Effect of Sodium Chloride on Meltability of Mozzarella Cheese
2014-03-07ZhangJianqiangLiHaoBianChunCaoRonganandZhangLiping
Zhang Jian-qiang, Li Hao, Bian Chun, Cao Rong-an, and Zhang Li-ping*
1Heilongjiang Bayi Agricultural University, 163319 Daqing, Heilongjiang, China;
2Harbin University,150086 Harbin, China
Effect of Sodium Chloride on Meltability of Mozzarella Cheese
Zhang Jian-qiang1, Li Hao1, Bian Chun2, Cao Rong-an1, and Zhang Li-ping1*
1Heilongjiang Bayi Agricultural University, 163319 Daqing, Heilongjiang, China;
2Harbin University,150086 Harbin, China
Meltability is one of the most important properties of Mozzarella cheese, as it is generally used in pizza and other foods. Mozzarella was prepared by no salted and immature production technology, and the effect of different addition amounts of salt on the meltability of mozzarella cheese was measured by Schreiber method and small amplitude oscillatory shear analysis (SAOSA) method. The results showed that different adding amounts of NaCl had significant influence on the meltability of Mozzarella cheese, and 2% NaCl addition was the best condition. The results measured by the methods of Schreiber and SAOSA were basically same: adding different amounts of NaCl had significant influence on the hardness and elasticity of mozzarella cheese, but no significant influence on the sticky. It was a good microscopic structure arrangement of Mozzarella cheese with 2% NaCl addition. Scanning electron micrographs showed that a space grid structure formed by casein had changed, and formed many uniform molecular holes. The results indicated that different addition amounts of salt had influence on meltability of no salted immature Mozzarella cheese, and this technology could be drastically shorten the processing time.
Mozzarella cheese, meltability, sodium chloride, SAOSA, Schreiber
Introduction
Mozzarella cheese originated in southern Italy, and it is one of typical Pasta Filate cheese (Fox et al., 2000). It is very important procedure that curd being kneaded and stretched in hot water, which make the cheese to a desired characteristics of smooth texture, meltability and stretchability (Early, 1998; Kibstedt, 1992; Chitpinityol and Crabbe, 1998). And a large number of Mozzarella cheese is used for pizza making. Adding NaCl to Mozzarella cheese can increase its flavor in the machining process, improve water activity, and control the growth of microorganism, thus it has the effect of the corrosion part. NaCl is critical for the development of water-holding properties and the solubilization of intact caseins during the early stage of Mozzarella cheese aging (Guo, 1997). However, adding NaCl may affect the protein structure, solution and polymerization, further drawing and meltability property. Guo et al. (1997) showed that increasing salt could reduce the content of Ca2+, and affect the casein calcium phosphate structure (Chitpinityol and Crabbe, 1998; Sheehan, 2004). High salt addition can reduce fat precipitation and moisture content of cheese which can get better meltability. While Rowney et al. (2004) showed no significant influence on the meltability of Mozzarella cheese with increasing NaCl addition.The objective of the current study was to evaluate the effect of different amounts of salt on the meltability of Mozzarella cheese, and to find a quick method of no salted and immature Mozzarella cheese production technology.
Materials and Methods
Cheese manufacture
The raw milk was standardised to obtain a proteinto-fat ratio of 1.2 : 1 and pasteurised at 63.5℃ for 30 min. The milk was cooled to 32℃ and inoculated with a starter culture (FD-DVS TCC-3, Chr Hansen, Denmark) consisting of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus at the level of 0.6%. After the acidity increased to 21°T, the chymosin (Stanmix 1150, Chr Hansen, Denmark) was diluted to 1 : 40 and added 3 g • 100 kg-1milk. The curd was cut after 40 min incubation and held for another 5 min. And the temperature was increased to 42℃with slow stirring, this process should be finished among 30 min. Whey was drained at 18°T. Curd was piled into two slabs and cut, and salting with 2% NaCl. The salted curd was dipped into 80℃ hot water with 3% NaCl, and stretched by hand leading to the center of the cheese to 75℃. The cheese was pressed into mould and allowed to cool for 1 h in 4℃ refrigerator, vacuum-wrapped and stored at 4℃.
