Synthesis and Identification of SG-BSA and SG-OVA
2014-03-07ZhuZeyaoZhanChunxiaandZhangQizhong
Zhu Ze-yao, Zhan Chun-xia, and Zhang Qi-zhong
1Institute of Hydrobiology, Jinan University, Guangzhou 510632, China
2Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, China
3Hainan Nongken Middle School, Haikou 570226, China
Synthesis and Identification of SG-BSA and SG-OVA
Zhu Ze-yao1,2, Zhan Chun-xia1,3, and Zhang Qi-zhong1*
1Institute of Hydrobiology, Jinan University, Guangzhou 510632, China
2Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, China
3Hainan Nongken Middle School, Haikou 570226, China
Hapten sulfaguanidine (SG) was coupled with carrier protein bovine serum albumin (BSA) to form a full antigen SGBSA by diazotization and glutaraldehyde methods. Ovalbumin (OVA) was used as protein carrier to couple with Hapten SG by glutaraldehyde method. Then, the immunogen and the coating antigen were purified by dialysis and gel exclusion chromatography. The conjugated ratio of SG to BSA in artificial antigen was 5.3 (using diazotization method) and 6.5 (glutaraldehyde method), and the conjugated ratio of SG to OVA in coating antigen was 2.3 (glutaraldehyde method) by UV-visible spectrophotometer. The coupling was successful according to the analysis of sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). BALB/c mice were immunized with the antigen (SG-BSA), and the titers of antiserum were tested to be 1 : 6 400 and 1 : 400 after three periods of immunities by indirect ELISA, which further identified the success of the synthesis of both immunogen SG-BSA and coating antigen SG-OVA.
sulfaguanidine (SG), immunogen, coating antigen, ELISA
Introduction
Sulfaguanidine [4-Amino-N-(diaminomethylene) benzenesulfonamide, SG] belongs to the family of sulfa drugs which are commonly used in the animal industry to treat enteritis and bacillary dysentery (Cui et al., 2004). As a favorable entero-antiseptic, sulfaguanidine has wide applications in fishery. However, SG is highly toxic (Harris et al., 1943), as it can cause kidney damages, blood abnormalities, and reduce the growth rate of mammalian species (Daft et al., 1942). So it is necessary to establish rapid method to detect sulfonamide residues in aquatic animals.
At present, the most common method to detect SG residues is chromatography, such as high performance liquid chromatography (HPLC) and high performance liquid chromatography coupled to a mass spectrometer (HPLC-MS) (Msagati et al., 2004; Maudens et al., 2004). However, these methods are time-consuming, labor-intensive and require expensive equipments that are available only in laboratories, and are not suitable for the convenient market monitoring (Fang et al., 2004). The determination method of enzyme-linked immunosorbent assay (ELISA) is demonstrated as a simple, rapid, cost-effective and highly sensitive method in quantitative detection of sulfonamide residue (Muldoon et al., 2000). In order to establish ELISA assay method, it is necessary to get the monoclonal antibodies against sulfaguanidine residues.
The molecular mass of sulfaguanidine (MW 232.26) is too low to induce production of specific antibody against it in an animal. But SG can become an immunogen after conjugation with bovine serum albumin (BSA). In this study, SG-BSA was synthesized with the diazotization method (Fleeker et al., 1985) or the glutaraldehyde method (Deborah et al., 1991), and SG-Ovalbumin (OVA) was synthesized by the glutaraldehyde method. Then synthesis of SG-BSA and SG-OVA was proved to be successful by identification experiments. This work laid a foundation for the preparation of monoclonal antibodies against sulfaguanidine residues.
Materials and Methods
Chemicals and reagents
Sulfaguanidine (SG), purity 99.6%, complete Freund's adjuvant (CFA), incomplete Freund's adjuvant (IFA), o-phenylenediamine (OPD), Tween-20, bisacrylamide, acrylamide and ovalbumin (OVA, MW 44000) were all purchased from Sigma Chemicals Co., USA. Bovine serum albumin (BSA, MW 67000) was purchased from Genview, USA. Sephadex-G75 medium was obtained from Pharmacia Company, Sweden. Goat anti-mouse IgG-horseradish-peroxidase (IgG-HRP) was purchased from Protein Tech Group. All other chemicals and organic solvents used were of analytical grade. Water was obtained from a MilliQ purification system (Millipore). The experimental animals were female BALB/C mice (7-week-old), obtained from the Center of Experimental Animals in the Medical College of Sun Yat-sen University, China.
