051-2056.
10 Moody AR, Murphy RE, Morgan PS, et al.Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia[J]. Circulation, 2003,107:3047-3052.
11 Murphy RE, Moody AR, Morgan PS, et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia[J]. Circulation, 2003,107:3053-3058.
12 Yamada N, Higashi M, Otsubo R, et al. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events[J]. AJNR Am J Neuroradiol, 2007, 28:287-292.
13 Takaya N, Yuan C, Chu BC, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques:a high-resolution magnetic resonance imaging study[J]. Circulation,2005, 111:2768-2775.
14 Sun J, Underhill HR, Hippe DS, et al. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage:a long-term time course study[J]. JACC Cardiovasc Imaging, 2012,5:798-804.
15 Sun J, Balu N, Hippe DS, et al. Subclinical carotid atherosclerosis:short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging[J].Radiology, 2013, 268:61-68.
16 Freilinger TM, Schindler A, Schmidt C, et al.Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke[J]. JACC Cardiovasc Imaging, 2012, 5:397-405.
17 Mitsumori LM, Hatsukami TS, Ferguson MS, et al.In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques[J]. J Magn Reson Imaging, 2003,17:410-420.
18 Cai JM, Hatsukami TS, Ferguson MS, et al.Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging[J]. Circulation, 2002, 106:1368-1373.
19 Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke[J]. Circulation, 2002, 105:181-185.
20 Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging[J]. Radiology, 2006,240:464-472.
21 U-King-Im JM, Tang TY, Patterson A, et al.Characterisation of carotid atheroma in symptomatic and asymptomatic patients using high resolution MRI[J]. J Neurol Neurosurg Psychiatry, 2008, 79:905-912.
22 Parmar JP, Rogers WJ, Mugler JR, et al. Magnetic resonance imaging of carotid atherosclerotic plaque in clinically suspected acute transient ischemic attack and acute ischemic stroke[J]. Circulation, 2010, 122:2031-2038.
23 Takaya N, Yuan C, Chu BC, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events:a prospective assessment with MRI-initial results[J]. Stroke, 2006, 37:818-823.
24 Kwee RM, van Oostenbrugge RJ, Mess WH, et al. MRI of carotid atherosclerosis to identify TIA and stroke patients who are at risk of a recurrence[J]. J Magn Reson Imaging, 2013, 37:1189-1194.
25 Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events[J]. J Vasc Surg, 2008, 47:337-342.
26 Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis:MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men[J]. Radiology,2009, 252:502-508.
27 Hosseini AA, Kandiyil N, Macsweeney ST, et al.Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke[J]. Ann Neurol, 2013, 73:774-784.
28 Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis[J]. Stroke,2007, 38:1633-1635.
29 Sadat U, Teng Z, Young VE, et al. Association between biomechanical structural stresses of atherosclerotic carotid plaques and subsequent ischaemic cerebrovascular events--a longitudinal in vivo magnetic resonance imaging-based finite element study[J]. Eur J Vasc Endovasc Surg, 2010, 40:485-491.
30 Teng Z, Sadat U, Huang Y, et al. In vivo MRI-based 3D mechanical stress-strain profiles of carotid plaques with juxtaluminal plaque haemorrhage:an exploratory study for the mechanism of subsequent cerebrovascular events[J]. Eur J Vasc Endovasc Surg, 2011, 42:427-433.
31 Kurosaki Y, Yoshida K, Endo H, et al. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events[J]. Neurosurgery, 2011,68:62-67.
32 Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke[J]. Cerebrovasc Dis, 2012, 34:343-350.
33 Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging[J]. J Am Coll Cardiol,2013, 62:1081-1091.
34 Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque mri and stroke risk:a systematic review and meta-analysis[J]. Stroke, 2013, 44:3071-3077.
35 Li ML, Xu WH, Song L, et al. Atherosclerosis of middle cerebral artery:Evaluation with high-resolution MR imaging at 3T[J]. Atherosclerosis, 2009, 204:447-452.
36 Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis:a 3-tesla MRI study[J].Neurology, 2010, 75:253-258.
37 Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage:prevalence and clinical relevance[J]. Ann Neurol, 2012, 71:195-198.
38 Wang J, Bornert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage(SNAP) imaging for carotid atherosclerotic disease evaluation[J]. Magn Reson Med, 2013, 69:337-345.
39 Sun J, Song Y, Chen H, et al. Adventitial perfusion and intraplaque hemorrhage:a dynamic contrast-enhanced MRI study in the carotid artery[J]. Stroke, 2013,44:1031-1036.