Physicochemical analysis
pH determination: using pH meter (HI4222, Cany Precision Instrument, China); moisture content: GB/ T5009.3-2003, national standard of China; fat content: GB/T5009.46-2003, national standard of China; total calcium content: adopting KMnSO4titration method; separable moisture content: according to reference of McMahon (Mcmahon, 1999); and salt determination: AgNO3titration method.
Meltability analysis
Schreiber test: specimen (7 mm thick) was sliced from a cheese block and cut into a circle with 1.7 mm in diameter. Then, the sample was put in a glass Petri dish with a 9 cm filter paper, placed at room temperature for 30 min and preheated to 100℃ for 1 h in the oven. The sample was taken out and put at room temperature for 30 min. The melted cheese collapsed diameter was determined by vernier calipers. The meltability area was calculated according to the equation, S=πR2(Guinee, 2000).
Small amplitude oscillatory shear method (SAOSA method): cheese sample was placed at room temperature for 30 min. A cheese cylinder (2 mm thick, 40 mm diameter) was placed in rheometer pallets (AR 1500ex, TA instruments, USA). The probe of the rheometer fell to the center surface of the cheese. Temperature sweep from 10℃ to 90℃ was performed with the speed of 3℃ • min-1. Measurement was performed at a shearing strain of 0.005 and a constant frequency of 1 Hz. It can get the elastic modulus G', viscosity modulus G", and softening point (G'=G") under different temperatures. These values were used to reflect the cheese meltability properties (Mizuno and Lucey, 2005; Kapoor, 2008).
According to equation of tan δ=G'/G", its corresponding point was softening point of cheese when the value of δ was 45, and the softening point corresponding temperature (Tsp) which represented cheese system viscous modulus became greater than the elastic modulus of the corresponding temperatures. Temperature scanning for cheese can get the result of Ea (activation energy) through the Arrhenius' curve. Activation energy can predict cheese in the heated rheological properties (Tunick, 2010; Gunasekaran, 1998) .
Complex modulus (G*) was obtained from G′and G″, and complex viscosity (η*) representing the resistance to flow was calculated as the followings:

Where, ω was the angular frequency. The Arrhenius equation was as the following:

Where, A was pre-exponential factor, R was gasconstant, and T was the absolute temperature.
Formula (3) could be transformed as the following:

Texture assessment
Sample pretreatment: the cheese sample shape was got by special sampler cuts, whose shape was cylindrical, with diameter 15 mm and height 10 mm for TPA test. All the samples were put in 0℃-4℃ refrigerator 30 min before the test in order to prevent the effect of temperature on the texture of cheese.
Plotting parameters: TPA secondary press method was used for this assessment using the Texture analyser (TA.XT2i, Stable Micro Systems, UK), specific parameters was as the followings: probe falling speed before the test: 5.0 mm • s-1; test probe falling speed: 1.0 mm • s-1; testing probe return rate: 5.0 mm • s-1; press deformation: 30%; trigger force: 0.20 N; and probe type: P/5.
Scanning electron microscopy (SEM)
The cheese was cut into slices (1×1×10 mm) and then fixed in 2.5 % (wt/vol) glutaraldehyde solution, kept in refrigerator for 6 h at 4-6℃. Then, it was washed with the gradient ethanol solution (30%, 50%, 70%, 90% and 100%) for 10 min, respectively, but repeated 100% ethanol solution 3 times. It was degreased 2 h by chloroform, and then washed by 100% ethanol 3 times. The sample was freeze dried, fixed after selection of scanning side, ion gradually gold-plated, and then shot in the scanning electron microscopy (S-4300, HITACHI, Japan) observation (Oberg,1993).