Preparation of SG-BSA and SG-OVA
In diazotization method, SG was dissolved in 10 cm3of aqueous hydrochloric acid solution (0.1 mol • dm-3) and this solution was cooled to 4℃ in refrigerator. Then NaNO2(0.1 mol • dm-3) was added dropwise until a KI-starch test paper became blue, indicating formation of the diazonium chloride salt of SG. This diazonium chloride salt solution was stirred for 1 h at 4℃ by a rotary mixer, and then a small amount of carbamide was added to stop reaction. Adjusted to pH 9.0 using NaOH (1.0 mol • dm-3), the diazonium chloride salt solution was added dropwise into 5 cm3of BSA solution (50 mg BSA in 5 cm3of pH 9.5 CBS aqueous solution) and stirred gently for 5 h at 4℃. The reaction solution was then dialyzed extensively against PBS for 3 days at 4℃ to remove free SG and other small molecules. PBS was changed every 12 h, and then the final solution was lyophilized and kept at –20℃.
According to the glutaraldehyde method, 15 cm3of 1, 4-Dioxane containing 50 mg SG was added to phosphate buffered saline (PBS) (15 cm3, 0.1 mol • dm-3, pH 7.2) containing 100 mg BSA or 70 mg OVA, and glutaraldehyde (0.2 cm3, 25%) was added to this mixed solution dropwise. Then the reaction solution was stirred gently for 5 h at room temperature, and it was dialyzed extensively against PBS for 3 days at 4℃to remove free SG and other small molecules. PBS was changed every 12 h. The final reaction solution was lyophilized and kept at –20℃.
Purification of SG-BSA and SG-OVA
In gel filtration chromatography (GFC), Sephadex-G75 chromatographic column (100 cm×1.5 cm) was equilibrated with 0.01 mol • dm-3PBS at a flow rate of 0.2 cm3• min-1overnight. Then, the conjugate dissolved in 0.01 mol • dm-3PBS (5 cm3) was loaded onto the chromatographic column, and went through the colum at the flow rate of 0.2 cm3• min-1. The eluting solution of target peak was collected between 400 min and 900 min. The final collecting sample was lyophilized and stored at –20℃ for the future use.
ldentification of SG-BSA and SG-OVA
SG-BSA, SG-OVA, standard BSA and standard OVA solutions were tested though UV spectrum scans from 200 nm to 400 nm wavelength (Tijssen, 1985). The concentrations of conjugates were calculated according to the formula: concentration (mg • cm-3) = 1.45×OD280–0.74×OD260. The molar absorbancy index was calculated by the following formulas, K=A/RCT,where, K was the molar absorbancy index, A was the absorbance, C was the concentration, and T was the thickness of colorimetric cylinder. Then molar ratio of conjugate was calculated by the following formula:
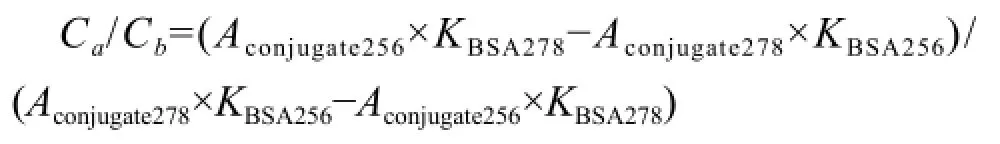
Where, Ca/Cbwas the molecule ratio of hapten to carrier protein, Aconjugatewas the absorbance of conjugate, KBSAwas the molar absorbancy index of BSA, and KSGwas the molar absorbancy index of SG.
