Effects of Allicin on Lipid Metabolism and Antioxidant Activity in Chickens
2014-03-07WangGongchenHanLuluWangJingLangWannanPanChuanyiandLiYanfei
Wang Gong-chen, Han Lu-lu, Wang Jing, Lang Wan-nan,, Pan Chuan-yi,, and Li Yan-fei*
1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2Harbin Pharmaceutical Group Bio-vaccine Co., Ltd., Harbin 150069, China
Effects of Allicin on Lipid Metabolism and Antioxidant Activity in Chickens
Wang Gong-chen1, Han Lu-lu1, Wang Jing1, Lang Wan-nan1,2, Pan Chuan-yi1,2, and Li Yan-fei1*
1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2Harbin Pharmaceutical Group Bio-vaccine Co., Ltd., Harbin 150069, China
To investigate the effects of allicin on chickens' lipid and antioxidant performance, Hy-laying hens' diets were replenished with 0 mg • kg-1, 50 mg • kg-1, 100 mg • kg-1, and 150 mg • kg-1allicin for 42 days, respectively. The alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TCHO), high density lipoprotein (HDL), and low density lipoprotein (LDL) levels were measured in chicken serum. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity and malondialdehyde (MDA) levels were measured in chicken serum and liver tissue homogenate. The results showed that the supplement dose of allicin tested did not significantly change the activity of ALT or AST (P>0.05); TG and CHOL levels decreased with the increase of allicin additive doses, and the difference between treatment groups and CG was significant (P<0.05), and there was the best effect with 100 mg • kg-1; allicin significantly reduced the content of MDA, and increased SOD and GSH-Px activities compared with CG (P<0.05), and 100 mg • kg-1of allicin resulted in the strongest SOD and GSH-Px activity. The antioxidant function test results of liver tissue homogenate were consistant with that of serum. Our findings indicated that allicin could enhance antioxidant capacity and reduce blood lipid level in chickens and 100 mg • kg-1was the optimal amount of allicin additives.
allicin, chicken, lipid metabolism, antioxidant capacity
Introduction
Allicin is one of the major active ingredients in garlic, and plays a role in antibacterial, antioxidant, anticancer, and anti-atherosclerosis activities, decreases cholesterol, and inhibits platelet aggregation (Kim and Chun, 2001). The impacts of allicin on lipid metabolism and antioxidant capacity are mostly tested on rat experiments. However, such researches on poultry are rarely, and the results are controversial. Allicin is reported as the main stimulant in garlic and sulfides. The decomposition product of allicin has cytotoxicity (Amagase et al., 2001). In this experiment, different doses of allicin were added in Hy-laying hens' diets to explore the effect of allicin on lipid metabolism and antioxidant capacity, and clarify the optimal amount of allicin additives in chickens. Our finding can provide a theoretical basis for allicin application in the poultry industry.
Materials and Methods
Chemicals
Allicin was purchased from Shandong Binzhou Institute of Animal Husbandry and Veterinary Project Development, with effective contents of 25%. Prior to use, the actual amount of allicin needed was calcu-lated, and then was mixed with the basal diet.
Experimental animals and treatment
A total of 80 healthy brown shell Hy-laying hen chickens at 1 day age with similar weights were selected for this study (Harbin Guangda Breeder Farm). After one week pre-fed, all chickens were randomly divided into four groups with 20 per group. 0 mg • kg-1(control group, CG), 50 mg • kg-1(lowdose group, LG), 100 mg • kg-1(middle dose group, MG), and 150 mg • kg-1(high dose group, HG) allicin were added to basal diets, respectively. Each group of chickens had free access to feed and water. The study lasted for 42 days, during the experiment, disinfection and immunization were performed according to regular breeding protocol. In the following morning of the last day of this study (off feed for 12 h), blood was collected from heart and centrifugated at 3 000 r • min-1for 10 min to obtain serum. Chickens were humanistic euthanized at the end of the study, and 0.5 g liver tissue of each chicken was homogenized to detect content of malondialdehyde (MDA), activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). The serum and liver tissues were stored at –70℃ before the detection of lipid metabolism and antioxidant capacity.
Testing index and method
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TCHO), high density lipoprotein (HDL), and low density lipoprotein (LDL) were measured with enzymatic and biochemical detection kit, and analyzed by automatic biochemical analyzer.
SOD, MDA, and GSH-Px were measured with xanthine oxidase method, thiobarbituric acid colorimetric method, and UV colorimetric biochemical assay kit, respectively, then analyzed by spectrophotometer.
Statistical analysis
Data obtained were presented as mean±standard deviation (X±SD). Microsoft Excel 2003 and DPSv7.05 data processing system were used for statistical analyses.
Results
Allicin effect on liver function parameters
Table 1 demonstrated that allicin additive did not significantly affect ALT and AST activities, or cause significant change of the trend.