Statistical analysis
Each experimental was performed 5 times. SAS 9.1 statistical software was used for One-way ANOVA analysis. Simplot 12.0 software was for rheological characteristic curve, and R statistical software analyzed activation energy. Finally, linear regression confidence interval of linear equation was obtained by Minitab16.0 software to reflect the two kinds of detection methods fitting.
Results and Discussion
Effect of salting on physicochemical characteristics of Mozzarella cheese
According to One-way ANOVA analysis by SAS 9.1, basic physical and chemical indicators of three different amounts of salt cheese (maturity 30 days) is shown in Table 1. It showed that salt addition had no significant difference on the protein content, fat content and pH of Mozzarella cheese, but had a significant effect on Ca2+and moisture content (p<0.05) with the dose dependent trend. The amount of salt showed positive correlation for the moisture content, and negative correlation for Ca2+content. Moisture content was the highest value of 6.34%, when the amount of salt was 3%. Mozzarella cheese with 3% NaCl addition contained the lowest moisture content and the highest salt content. The reason was that high osmotic pressure of Mozzarella cheese with high salt resulted in the internal water outward flow continuously, and NaCl infiltrated to cheese inside.
Schreiber test
The photographs of meltability of Mozzarella cheese with different dose salt are shown in Fig. 1. The meltability area is shown in Fig. 2. The dose of salt had significant effects on the meltability of cheese (p<0.05). The cheese meltability increased as the amount of salt increasing from 1% NaCl to 2% NaCl, and decreased from 2% NaCl to 3% NaCl. It exhibited the best meltability with 2% NaCl, as different salt addition amounts