SDS-PAGE
The conjugates were dissolved with Tris-HCl sample buffer (10 mmol • dm-3Tris-HCl, pH 6.8, 0.1% bromophenol blue, 2% w/v SDS, 10% glycerol, 0.2 mol • dm-3DTT) and denatured for 3-5 min at 100℃, and then loaded onto the gels including 5% spacer gel (4.83% w/v acrylamide, 0.17% w/v bisacrylamide, 10% w/v SDS, 0.126 mol • dm-3Tris • HCl, pH 6.8, 10% w/v ammonium persulfate, and 0.1% v/v TEMED), 15% separation gel (14.5% w/v acrylamide, 0.5% w/v bisacrylamide, 10% w/v SDS, 0.375 mol • dm-3Tris • HCl, pH 8.8, 10% w/v ammonium persulfate, and 0.1% v/v TEMED). Electrophoresis was carried out in Tris-Gly electrophoresis buffer (25 mmol • dm-3Tris, 250 mmol • dm-3glycine, pH 8.4, 0.5% w/v SDS). First, the constant voltage was 8 V • cm-1, then increased to 15 V • cm-1after bromophenol blue was added into the separation gel. Gels were stained with the Coomassie Brilliant Blue R-250 solution (0.5% w/v Coomassie Brilliant Blue R-250, 45% v/v methanol, 10% v/v acetic acid) overnight, then shaken and destained for 2 h with the destaining solution (25% v/v methanol, 10% v/v acetic acid). Gels were scanned and analyzed by protein analysis system after destaining.
Immunization of animals
Experimental animals
BALB/c female mice (7-week-old) were immunized with SG-BSA conjugates synthesized by the diazotization and glutaraldehyde methods, respectively. The mice were injected intraperitoneally at a dose of 100 μg conjugate with Freund's complete adjuvant for the first time and with Freund's incomplete adjuvant for the other two times at a 2-week interval. Serum samples were collected from the tail vein of anaesthetized mice on the 7th day after the third immunization, and antibody titers were determined by indirect ELISA using SG-OVA as the coating antigen.
Indirect ELISA
96 wells-microtitre plates were coated with SG-OVA in carbonate buffer (0.1 mol • dm-3, pH 9.6) (0.1 cm3• well-1) by overnight incubation at 4℃. Plates were washed three times with PBS (0.01 mol • dm-3, pH 7.4) containing 1% Tween 20 (PBST), and then 0.2 cm3• well-1of 5% skim milk in PBS (0.01 mol • m-3, pH 7.4) was added to each well to block the plastic surface and eliminate nonspecific binding. After incubating for 2 h at 37℃, plates were washed three times with PBST. Antiserum (φr=1 : 200, 1 : 400, 1 : 800, 1 : 1 600, 1 : 3 200, 1 : 6 400, 1 : 12 800) was then added to each well (0.1 cm3• well-1) and incubated for 1 h at 37℃. After incubation, plates were washed three times with PBST, and goat anti-mouse IgG marked with Horseradish Peroxidase (HRP) (1 : 10 000) was added (0.1 cm3• well-1), and incubated for 1 h at 37℃. Plates were washed three times with PBST. A 0.1 cm3POD solution was added to each well and incubated for 8-15 min at 37℃ in the dark. H2SO4(2 mol • m-3) was added to stop the reaction and the absorbance value at 492 nm was determined by microplate reader.
Results
In diazo-reaction, SG diazonium salt was linked to tyrosine groups, histidine groups and tryptophan groups of BSA (Fig. 1).
UV spectrum (Fig. 2) showed that the major absorbance peaks of SG were at 259 nm, while those of BSA was at 278 nm and 206 nm. The spectrum of SG-BSA (by the method of diazotization) had two absorbance peaks. The first absorbance peak of 260 nm indicated that the absorbance peak of BSA at 278 nm had shifted to the absorbance peak of SG at 259 nm.The second absorbance peak at 340 nm was the peak of azo-bond, which indicated that SG diazonium salt was linked to BSA successfully. The spectrum of SGBSA or SG-OVA conjugates (by the glutaraldehyde method) (Figs. 2 and 3) also had two absorbance peaks. The first absorbance peak at 268 nm indicated that the absorbance peak of protein at 278 nm had shifted to the absorbance peak of SG at 259 nm. The second peak at 211 nm indicated that the complete peptide bond existed in the conjugates. Those confirmed that SG had been conjugated to the carrier proteins successfully. Molar ratio was 6.5 for SG-BSA (by the glutaraldehyde method), 5.3 SG-BSA (by the method of diazotization), and 2.3 for SG-OVA.