Table 1 Evaluation index of liver function (UI • L-1)
Effects of allicin on lipid metabolism
TG and TCHO levels decreased with the increase of allicin additive doses, and significant differences were observed between treatment groups and CG. HDL levels increased with the increase of allicin additive doses, but there was no significant difference between treatment groups and CG. LDL level in HG was significantly lower than that in CG, but there was no significant difference in LG and MG (Table 2).
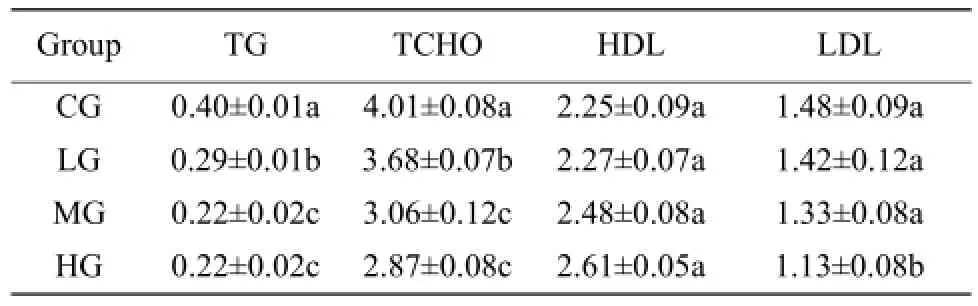
Table 2 Effect of allicin on lipid metabolism (μmol • L-1)
Effect of allicin on antioxidant activity
In serum and liver tissue, SOD and GSH-Px activities increased with the increased doses of allicin additive, and there was the strongest effect in MG. MDA in all the treatment groups were all significantly lowerthan in CG, and such reduction was dose-dependent (Tables 3 and 4).

Table 3 Effect of allicin on antioxidant capacity in serum

Table 4 Effect of allicin on antioxidant capacity in liver tissue
Discussion
Aminotransferase catalyzes the transformation of amino in amino acid, and plays an important role in the process of sugar, protein, and fat transformation (Gu et al., 2006). ALT and AST are abundant in liver cells. When liver cells were damaged, ALT and AST were released into the blood. Thus, ALT and AST levels in serum were often referred to determine poultry liver function and the health status. ALT and AST standard levels were rarely reported. A number of studies showed that ALT and AST levels in chicken serum differed slightly by performance (such as chickens and hens), species, and genders (Chen et al., 1998). In this study, serum ALT and AST levels were not significantly different among treatment groups. These results were consistent with results from the study done by Hong et al. (2005) on Bovans brown hens, and Lee et al. (2011) on C57BL/6J mice. Therefore, we could conclude that allicin did not have direct impacts on ALT and AST levels of healthy chicken. In addition, this study also indicated that the dose of allicin we used was within a reasonable range, since no cytotoxicity and damage to the liver cells were observed.
Cholesterol absorption in small intestine can be inhibited by saponins, which is component in allicin, saponins can also reduce plasma cholesterol level. Zhang et al. (2007) showed allicin was able to reduce TG and TCHO levels in experimental hyperlipidemic mice. Plasma TG and TCHO levels significantly decreased in AA broilers after they were fed with diets with allicin additive (Wan, 2003; Zhu, 2004). Results of the study showed allicin at different doses could significantly reduce TG and TCHO levels. This further illustrated that garlic had lipid-reducing effect. The mechanism, on one hand, may be the metabolism and transformation between lipoproteins were facilitated; on the other hand, may due to the inhibition of intestinal absorption of cholesterol, reduction of cholesterol synthesis in the liver, and enhancement of serum and liver TG decomposition rate. The results showed that allicin significantly reduced blood LDL levels in hypercholesterolemia animal and hyperlipidemia patients (Matsuura, 2001; Kojuri et al., 2007), which had unanimous conclusion. When the amount of additive allicin was 150 mg • kg-1, serum LDL level in chickens significantly declined. Studies by Zhang et al. (2007) considered that allicin elevated HDL levels in experimental hyperlipidemia mice significantly. However, in this study, HDL levels showed no significant difference between any groups with different doses of allicin additive, though a rising trend of HDL content was observed along with increasing amount of allicin additives. Such different conclusions might be related to different animal species, and cholesterol metabolism pathways and extent varied in different physiological stages of these two species.