made an ion exchange process between Na+and Ca2+, which led to the loss of Ca2+and further made the casein micelle gel property be weaking. High amount of NaCl would cause little free oil, because protein hydrolysis became more and more fully. The degradation product of casein changed the original cheese reticular structure characteristics. Small fat globules embedded in softening protein in the outer wall and it was difficult to link together form free oil.
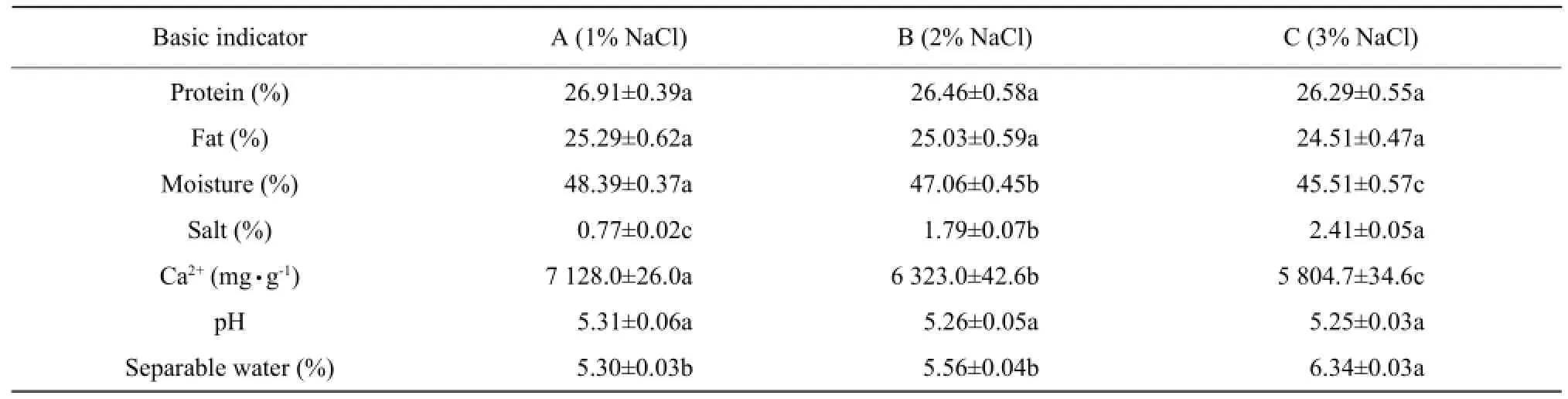
Table 1 Physicochemical characteristics of Mozzarella cheese with different amounts of salt (maturity 30 days)

Fig. 1 Meltability of Mozzarella cheese with different amounts of salt
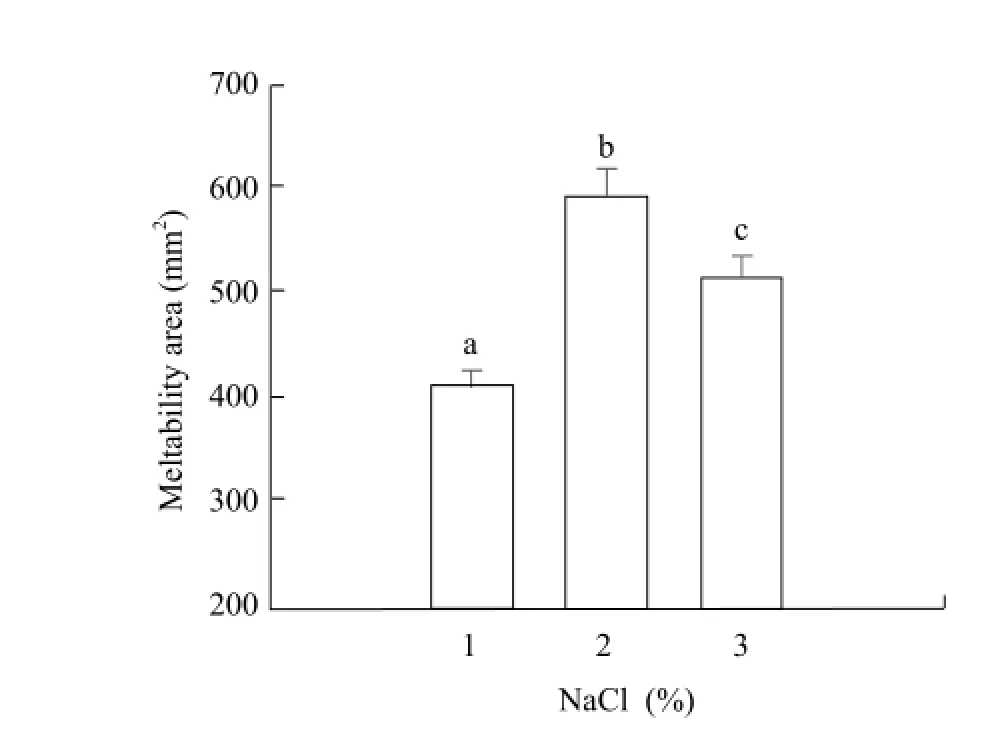
Fig. 2 Meltability area of Mozzarella cheese
Influence of salt addition on rheological property of Mozzarella cheese
The results of the rheological properties of Mozzarella cheese salt are shown in Fig. 3. The temperature dosedependently suppressed the value of G' and G" for all the treatments. The value of G' was higher than G" as the range of the temperature changing from 10℃ to 60℃, which indicated that cheese had more elastic than viscous. The rate of decreasing of G' and G" became slowly when the temperature was from 70℃ to 80℃, but the viscous modulus was higher than the elastic modulus. This change indicated a phase transition from an unmelted cheese to a melted cheese, namely the intersection point of G' and G" was meltability point of the cheese. In comparison of three groups, 1% salt treatment exhibited the lowest δ of Mozzarella cheese at the same temperature. These results were in agreement with previous studies by similar methods on Mozzarella (Udyarajan, 2007), Cheddar (Guineee, 2000) and Gaziantep cheeses (Kahyaoglu, 2007). So further evaluation can be done.