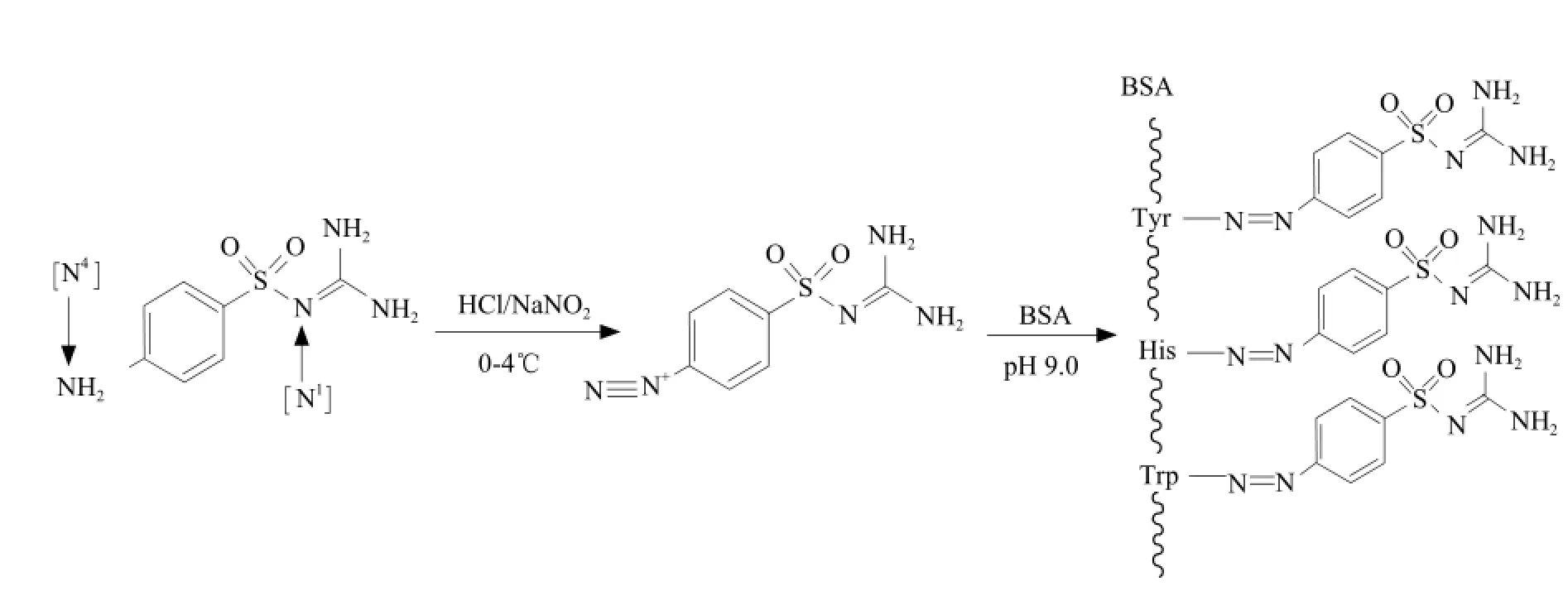
Fig. 1 Synthesis of SG-BSA with method of diazotization
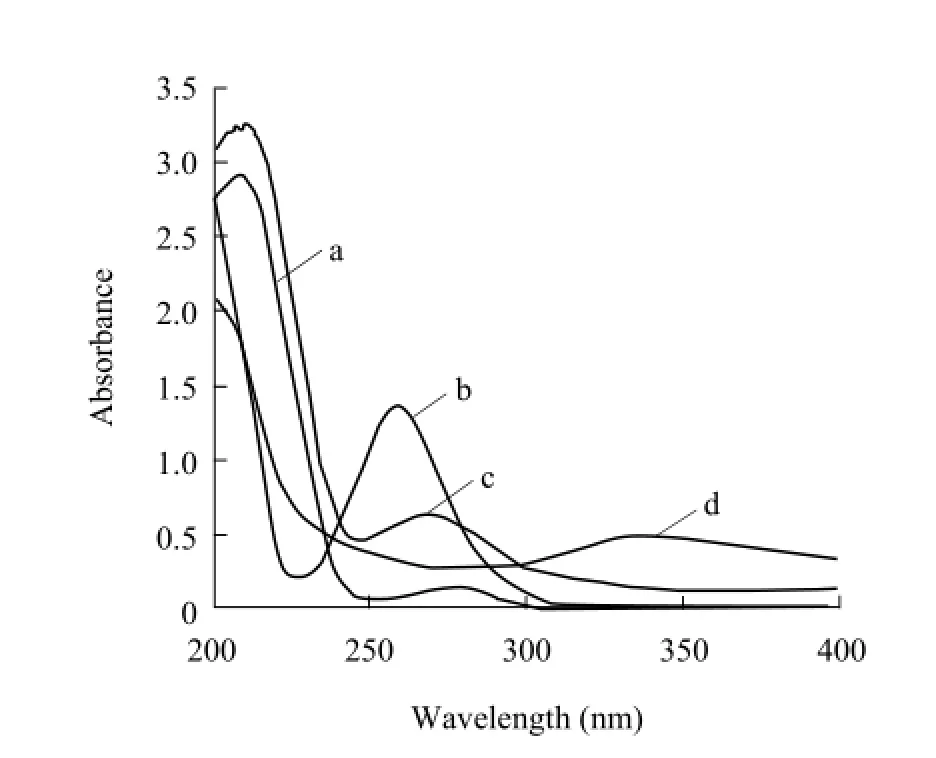
Fig. 2 UV scanning spectrum of immunogen SG-BSA
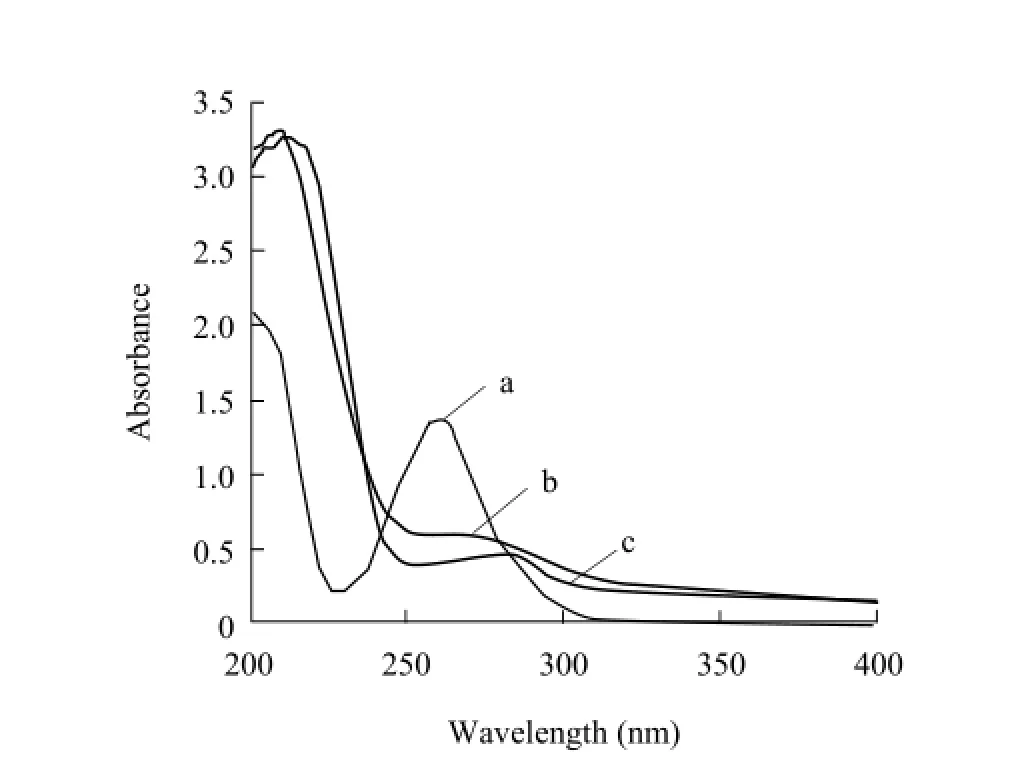
Fig. 3 UV scanning spectrum of coating antigen SG-OVA
The electrophoretic results showed that the band (Lane1) of SG-BSA synthesized with the diazotization method had tailing phenomenon and was lower than that of BSA in spite of the similar molecular mass between SG-BSA and BSA (66.2 ku) (Lane M) (Fig. 4). The band (Lane 2) of SG-BSA synthesized with the glutaraldehyde method was apparently lower than that of BSA (66.2 ku) (Lane M) (Fig. 4). These results indicated that the two coupling methods were both successful.