Antioxidant activity of allicin is related to effectively scavenging oxygen free radicals effect. Zhang and Shi (2002) observed that allicin could effectively remove • O2-and • OH radicals, and also showed a significant dose-dependent relationship. Garlic and its extracts had strong antioxidant activity. SOD, asan oxygen radical scavenger, may indirectly reflect the levels of oxygen free radicals and lipid peroxides content in the body. MDA is a product of membrane lipid peroxidation. Increased MDA indicated peroxidation enhancement caused by tissue damage and reduced antioxidant mechanisms, and also could indirectly reflect the degree of cell damage. Therefore, mediated by lipid peroxidation (Kawamura et al., 1995; Hsu et al., 2009; Cuzzocrea and Reiter, 2001). In addition, Zheng and Ding (2001) investigated that allicin significantly increased the activity of the liver tissue GSH, and reduced MDA levels. In the study of nicotine-induced lipid peroxidation injury in mice, Helen et al. (1999) found that GSH-Px activity increased in mice taking garlic oil. In this study, serum SOD and GSH-Px activities in chickens fed by different doses of allicin increased significantly than those in the control group, and when the additive amount was 100 mg • kg-1, the increasing effect peaked. In addition, MDA level significantly decreased with additive allicin amounts. Thus, we inferred that allicin, as a feed additive, could significantly improve the antioxidant capacity of chickens and reduce lipid peroxidation damages.
Conclusions
Allicin enhanced antioxidant capacity and reduced blood lipid level in chickens, and appeared the strongest effects with 100 mg • kg-1of allicin.
Amagase H, Petesch B L, Matsuura H, et al. 2001. Intake of garlic and its bioactive components. The Journal of Nutrition, 131(3): 955-962.
Chen G H, Bian J C, Wang K H, et al. 1998. Comparsion on activity of plasma AKP, GPT, CPK in Chinese antive chickens. The Journal of Northwest Agricultural University, 26(4): 66-70.
Cuzzocrea S, Reiter R J. 2001. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. European Journal of Pharmacology, 426(1-2): 1-10.
Gu Y F, Liu A H, Liu D Y, et al. 2006. Effects of cysteamine on the part physiological and biochemical parameters of broilers. Cereal & Feed Industry, 8: 34-36.
Helen ARajasree C R, Krishnakumar K, Augusti K T, et al. 1999. Antioxidant role of oils isolated from garlic (Allium sativum Linn) and onion (Allium cepa Linn) on nicotine-induced lipid peroxidation. Veterinary and Human Toxicology, 41(5): 316-319.
Hong W, Gong Y S, Li J G, et al. 2005. Effects of allicinon on production capacity caecum microorganism and serum biochemical index in laying hens. Progress in Veterinary Medicine, 26(9): 85-87.
Hsu Y W, Tsai C F, Chenb W K, et al. 2009. Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food and Chemical Toxicology, 47(9): 2281-2288.
Kawamura E, Yamakana N, Tomo F, et al. 1995. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury. Hepatology, 21(4): 1138-1143.
Kim H J, Chun H S. 2001. Biological functions of organosulfur of compounds in allium vegetables. Journal of the Korean Society of Food Science and Nutrition, 28(6): 1412-1423.
Kojuri J, Vosough I A R, Akrami M. 2007. Effects of anethum graveolens and garlic on lipid profile in hyperlipidemic patients. Lipids Health Dis, 6(5): 1-5.
Lee M S, Kim I H, Kim C T, et al. 2011. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. Nutr, 141: 1947-1953.
Matsuura H. 2001. Saponins in garlic as modifiers of the risk of cardiovascular disease. The Journal of Nutrition, 131(3): 1000-1005.
Wan J Y. 2003. Effects of some Chinese medical herbs on blood biochemical index of chickens. Animal Husbandry & Veterinary Medicine, 35(11): 8-10.
Zhang J L, Shi Z X. 2002. An experimental study of scavenging oxygen free radicals with garlicin. Journal of China-Japan Friendship Hospital, 16(5-6): 298-300.
Zhang T Y, Tong X Q, Liu X Y. 2007. Study on the anithyperlipidemia effect of allicin and the mechanism. Chinese Journal of Experimental Traditional Medical Formulae, 13(2): 32-35.
Zheng M, Ding H. 2001. Protective effect of garlicin on experimental liver injury in mice. Chinese Traditional and Herbal Drugs, 32(5): 440-442.
Zhu L X. 2004. Protective effect of garlicin on hepatic fibrosis induced by dimethylnitrosamine in rats. Chinese Traditional and Herbal Drugs, 35(2): 1384-1387.
Q493; S831
A
1006-8104(2014)-03-0046-04
Received 26 February 2014
Wang Gong-chen (1984-), male, Master, engaged in the research of veterinary medicine. E-mail: wanggongchen@163.com
* Corresponding author. Li Yan-fei, Ph. D, professor, engaged in the research of veterinary medicine. E-mail: yanfeili200@126.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effect of Seed Soaking with Exogenous Proline on Seed Germination of Rice Under Salt Stress
- Physiological Changes and Cold Tolerance of Three Camphor Species During Natural Winter Temperature Fluctuations
- Effects of Methylated Soybean Oil Adjuvant on Fomesafen Efficacy to Weeds
- Method for Isolating Mitochondrial DNA from Etiolated Tissue of Cabbage
- Lentivirus Mediated Gene Manipulation in Trophectoderm of Porcine Embryos
- Design and Implementation of Stud-farm Daily Management System Based on C/S Structure