The softening point is shown in Fig. 4. During the heating process from 20℃ to 80℃, Mozzarella cheese of 2% salt treatment achieved its softening point at 51.4℃, and δ was relatively high. The viscosity changed, which demonstrated its good liquidity(Gunasekaran, 1998), and the meltability was the best of the three samples. Mozzarella cheese of 1% and 3% NaCl showed the softening points of 59.5℃ and 53.7℃ respectively, and then began to melt during the meltability range from 20℃ to 80℃. The faster the sample reached its softening point, the earlier it melted and the greater the distance flew, and then the better its meltability. However, Mozzarella cheese with 1% salt addition contained more water. Its meltability was less than Mozzarella cheese of 3% salt treatment. Therefore, moisture content could not decide the meltability of cheese. This was the result equation, y=lnA–(Ea/R)×x, calculated by equation of lnη*=lnA–Ea/RT and lnη*=y, 1/T=x, according to different temperature points of G' and G". Three canonical plottings are shown in Fig. 5.
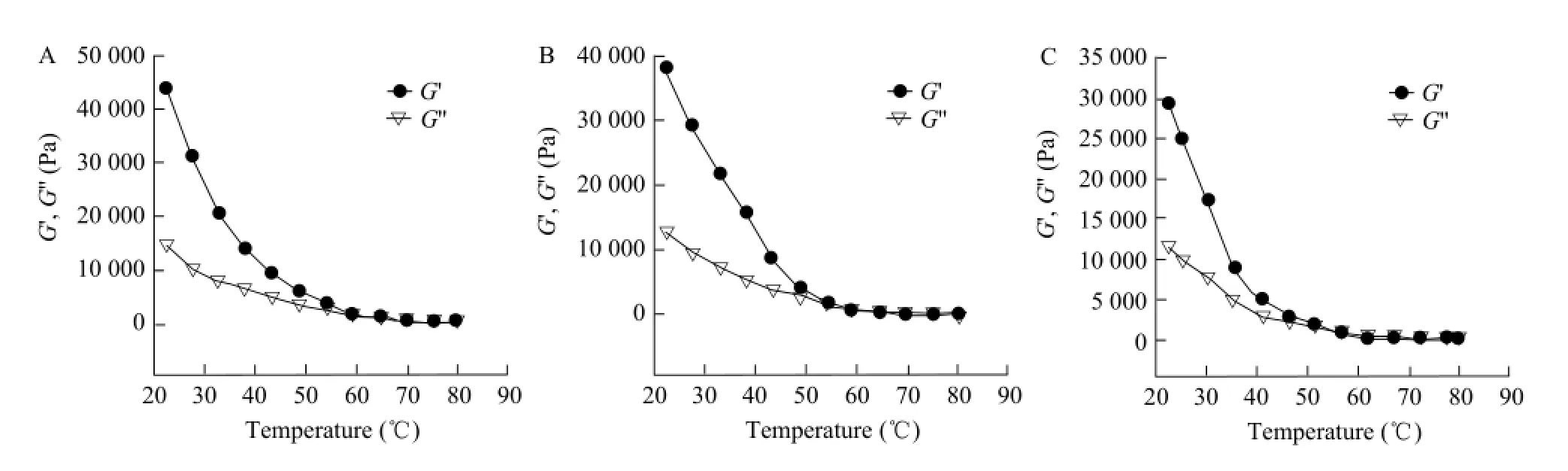
Fig. 3 Rheological properties of Mozzarella cheese with different amounts of salt
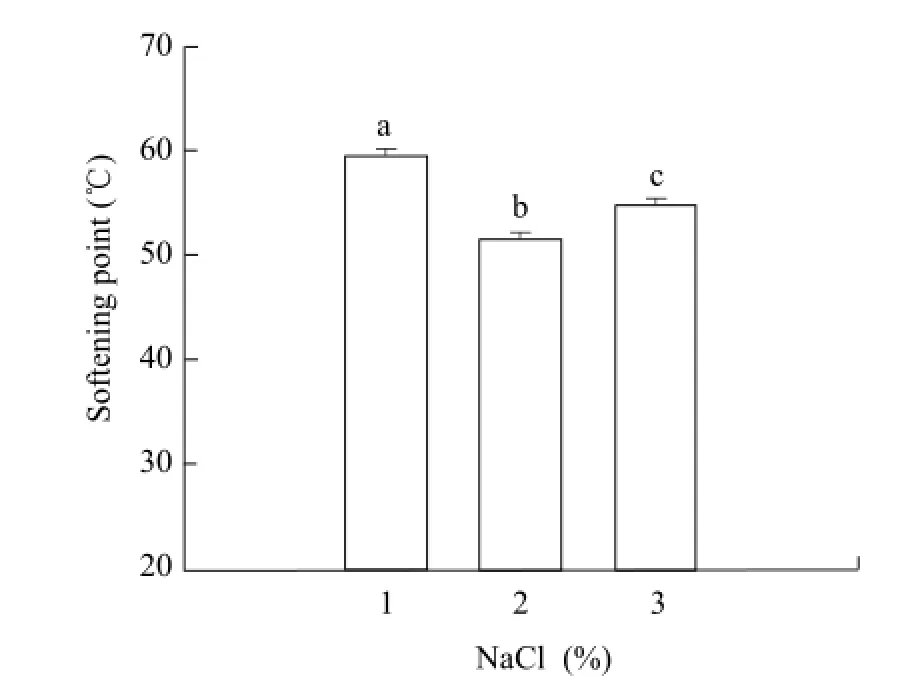
Fig. 4 Softening point of Mozzarella cheese added different amounts of salt
According to the above formula, three groups of Ea were as the followings: 60.8, 61.3, 62.7, 73.4, 74.1, 73.2, 68.1, 69.3 and 68.5 kJ • mol-1, respectively, which demonstrated that there was a close relationship between Ea and protein content, moisture content. Higher Ea was caused by strong protein network, and NaCl promoted hydration character to the casein of cheese. NaCl could decrease surface hydrophobic effects of protein and increase the water binding capacity of protein. So it led to whey