The antibody titer was defined as the reciprocal of the antiserum dilution that gave an absorbance value 2.1 times than that of the negative control at 492 nm. The results of indirect ELISA showed thatimmunization of mice with SG-BSA could elicit significant immune response. The antibody titer induced by SG-BSA was 1 : 6 400 (by the diazotization method) and 1 : 400 (by the glutaraldehyde method).
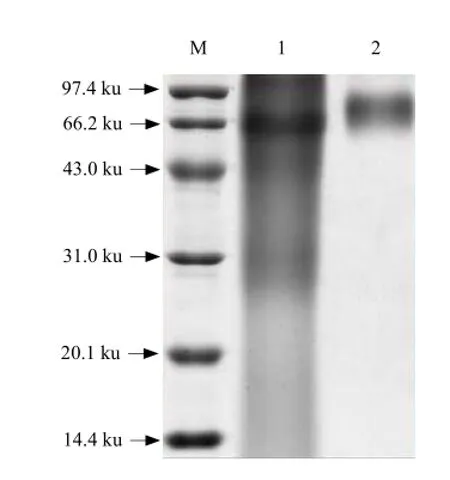
Fig. 4 SDS-PAGE illustration of SG-BSA
Discussion
Sulfaguanidine (MW 232.26) is a micro-molecule compound that is too small to induce production of specific antibody against it in an animal, but SG can become an immunogen after conjugation with bovine serum albumin (BSA). Like the other sulphonamide, SG contains an aromatic amine group (N4position) and an amide group (N1position) (Verdel et al., 2006). If N4-amino group was linked with BSA, specific antibody against SG could be synthesized. If N1-amino group was linked with BSA, antibody cluster against sulphonamide could be synthesized (Li et al., 2002). In this study, we synthesized SG-BSA with the method of diazotization, therefore, N4-amino group of SG was linked with BSA, and the specific antibody against SG could be obtained.
Some studies indicated that molecule conjugate ratio could contribute to generation of antibody. Schneider et al. (1992) recently demonstrated that the optimal molecule conjugate ratio was 10-20. Hodgson et al. (1991) showed while the molecule conjugate ratio was 3-25, the hapten-carrier protein could result in the best immune response. However, Little et al. (1969) reported that one molecule conjugate ratio was enough to make the specific antibody generation. In our study, Molar ratios of SG-BSA were 6.5 (by the glutaraldehyde method) and 5.3 (by the diazotization method), respectively. But antibody titer (1 : 6 400) induced by SG-BSA synthesized with the diazotization method was higher than that (1 : 400) with the glutaraldehyde method. These results suggested that the molecule conjugate ratio did not have a crucial effect on the generation of antibody, and antibody titer might have something to do with solubility of artificial immunogen (Parker et al., 1981).
In the view of molecular structure, SG diazonium salt became SG-BSA after being linked to tyrosine groups, histidine groups and tryptophane groups of BSA in the method of diazotization. The glutaraldehyde belongs to the bifunctional agents and its aldehyde groups can couple free amino groups to form conjugates. However, self-coupling is easy to happen by using the glutaraldehyde method, which brings a lot of difficulties to the purification of conjugates. From what has been stated above, the method of diazotization is much better than the glutaraldehyde method to synthesize SG-BSA, which agrees with the previous studies done by Cao et al (2004).
Cao S F, Cheng M R, Liu Y D, et al. 2004. The study of comparison on two sulfadimethoxine immunizing antigens constructed with two different methods. Chinese Journal of Preventive Veterinary Medicine, 26(4): 301-303.
Cui X J, Zhang Z Y, Ren Y L, et al. 2004. Quality control of the powder pharmaceutical samples of sulfaguanidine by using NIR reflectance spectrometry and temperature-constrained cascade correlation networks. Talanta, 64(4): 943-948.