protein in the protein linear structure entering into the network of the protein, which enhanced protein network structure. High concentration of NaCl induced the change of cheese external osmotic pressure and affected the hydration property of the protein, resulting in influence of the protein network structure (McMahon, 1999).
The fitting graph is shown in Fig. 6. There was a linear relation of cheese meltability assessed by Schreiber test and SAOSA with correlation coefficient of 99.0% except one point in the confidence interval of 95.0%. In conclusion, the two methods had consistent results. These results were in agreement with previous study reported by Xi et al ( 2011).
lnfluence of different doses salt on texture of Mozzarella cheese
The texture result of cheese (maturity 30 days) isshown in Table 2. It demonstrated that different doses of salt had significant influence on hardness and elastic of Mozzarella cheese (p<0.05), but no significant influence on stick. It exhibited the highest hardness and elastic character of Mozzarella cheese with 3% salt addition. The reason was that the high concentration of NaCl inhibited the growth of starter culture and protease activity, prevented the free water and partial proteins combined to become hydrated proteins (Cervantes, 1983) which improved the hardness and elastic increased; another reason was that the cheese was of high NaCl concentration leading to high osmotic pressure, resulting in internal moisture outward flow continuously. It decreased the moisture content of cheese, further improved hardness and elastic of cheese.
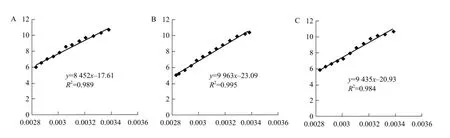
Fig. 5 Arrhenius equation standard graph of Mozzarella cheese added different amounts of salt A, 1% NaCl addition; B, 2% NaCl addition; C, 3% NaCl addition.
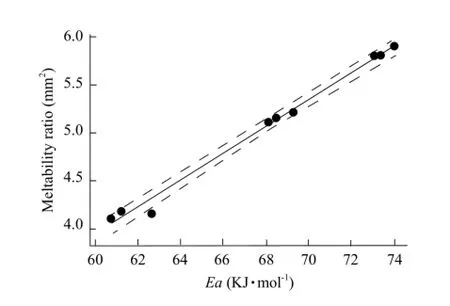
Fig. 6 Fitting graph of Mozzarella cheese with different amounts of salt meltability by Schreiber method and SAOSA method
Influence of salt addition on microscopic structure and meltability of Mozzarella cheese
The photograph of scanning electron microscope is shown in Fig. 7. It was in good order for the microscopic structure arrangement of Mozzarella cheese with 2% NaCl addition. The space grid structure which was formed by casein had changed, and led to many uniform molecular holes. For other two cheese with 1% and 3% NaCl addition, they were not in good order for the microscopic structure arrangement. It had shaped a big molecular hole by the space grid structure of the protein and the structure of protein became loose. NaCl could increase the hydration of the casein in cheese. The more salt, the higher hydration of casein, and then the more expanded of casein matrix in cheese. The high concentration salt inhibited the hydrolization of the protein. So proteolysis was asymmetrical. The degradation product of casein changed the primary character of structure and formed larger hole. However, the low concentration of salt could promote the activity of microflora in cheese and increase the proteolysis. So the microscopic structure changed and bigger hole formed. The appropriate concentration of NaCl could affect the emulsification of casein (Kindstedt, 1992). The proteolysis was symmetrical, the microscopic structure was in good order and many uniform molecular holes shaped. So the meltability of the cheese was the best for 2% NaCl addition in all the treatments.
Table 2 Influence of NaCl addition on texture of Mozzarella cheese
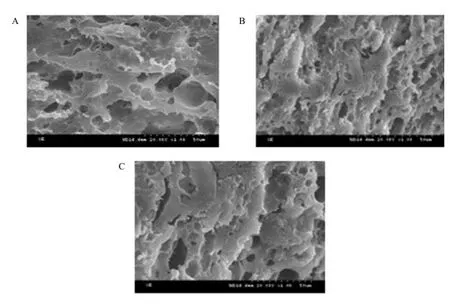
Fig. 7 Scanning electron micrographs of different amounts of salt in Mozzarella cheese
Conclusions
The results indicated that NaCl addition amounts had no significant effects on the protein, fat and pH, but had significant effects on Ca2+and moisture content of Mozzarella cheese. It affected meltability of Mozzarella cheese significantly. The best NaCl addition amount was 2%, and given the best meltability of Mozzarella cheese characters. The results of Schreiber and SAOSA showed a good linear relation, which indicated the results were reliable. The salt addition had significant effects on the hardness and elasticity of cheese, but no significant effects on sticky. It was in good order for the microscopic structure arrangement of Mozzarella cheese with 2% NaCl addition. If we made Mozzarella cheese through no salted and immature production technology, and then took 2% NaCl to modify the cheese, we could get a better meltability of mozzarella cheese. This processing technology could shorten the time of Mozzarella cheese processing.
Cervantes M A, Lund D B, Olson N F. 1983. Effects of salt concentration and freezing on Mozzarella cheese texture. Journal of Dairy Science, 66(2): 204-213.
Chitpinityol S, Crabbe M J C. 1998. Chymosin and aspartic proteinases. Food Chemistry, 61(4): 395-418.
Fox P F, Mcsweeney P L H, Cogan T M, et al. 2000. Fundamentals ofcheese science. Aspen Publishers Inc, Gaitherburg. pp. 7-8.
Guinee T P, Auty M A E, Mullins C, et al. 2000. Preliminary observations on effects of fat content and degree of fat emulsification on the structure-functional relationship of Cheddar-type cheese. Journal of Texture Studies, 31(6): 645-663.
Guinee T P. 2000. The functionality of cheese as an ingredient: a review. Journal Dairy Technology, 57(2): 79-91.