Daft F S, Ashburn L L, Sebrell W H. 1942. Biotin deficiency and other changes in rats given sulfanilylguanidine or succinyl sulfathiazole in purified diets. Science, 96(2492): 321-322.
Deborah E D, Stanley E K. 1991. Competitive direct enzyme-linkedimmunosorbent screening assay for the detection of sulfamethazine contamination of animal feed. Assoc Off Anal Chem, 74(5): 784-789.
Fang C, Wang H D, Wang Z Y, et al. 2004. Study on the residues of sulfonamides by enzyme immunoassay. Shanghai Journal of Animal Husbandry and Veterinary Medicine, 2: 16-18.
Fleeker J R, Lovett L J. 1985. Drug residues in animal tissues. Enzyme immunoassay for screening sulfamethazine residues in swineblood. J Assoc off Anal Chem, 68(2): 172-174.
Harris J S, Kohn H I. 1943. The chronic toxicity of the sulfonamides for the growing rat as influenced by the type of diet, the addition of feces to the diet, and appetite. Journal of Pharmacology And Experimental Therapeutics, 78(1): 56-64.
Hodgson E, Roc R M, Motoyama N. 1991. Pesticides and the future: toxicological studies of risks and benefits. North Carolina State University, Raleigh N C. pp. 273-287.
Li J S, Qiu Y M, Wang C. 2002. Analysis of veterinary drugs residue. Press of shanghai Scientific Technology, Shanghai. pp. 251-252.
Little J R, Counts R B. 1969. Affinity and heterogeneity of antibodies induced by epsilon-2, 4-dinitrophenylinsulin. Biochemistry, 8(7): 2729-2736.
Maudens K E, Zhang G F, Lambert W E. 2004. Quantitative analysis of twelve sulfonamides in honey after acidic hydrolysis by highperformance liquid chromatography with post-column derivatization and fluorescence detection. J Chromatogr A, 1047(1): 85-92.
Msagati T A M, Nindi M M. 2004. Multiresidue determination of sulfonamides in a variety of biological matrices by supported liquid membrane with high pressure liquid chromatography-electrospray mass spectrometry detection. Talanta, 64(1): 87-100.
Muldoon M T, Holtzapple C K, Deshpande S S, et al. 2000. Development of a monoclonal antibody-based cELISA for the analysis of sulfadimethoxine. 1. Development and characterization of monoclonal antibodies and molecular modeling studies of antibody recognition. J Agric Food Chem, 48(2): 537-544.
Parker C W. 1981. Radioimmunoassay of biologically active compounds. Press of Science, Beijing. pp. 165.
Schneider P, Hammock B D. 1992. Influence of the ELISA format and the hapten-enzyme conjugate on the sensitivity of an immunoassay for S-triazine herbicides using monoclonal antibodies. J Agric Food Chem, 40(3): 525-530.
Tijssen P. 1985. Practice and theory of enzyme immunoassay. Elsevier Amsterdam, New York. pp. 27.
Verdel B M, Souverein P C, Egberts A C G, et al. 2006. Difference in risks of allergic reaction to sulfonamide drugs based on chemical structure. The Annals of Pharmacotherapy, 40(6): 1040-1046.
R392.11
A
1006-8104(2014)-02-0062-06
Received 3 November 2013
Zhu Ze-yao (1982-), male, Master, research assistant, engaged in the research of antibody and aquatic product safety. E-mail: zhuzy007@163.com
* Corresponding author. Zhang Qi-zhong, Ph. D, professor, engaged in the research of quality and safety of aquatic products. E-mail: zqz666@sina. com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regulation of Foliar Application DCPTA on Growth and Development of Maize Seedling Leaves in Heilongjiang Province
- Comparison of Physiological Properties Between Dwarf and Vinetype Cucumbers (Cucumis sativus Linn.)
- Effects of Substitute Media on Development of Potted Cyclamen percicum Mill.
- Expression of HSP72 in Mouse Preimplantation Embryos with Heat Shock
- Effects of Maternal Dietary Energy Restriction on Fat Deposition of Offspring
- Effects of Dietary Protein and Temperature on Growth and Flesh Quality of Songpu Mirror Carp