Gunasekaran S, Kuo M I, Wang Y C. 1998. Evaluating melt characteristics of Mozzarella cheese by a linear viscoelastic test. Australian Journal of Dairy Technology, 53(2): 111.
Guo M R, Gilmore J A, Kindstedt P S, et al. 1997. Effect of sodium chloride on the serum phase of Mozzarella cheese. Journal of Dairy Science, 80: 3092-3098.
Kahyaoglu T, Kaya S, Kaya A. 2005. Effects of fat reduction and curd dipping temperature on viscoelasticity, texture and appearance of Gaziantep cheese. Food Science and Technology International, 11(3): 191-198.
Kapoor R, Metzger L E. 2008. Process cheese: scientific and technological aspects a review. Comprehensive Reviews in Food Science and Food Safety, 7(2): 194-214.
Kindstedt P S, Kiely L J, Gilmore J A. 1992. Variation in composition and functional properties within brine salted Mozzarella cheese. Journal of Dairy Science, 75(11): 2913-2921.
Kinstedt P S. Kiely L J, Gilmore J A, 1992. Variation in composition and functional properties within brine-salted Mozzarella cheese. Journal of Dairy Science, 75(11): 2913-2921.
Kuo M I, Gunasekaran S. 2009. Effect of freezing and frozen storage on microstructure of Mozzarella and pizza cheeses. LWT-Food Science and Technology, 42(1): 9-16.
McMahon D J, Fife R L, Oberg C J. 1999. Water partitioning in Mozzarella cheese and its relationship to cheese meltability. Journal of Dairy Science, 82(7): 1361-1369.
Mizuno R, Lucey J A. 2005. Effect of two types of emulsifying salts on the functionality of pasta cheese. Journal of Dairy Science, 8(10): 3411-3425.
Early R. 1998. The technology of dairy products. 2rd ed. B1ackie Academic Professional, London. pp. 20-21.
Rowney M K, Roupas P, Hick Y M W, et al. 2004. Salt-induced structural changes in 1-day old Mozzarella cheese and the impact upon free oil formation. International Dairy Journal, 14(9): 809-816.
Sheehan J J, Guinee T P. 2004. Effect of pH and calcium level on the biochemical, textural and functional properties of reducedfat Mozzarella cheese. International Dairy Journal, 14(2): 161-172.
Tunick M H. 2010. Activation energy measurements in rheological analysis of cheese. International Dairy Journal, 20(10): 680-685.
Udyarajan C T, Horne D S, Lucey J A. 2007. Use of time-temperature superposition to study the rheological properties of cheese during heating and cooling. International Journal of Food Science & Technology, 42(6): 686-698.
Xi X M, Bryony J, Lu Z, et al. 2011. Correlating Mozzarella cheese properties to production processes by rheological, mechanical and microstructure study: meltability study and activation energy. Food Science, 1: 536-544.
TS252.53
A
1006-8104(2014)-03-0068-08
Received 18 February 2014
Supported by 12th Five-year Plan of National Ministry of Science and Technology (2011BAD09B02); National Ministry of Science and Technology (2009GJB20010)
Zhang Jian-qiang (1982-), Master, research assistant, engaged in the research of dairy science. E-mail: zjq049@163.com
* Corresponding author. Zhang Li-ping, professor, engaged in the research of agricultural product processing. E-mail: zip77@126.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effect of Seed Soaking with Exogenous Proline on Seed Germination of Rice Under Salt Stress
- Physiological Changes and Cold Tolerance of Three Camphor Species During Natural Winter Temperature Fluctuations
- Effects of Methylated Soybean Oil Adjuvant on Fomesafen Efficacy to Weeds
- Method for Isolating Mitochondrial DNA from Etiolated Tissue of Cabbage
- Lentivirus Mediated Gene Manipulation in Trophectoderm of Porcine Embryos
- Effects of Allicin on Lipid Metabolism and Antioxidant Activity in Chickens
